Extending Landsat 8: Retrieval of an Orange contra-Band for Inland Water Quality Applications
Abstract
:1. Introduction
2. Theory
3. Data and Methods
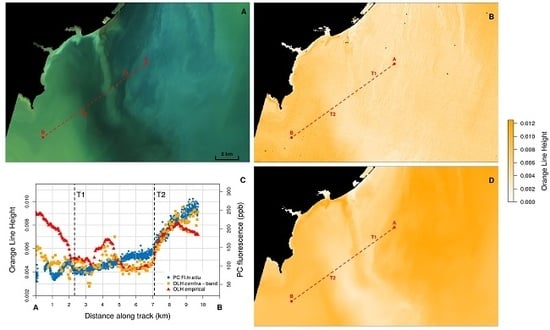
3.1. Analysis
3.2. In Situ Dataset
3.2.1. Dutch Lakes
3.2.2. Belgian Lakes
3.2.3. Radiometric Measurements
3.2.4. Pigment Concentration
4. Results
4.1. Calibration and Validation of the Orange contra-Band Retrieval
4.2. Sensitivity to Errors in the Input Data
4.3. Generality of the OLI Orange contra-Band Algorithm
4.4. Cross-Validation with OLCI/Sentinel-3 Imagery
4.5. Contribution of New Information from the Orange contra-Band
4.6. Example Application: Lake Erie Cyanobacteria Bloom
5. Discussion
6. Conclusions
- The relative spectral profiles of the SRFs are equivalent within their overlapping regions.
- The spectrally narrower band(s) is(are) completely contained in the broader band.
- Measurements of the overlapping bands are collocated in space and time.
- There is no superposition between the narrower bands.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Atmospheric compensation |
| CA | Coastal/Aerosol (band 1 of OLI) |
| CDOM | Chromophoric dissolved organic |
| Chl | Chlorophyll |
| CV | Coefficient of variation |
| Diff. | Difference |
| DSF | Dark Spectrum Fitting |
| Equiv. | Equivalent |
| FWHM | Full Width at Half Maximum |
| MERIS | Medium Resolution Imaging Spectrometer |
| MS | Multispectral |
| NA | Not available |
| NAP | Non-Algal Particles |
| NIR | Near Infrared |
| OC | Ocean Colour |
| OLCI | Ocean and Land Colour Instrument |
| OLH | Orange line height |
| OLI | Operational Land Imager |
| OWT | Optical Water Types |
| Pan | Panchromatic |
| PC | Phycocyanin |
| PCI | Phycocyanin index |
| SD | Standard deviation |
| SNR | Signal to noise ratio |
| SRF | Spectral response function |
| SWIR | Shortwave Infrared |
| TOA | Top of atmosphere |
Appendix A. Generalization and Derivation of the contra-Band Analytical Algorithm
References
- CEOS. Feasibility Study for an Aquatic Ecosystem Earth Observing System; Technical Report; Commonwealth Scientific and Industrial Research Organisation (CSIRO): Canberra, Australia, 2018. [Google Scholar]
- IOCCG. Earth Observations in Support of Global Water Quality Monitoring; Technical Report; International Ocean-Colour Coordinating Group (IOCCG): Darmouth, NS, Canada, 2018. [Google Scholar]
- Muller-Karger, F.E.; Hestir, E.; Ade, C.; Turpie, K.; Roberts, D.A.; Siegel, D.; Miller, R.J.; Humm, D.; Izenberg, N.; Keller, M.; et al. Satellite sensor requirements for monitoring essential biodiversity variables of coastal ecosystems. Ecol. Appl. 2018, 28, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Andreadis, K.M.; Schumann, G.J.; Pavelsky, T. A simple global river bankfull width and depth database. Water Resour. Res. 2013, 49, 7164–7168. [Google Scholar] [CrossRef]
- Vanhellemont, Q.; Ruddick, K.G. Turbid wakes associated with offshore wind turbines observed with Landsat 8. Remote Sens. Environ. 2014, 145, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Franz, B.A.; Bailey, S.W.; Kuring, N.; Werdell, P.J. Ocean color measurements with the Operational Land Imager on Landsat-8: Implementation and evaluation in SeaDAS. J. Appl. Remote Sens. 2015, 9, 096070. [Google Scholar] [CrossRef]
- Trinh, R.C.; Fichot, C.G.; Gierach, M.M.; Holt, B.; Malakar, N.K.; Hulley, G.; Smith, J. Application of Landsat 8 for Monitoring Impacts of Wastewater Discharge on Coastal Water Quality. Front. Mar. Sci. 2017, 4, 329. [Google Scholar] [CrossRef] [Green Version]
- Vanhellemont, Q.; Ruddick, K.G. Advantages of high quality SWIR bands for ocean colour processing: Examples from Landsat-8. Remote Sens. Environ. 2015, 161, 89–106. [Google Scholar] [CrossRef] [Green Version]
- Lee, Z.; Shang, S.; Qi, L.; Yan, J.; Lin, G. A semi-analytical scheme to estimate Secchi-disk depth from Landsat-8 measurements. Remote Sens. Environ. 2016, 177, 101–106. [Google Scholar] [CrossRef]
- Ogashawara, I.; Li, L.; Moreno-Madriñán, M.J. Slope algorithm to map algal blooms in inland waters for Landsat 8/Operational Land Imager images. J. Appl. Remote Sens. 2016, 11, 012005. [Google Scholar] [CrossRef] [Green Version]
- Olmanson, L.G.; Brezonik, P.L.; Finlay, J.C.; Bauer, M.E. Comparison of Landsat 8 and Landsat 7 for regional measurements of CDOM and water clarity in lakes. Remote Sens. Environ. 2016, 185, 119–128. [Google Scholar] [CrossRef]
- Stumpf, R.P.; Wynne, T.T.; Baker, D.B.; Fahnenstiel, G.L. Interannual Variability of Cyanobacterial Blooms in Lake Erie. PLoS ONE 2012, 7, e42444. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Song, K. Bio-optical Modeling of Phycocyanin. In Bio-optical Modeling and Remote Sensing of Inland Waters; Mishra, D.R., Ogashawara, I., Gitelson, A.A., Eds.; Elsevier: Chippenham, UK, 2017; Chapter 8; pp. 233–262. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Ruiz-Verdú, A.; Domínguez-Gómez, J.A.; Peña-Martinez, R.; Peters, S.W.; Gons, H.J. Influence of phytoplankton pigment composition on remote sensing of cyanobacterial biomass. Remote Sens. Environ. 2007, 106, 414–427. [Google Scholar] [CrossRef]
- Kutser, T.; Metsamaa, L.; Strömbeck, N.; Vahtmäe, E. Monitoring cyanobacterial blooms by satellite remote sensing. Estuarine Coast. Shelf Sci. 2006, 67, 303–312. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Robertson, L. An algorithm for detecting trophic status (chlorophyll-a), cyanobacterial-dominance, surface scums and floating vegetation in inland and coastal waters. Remote Sens. Environ. 2012, 124, 637–652. [Google Scholar] [CrossRef]
- Wernand, M.R.; Shimwell, S.J.; De Munck, J.C. A simple method of full spectrum reconstruction by a five-band approach for ocean colour applications. Int. J. Remote Sens. 1997, 18, 1977–1986. [Google Scholar] [CrossRef]
- Lee, Z.; Shang, S.; Hu, C.; Zibordi, G. Spectral interdependence of remote-sensing reflectance and its implications on the design of ocean color satellite sensors. Appl. Opt. 2014, 53, 3301. [Google Scholar] [CrossRef]
- Sun, D.; Hu, C.; Qiu, Z.; Wang, S. Reconstruction of hyperspectral reflectance for optically complex turbid inland lakes: Test of a new scheme and implications for inversion algorithms. Opt. Express 2015, 23, A718. [Google Scholar] [CrossRef]
- Sun, D.; Hu, C.; Qiu, Z.; Shi, K. Estimating phycocyanin pigment concentration in productive inland waters using Landsat measurements: A case study in Lake Dianchi. Opt. Express 2015, 23, 3055–3074. [Google Scholar] [CrossRef] [Green Version]
- Simis, S.G.H.; Kauko, H.M. In vivo mass-specific absorption spectra of phycobilipigments through selective bleaching. Limnol. Oceanogr. Methods 2012, 10, 214–226. [Google Scholar] [CrossRef]
- Beck, R.; Zhan, S.; Liu, H.; Tong, S.; Yang, B.; Xu, M.; Ye, Z.; Huang, Y.; Shu, S.; Wu, Q.; et al. Comparison of satellite reflectance algorithms for estimating chlorophyll-a in a temperate reservoir using coincident hyperspectral aircraft imagery and dense coincident surface observations. Remote Sens. Environ. 2016, 178, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Nikolakopoulos, K.G. Comparison of Nine Fusion Techniques for Very High Resolution Data. Photogramm. Eng. Remote Sens. 2008, 74, 647–659. [Google Scholar] [CrossRef] [Green Version]
- Hoepffner, N.; Sathyendranath, S. Determination of the major groups of phytoplankton pigments from the absorption spectra of total particulate matter. J. Geophys. Res. 1993, 98, 22789. [Google Scholar] [CrossRef]
- Wang, G.; Lee, Z.; Mishra, D.R.; Ma, R. Retrieving absorption coefficients of multiple phytoplankton pigments from hyperspectral remote sensing reflectance measured over cyanobacteria bloom waters. Limnol. Oceanogr. Methods 2016, 14, 432–447. [Google Scholar] [CrossRef] [Green Version]
- Pahlevan, N.; Lee, Z.; Wei, J.; Schaaf, C.B.; Schott, J.R.; Berk, A. On-orbit radiometric characterization of OLI (Landsat-8) for applications in aquatic remote sensing. Remote Sens. Environ. 2014, 154, 272–284. [Google Scholar] [CrossRef]
- Pahlevan, N.; Sarkar, S.; Franz, B.A.; Balasubramanian, S.V.; He, J. Sentinel-2 MultiSpectral Instrument (MSI) data processing for aquatic science applications: Demonstrations and validations. Remote Sens. Environ. 2017, 201, 47–56. [Google Scholar] [CrossRef]
- Gregg, W.W.; Carder, K.L. A simple spectral solar irradiance model for cloudless maritime atmospheres. Limnol. Oceanogr. 1990, 35, 1657–1675. [Google Scholar] [CrossRef]
- Zibordi, G.; Voss, K.J. Geometrical and spectral distribution of sky radiance: Comparison between simulations and field measurements. Remote Sens. Environ. 1989, 27, 343–358. [Google Scholar] [CrossRef]
- Castagna, A.; Carol Johnson, B.; Voss, K.; Dierssen, H.M.; Patrick, H.; Germer, T.A.; Sabbe, K.; Vyverman, W. Uncertainty in global downwelling plane irradiance estimates from sintered polytetrafluoroethylene plaque radiance measurements. Appl. Opt. 2019, 58, 4497. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L.; Lee, Z. Uncertainties of SeaWiFS and MODIS remote sensing reflectance: Implications from clear water measurements. Remote Sens. Environ. 2013, 133, 168–182. [Google Scholar] [CrossRef]
- Vanhellemont, Q.; Ruddick, K. Atmospheric correction of metre-scale optical satellite data for inland and coastal water applications. Remote Sens. Environ. 2018, 216, 586–597. [Google Scholar] [CrossRef]
- Vanhellemont, Q. Adaptation of the dark spectrum fitting atmospheric correction for aquatic applications of the Landsat and Sentinel-2 archive. Remote Sens. Environ. 2019, 225, 175–192. [Google Scholar] [CrossRef]
- Zibordi, G.; Mélin, F.; Berthon, J.F.; Holben, B.; Slutsker, I.; Giles, D.; D’Alimonte, D.; Vandemark, D.; Feng, H.; Schuster, G.; et al. AERONET-OC: A Network for the Validation of Ocean Color Primary Products. J. Atmos. Ocean. Technol. 2009, 26, 1634–1651. [Google Scholar] [CrossRef]
- Spyrakos, E.; O’Donnell, R.; Hunter, P.D.; Miller, C.; Scott, M.; Simis, S.G.H.; Neil, C.; Barbosa, C.C.F.; Binding, C.E.; Bradt, S.; et al. Optical types of inland and coastal waters. Limnol. Oceanogr. 2017, 63. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.D.; Ramaswamy, L.; Lawson, V. CyanoTRACKER: A Citizen Science Project for Reporting Harmful Algal Blooms. In Proceedings of the 2016 IEEE 2nd International Conference on Collaboration and Internet Computing (CIC), Pittsburgh, PA, USA, 1–3 November 2016; pp. 391–397. [Google Scholar] [CrossRef]
- Fu, G.; Baith, K.S.; McClain, C.R. SeaDAS: The SeaWiFS Data Analysis System. In Proceedings of the Fourth Ocean Remote Sensing Conference, Qingdao, China, 28–31 July 1998; pp. 73–77. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Descy, J.P.; Pirlot, S.; Verniers, G.; Viroux, L.; Lara, Y.; Wilmotte, A.; Vyverman, W.; Vanormelingen, P.; Van Wichelen, J.; Van Gremberghe, I.; et al. B-BLOOMS 2-Cyanobacterial Blooms: Toxicity, Diversity, Modeling and Management; Technical Report; Belgian Science Policy: Brussels, Belgium, 2011.
- Lee, Z.; Pahlevan, N.; Ahn, Y.H.; Greb, S.; O’Donnell, D. Robust approach to directly measuring water-leaving radiance in the field. Appl. Opt. 2013, 52, 1693. [Google Scholar] [CrossRef] [PubMed]
- Barsi, J.; Lee, K.; Kvaran, G.; Markham, B.; Pedelty, J. The Spectral Response of the Landsat-8 Operational Land Imager. Remote Sens. 2014, 6, 10232–10251. [Google Scholar] [CrossRef] [Green Version]
- Mueller, J.L.; Morel, A.; Frouin, R.; Davis, C.; Arnone, R.; Carder, K.; Lee, Z.; Steward, R.G.; Hooker, S.; Mobley, C.D.; et al. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 4, Volume III: Radiometric Measurements and Data Analysis Protocols; Technical Report; NASA, Goddard Space Flight Space Center: Greenbelt, MD, USA, 2003.
- Rijstenbil, J.W. Effects of UVB radiation and salt stress on growth, pigments and antioxidative defence of the marine diatom Cylindrotheca closterium. Mar. Ecol. Prog. Ser. 2003, 254, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, A. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Morfitt, R.; Barsi, J.; Levy, R.; Markham, B.; Micijevic, E.; Ong, L.; Scaramuzza, P.; Vanderwerff, K. Landsat-8 Operational Land Imager (OLI) radiometric performance on-orbit. Remote Sens. 2015, 7, 2208–2237. [Google Scholar] [CrossRef] [Green Version]
- Donlon, C.; Berruti, B.; Buongiorno, A.; Ferreira, M.H.; Féménias, P.; Frerick, J.; Goryl, P.; Klein, U.; Laur, H.; Mavrocordatos, C.; et al. The Global Monitoring for Environment and Security (GMES) Sentinel-3 mission. Remote Sens. Environ. 2012, 120, 37–57. [Google Scholar] [CrossRef]
- OLCI Expert Support Laboratories. S3 OLCI Cyclic Performance Report; Technical Report 44; European Space Agency: Paris, France, 2019. [Google Scholar]
- Moore, T.S.; Mouw, C.B.; Sullivan, J.M.; Twardowski, M.S.; Burtner, A.M.; Ciochetto, A.B.; McFarland, M.N.; Nayak, A.R.; Paladino, D.; Stockley, N.D.; et al. Bio-optical Properties of Cyanobacteria Blooms in Western Lake Erie. Front. Mar. Sci. 2017, 4, 300. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Hu, C.; Duan, H.; Cannizzaro, J.; Ma, R. A novel MERIS algorithm to derive cyanobacterial phycocyanin pigment concentrations in a eutrophic lake: Theoretical basis and practical considerations. Remote Sens. Environ. 2014, 154, 298–317. [Google Scholar] [CrossRef]
- Dekker, A.G. Detection of Optical Water Quality Parameters for Eutrophic Waters by High Resolution Remote Sensing. Ph.D. Thesis, Vrije University, Amsterdam, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Irons, J.R.; Dwyer, J.L.; Barsi, J.A. The next Landsat satellite: The Landsat Data Continuity Mission. Remote Sens. Environ. 2012, 122, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Pahlevan, N.; Schott, J.R.; Franz, B.A.; Zibordi, G.; Markham, B.; Bailey, S.; Schaaf, C.B.; Ondrusek, M.; Greb, S.; Strait, C.M. Landsat 8 remote sensing reflectance (R rs ) products: Evaluations, intercomparisons, and enhancements. Remote Sens. Environ. 2017, 190, 289–301. [Google Scholar] [CrossRef]
- Wang, G.; Lee, Z.; Mouw, C. Multi-Spectral Remote Sensing of Phytoplankton Pigment Absorption Properties in Cyanobacteria Bloom Waters: A Regional Example in the Western Basin of Lake Erie. Remote Sens. 2017, 9, 1309. [Google Scholar] [CrossRef] [Green Version]










| Id | System | Region | Country | Coordinates | Matchups |
|---|---|---|---|---|---|
| 1 | Kremenchuk Reservoir | Eastern Europe | Ukraine | 24 (19) | |
| 2 | Rybinsk Reservoir | Eastern Europe | Russia | 7 (6) | |
| 3 | Tsimlyansk Reservoir | Eastern Europe | Russia | 17 (8) | |
| 4 | Somasila Reservoir | Asia | India | 10 (3) | |
| 5 | Lake Dianchi | Asia | China | 7 (6) | |
| 6 | Lake Burrumbeet | Oceania | Australia | 13 (5) | |
| 7 | Bloemhof Dam | Africa | South Africa | 21 (3) | |
| 8 | Laguna Mar Chiquita | South America | Argentina | 11 (11) | |
| 9 | Lake Ilopango | Central America | El Salvador | 18 (15) | |
| 10 | Lake Erie | North America | US and Canada | 4 (3) |
| Chl a | Chl b | Chl c | Carotenoids | PC | PE | |
|---|---|---|---|---|---|---|
| Concentration (mg m) | ||||||
| Netherlands | 1.90–334.18 (36.44) | 0.15–6.75 (2.51) | 0.00–8.82 (0.72) | 0.81–47.65 (11.38) | 0.01–329.41 (22.04) | 0.33–17.54 (4.16) |
| Belgium | 0.63–382.72 (12.98) | 0.00–11.33 (0.89) | 0.00–9.26 (0.47) | 0.26–39.37 (5.42) | NA | NA |
| Ratio to Chl a (unitless) | ||||||
| Netherlands | 1 | 0.00–0.16 (0.07) | 0.00–0.23 (0.02) | 0.14–0.78 (0.31) | 0.00–2.99 (0.68) | 0.02–1.61 (0.11) |
| Belgium | 1 | 0.00–0.44 (0.08) | 0.00–0.22 (0.05) | 0.06–0.94 (0.48) | NA | NA |
| CA | Blue | Green | Red | NIR | Pan | |
|---|---|---|---|---|---|---|
(unitless) | 284 | 321 | 223 | 113 | 45 | 112 |
(W mm sr) | 51.2 | 36.6 | 21.1 | 9.1 | 2.8 | 15.1 |
(W mm) | 1167.4 | 1263.1 | 1125.5 | 1008.4 | 649.8 | 1086.7 |
(sr) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagna, A.; Simis, S.; Dierssen, H.; Vanhellemont, Q.; Sabbe, K.; Vyverman, W. Extending Landsat 8: Retrieval of an Orange contra-Band for Inland Water Quality Applications. Remote Sens. 2020, 12, 637. https://doi.org/10.3390/rs12040637
Castagna A, Simis S, Dierssen H, Vanhellemont Q, Sabbe K, Vyverman W. Extending Landsat 8: Retrieval of an Orange contra-Band for Inland Water Quality Applications. Remote Sensing. 2020; 12(4):637. https://doi.org/10.3390/rs12040637
Chicago/Turabian StyleCastagna, Alexandre, Stefan Simis, Heidi Dierssen, Quinten Vanhellemont, Koen Sabbe, and Wim Vyverman. 2020. "Extending Landsat 8: Retrieval of an Orange contra-Band for Inland Water Quality Applications" Remote Sensing 12, no. 4: 637. https://doi.org/10.3390/rs12040637