Combining Laser-Induced Breakdown Spectroscopy (LIBS) and Visible Near-Infrared Spectroscopy (Vis-NIRS) for Soil Phosphorus Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of the Soil Samples
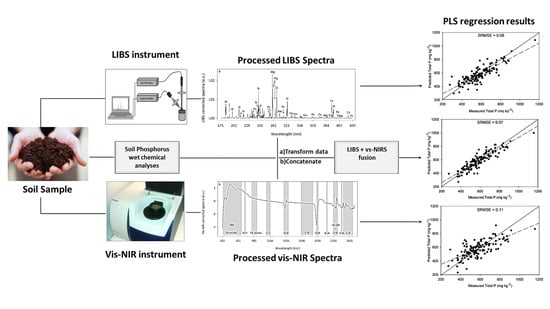
2.2. Wet Chemical P Analyses
2.3. Laser-Induced Breakdown Spectroscopy Analyses
2.4. Visible and Near-Infrared Spectroscopy Analyses
2.5. Spectra Preprocessing
2.6. Variable Selection
2.7. Regression Modeling for P Determination
3. Results and Discussion
3.1. Soil Samples and Their Characteristics
3.2. LIBS Results
3.2.1. PLS Regression Models
3.2.2. LIBS Wavelengths Selected for P Determination
3.3. Vis–NIR Results
3.3.1. PLS Regression Models
3.3.2. Vis-NIRS Wavelengths Selected for P Determination
3.4. Combined LIBS-Vis-NIRS Results
3.4.1. PLS Regression Models
3.4.2. Combined LIBS-Vis-NIR Wavelengths Selected for P Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Phosphorus. In Handbook of Plant Nutrition; Hopkins, B.G., Ed.; CRC Press: London, UK, 2015; ISBN 9781439881989. [Google Scholar]
- Rubæk, G.H.; Kristensen, K.; Olesen, S.E.; Østergaard, H.S.; Heckrath, G. Phosphorus accumulation and spatial distribution in agricultural soils in Denmark. Geoderma 2013, 209–210, 241–250. [Google Scholar] [CrossRef]
- Ringeval, B.; Nowak, B.; Nesme, T.; Delmas, M.; Pellerin, S. Contribution of anthropogenic phosphorus to agricultural soil fertility and food production. Glob. Biogeochem. Cycles 2014, 28, 743–756. [Google Scholar] [CrossRef] [Green Version]
- Frossard, E.; Condron, L.M.; Oberson, A.; Sinaj, S.; Fardeau, J.C. Processes governing phosphorus availability in temperate soils. J. Environ. Qual. 2000, 29, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, S.K.; De Datta, S.K. Chemistry of Phosphorus Transformations in Soil. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1991; pp. 1–120. [Google Scholar]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms. In Phosphorus in Action; Bünemann, E., Oberson, A.F.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–198. [Google Scholar]
- De Jonge, L.W.; Moldrup, P.; Rubaek, G.H.; Schelde, K.; Djurhuus, J. Particle Leaching and Particle-Facilitated Transport of Phosphorus at Field Scale. Vadose Zone J. 2004, 3, 462–470. [Google Scholar] [CrossRef]
- Norgaard, T.; Moldrup, P.; Olsen, P.; Vendelboe, A.L.; Iversen, B.V.; Greve, M.H.; Kjaer, J.; de Jonge, L.W. Comparative Mapping of Soil Physical-Chemical and Structural Parameters at Field Scale to Identify Zones of Enhanced Leaching Risk. J. Environ. Qual. 2013, 42, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Johnston, A.E.; Poulton, P.R. Phosphorus in Agriculture: A Review of Results from 175 Years of Research at Rothamsted, UK. J. Environ. Qual. 2019, 48, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Sharpley, A.; Jarvie, H.P.; Buda, A.; May, L.; Spears, B.; Kleinman, P. Phosphorus Legacy: Overcoming the Effects of Past Management Practices to Mitigate Future Water Quality Impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef] [Green Version]
- Withers, P.J.A.; Vadas, P.A.; Uusitalo, R.; Forber, K.J.; Hart, M.; Foy, R.H.; Delgado, A.; Dougherty, W.; Lilja, H.; Burkitt, L.L.; et al. A Global Perspective on Integrated Strategies to Manage Soil Phosphorus Status for Eutrophication Control without Limiting Land Productivity. J. Environ. Qual. 2019, 48, 1234. [Google Scholar] [CrossRef] [Green Version]
- Fixen, P.E.; Grove, J.H. Testing Soils for Phosphorus. Soil Test. Plant Anal. 1990, 141–180. [Google Scholar] [CrossRef]
- Jordan-Meille, L.; Rubæk, G.H.; Ehlert, P.A.I.; Genot, V.; Hofman, G.; Goulding, K.; Recknagel, J.; Provolo, G.; Barraclough, P. An overview of fertilizer-P recommendations in Europe: Soil testing, calibration and fertilizer recommendations. Soil Use Manag. 2012, 28, 419–435. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; van Dijk, K.C. Phosphorus management in Europe in a changing world. Ambio 2015, 44, 180–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos-Moreira, M.; Lemercier, B.; Dupas, R.; Michot, D.; Viaud, V.; Akkal-Corfini, N.; Louis, B.; Gascuel-Odoux, C. High-resolution mapping of soil phosphorus concentration in agricultural landscapes with readily available or detailed survey data. Eur. J. Soil Sci. 2017, 68, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Delmas, M.; Saby, N.; Arrouays, D.; Dupas, R.; Lemercier, B.; Pellerin, S.; Gascuel-Odoux, C. Explaining and mapping total phosphorus content in French topsoils. Soil Use Manag. 2015, 31, 259–269. [Google Scholar] [CrossRef]
- Kovar, J.L.; Pierzynski, G.M. Methods of phosphorus analysis for soils, sediments, residuals, and waters. In Southern Cooperative Series Bulletin; Virginia Tech University: Blacksburg, VA, USA, 2009; p. 110. [Google Scholar]
- Harmon, R.S.; Russo, R.E.; Hark, R.R. Applications of laser-induced breakdown spectroscopy for geochemical and environmental analysis: A comprehensive review. Spectrochim. Acta Part B At. Spectrosc. 2013, 87, 11–26. [Google Scholar] [CrossRef]
- Cremers, D.A.; Radziemski, L.J. Handbook of Laser-Induced Breakdown Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780470093016. [Google Scholar]
- Senesi, G.S.; Senesi, N. Laser-induced breakdown spectroscopy (LIBS) to measure quantitatively soil carbon with emphasis on soil organic carbon. A review. Anal. Chim. Acta 2016, 938, 7–17. [Google Scholar] [CrossRef]
- Capitelli, F.; Colao, F.; Provenzano, M.R.; Fantoni, R.; Brunetti, G.; Senesi, N. Determination of heavy metals in soils by Laser Induced Breakdown Spectroscopy. Geoderma 2002, 106, 45–62. [Google Scholar] [CrossRef]
- Bousquet, B.; Sirven, J.B.; Canioni, L. Towards quantitative laser-induced breakdown spectroscopy analysis of soil samples. Spectrochim. Acta-Part B At. Spectrosc 2007, 1582–1589. [Google Scholar] [CrossRef]
- Senesi, G.S.; Dell’Aglio, M.; Gaudiuso, R.; De Giacomo, A.; Zaccone, C.; De Pascale, O.; Miano, T.M.; Capitelli, M. Heavy metal concentrations in soils as determined by laser-induced breakdown spectroscopy (LIBS), with special emphasis on chromium. Environ. Res. 2009, 109, 413–420. [Google Scholar] [CrossRef]
- Harris, R.D.; Cremers, D.A.; Ebinger, M.H.; Bluhm, B.K. Determination of Nitrogen in Sand Using Laser-Induced Breakdown Spectroscopy. Appl. Spectrosc. 2004, 58, 770–775. [Google Scholar] [CrossRef]
- Sallé, B.; Cremers, D.A.; Maurice, S.; Wiens, R.C.; Fichet, P. Evaluation of a compact spectrograph for in-situ and stand-off Laser-Induced Breakdown Spectroscopy analyses of geological samples on Mars missions. Spectrochim. Acta Part B At. Spectrosc. 2005, 60, 805–815. [Google Scholar] [CrossRef]
- Ferreira, E.C.; Milori, D.M.B.P.; Ferreira, E.J.; Dos Santos, L.M.; Martin-Neto, L.; de Araújo Nogueira, A.R. Evaluation of laser induced breakdown spectroscopy for multielemental determination in soils under sewage sludge application. Talanta 2011, 85, 435–440. [Google Scholar] [CrossRef]
- Díaz, D.; Hahn, D.W.; Molina, A. Evaluation of laser-induced breakdown spectroscopy (LIBS) as a measurement technique for evaluation of total elemental concentration in soils. Appl. Spectrosc. 2012. [Google Scholar] [CrossRef]
- Sanchez, S.; Knadel, M.; Labouriau, R.; Rubaek, G.H.; Heckrath, G. EXPRESS: Total Phosphorus Determination in Soils Using Laser-Induced Breakdown Spectroscopy: Evaluating Different Sources of Matrix Effects. Appl. Spectrosc. 2020, 1–12. [Google Scholar] [CrossRef]
- Lu, C.; Wang, L.; Hu, H.; Zhuang, Z.; Wang, Y.; Wang, R.; Song, L. Analysis of total nitrogen and total phosphorus in soil using laser-induced breakdown spectroscopy. Chin. Opt. Lett. 2013. [Google Scholar] [CrossRef]
- Guo, G.; Niu, G.; Shi, Q.; Lin, Q.; Tian, D.; Duan, Y. Multi-element quantitative analysis of soils by laser induced breakdown spectroscopy (LIBS) coupled with univariate and multivariate regression methods. Anal. Methods 2019, 11, 3006–3013. [Google Scholar] [CrossRef]
- Xu, X.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. Fast and Simultaneous Determination of Soil Properties Using Laser-Induced Breakdown Spectroscopy (LIBS): A Case Study of Typical Farmland Soils in China. Soil Syst. 2019, 3, 66. [Google Scholar] [CrossRef] [Green Version]
- Erler, A.; Riebe, D.; Beitz, T.; Löhmannsröben, H.G.; Gebbers, R. Soil nutrient detection for precision agriculture using handheld laser-induced breakdown spectroscopy (LIBS) and multivariate regression methods (PLSR, lasso and GPR). Sensors 2020, 20, 418. [Google Scholar] [CrossRef] [Green Version]
- Villas-Boas, P.R.; de Menezes Franco, M.A.; Martin-Neto, L.; Gollany, H.T.; Milori, D.M.B.P. Applications of Laser-Induced Breakdown Spectroscopy for Soil Characterization, Part II: Review of Elemental Analysis and Soil Classification. Eur. J. Soil Sci. 2019, 71, 805–818. [Google Scholar] [CrossRef]
- Ferreira, E.C.; Neto, J.A.G.; Milori, D.M.B.P.; Ferreira, E.J.; Anzano, J.M. Laser-induced breakdown spectroscopy: Extending its application to soil pH measurements. Spectrochim. Acta Part B At. Spectrosc. 2015, 110, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Segnini, A.; Xavier, A.A.P.; Otaviani-Junior, P.L.; Ferreira, E.C.; Watanabe, A.M.; Sperança, M.A.; Nicolodelli, G.; Villas-Boas, P.R.; Oliveira, P.P.A.; Milori, D.M.B.P. Physical and Chemical Matrix Effects in Soil Carbon Quantification Using Laser-Induced Breakdown Spectroscopy. Am. J. Anal. Chem. 2014. [Google Scholar] [CrossRef] [Green Version]
- Villas-Boas, P.R.; Romano, R.A.; de Menezes Franco, M.A.; Ferreira, E.C.; Ferreira, E.J.; Crestana, S.; Milori, D.M.B.P. Laser-induced breakdown spectroscopy to determine soil texture: A fast analytical technique. Geoderma 2016, 263, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Knadel, M.; Gislum, R.; Hermansen, C.; Peng, Y.; Moldrup, P.; de Jonge, L.W.; Greve, M.H. Comparing predictive ability of laser-induced breakdown spectroscopy to visible near-infrared spectroscopy for soil property determination. Biosyst. Eng. 2017. [Google Scholar] [CrossRef]
- Villas-Boas, P.R.; Franco, M.A.; Martin-Neto, L.; Gollany, H.T.; Milori, D.M.B.P. Applications of laser-induced breakdown spectroscopy for soil analysis, part I: Review of fundamentals and chemical and physical properties. Eur. J. Soil Sci. 2019. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1987; ISBN 091325049X. [Google Scholar]
- Nocita, M.; Stevens, A.; van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthès, B.; Dor, E.B.; Brown, D.J.; Clairotte, M.; Csorba, A.; et al. Soil Spectroscopy: An Alternative to Wet Chemistry for Soil Monitoring. Adv. Agron. 2015, 132, 139–159. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, B.; Rossel, R.A.V.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. Adv. Agron. 2010, 107, 163–215. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.N. Spectroscopy of Rocks and Minerals and Principles of Spectroscopy. In Remote Sensing for the Eartb Sciences: Manual of Remote Sensing; Wiley: Hoboken, NJ, USA, 1999; Volume 3, ISBN 0471-29405-5. [Google Scholar]
- Bogrekci, I.; Lee, W.S. Spectral soil signatures and sensing phosphorus. Biosyst. Eng. 2005, 92, 527–533. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Kuang, B. On-line visible and near infrared spectroscopy for in-field phosphorous management. Soil Tillage Res. 2016, 155, 471–477. [Google Scholar] [CrossRef]
- He, Y.; Huang, M.; García, A.; Hernández, A.; Song, H. Prediction of soil macronutrients content using near-infrared spectroscopy. Comput. Electron. Agric. 2007, 58, 144–153. [Google Scholar] [CrossRef]
- Maleki, M.R.; Van Holm, L.; Ramon, H.; Merckx, R.; De Baerdemaeker, J.; Mouazen, A.M. Phosphorus Sensing for Fresh Soils using Visible and Near Infrared Spectroscopy. Biosyst. Eng. 2006, 95, 425–436. [Google Scholar] [CrossRef]
- Morón, A.; Cozzolino, D. Measurement of phosphorus in soils by near infrared reflectance spectroscopy: Effect of reference method on calibration. Commun. Soil Sci. Plant Anal. 2007, 38, 1965–1974. [Google Scholar] [CrossRef]
- Niederberger, J.; Todt, B.; Boča, A.; Nitschke, R.; Kohler, M.; Kühn, P.; Bauhus, J. Use of near-infrared spectroscopy to assess phosphorus fractions of different plant availability in forest soils. Biogeosciences 2015, 12, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Pätzold, S.; Leenen, M.; Frizen, P.; Heggemann, T.; Wagner, P.; Rodionov, A. Predicting plant available phosphorus using infrared spectroscopy with consideration for future mobile sensing applications in precision farming. Precis. Agric. 2019. [Google Scholar] [CrossRef] [Green Version]
- De Souza, M.F.; Franco, H.C.J.; Do Amaral, L.R. Estimation of soil phosphorus availability via visible and near-infrared spectroscopy. Sci. Agric. 2020, 77, 1–6. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.W.; Holmes, M.; Hanpin, M. Variables selection methods in near-infrared spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, R.; Li, S.; Chen, S.; Jiang, Q.; Zhou, L.; Shi, Z. Multi-sensor fusion for the determination of several soil properties in the Yangtze River Delta, China. Eur. J. Soil Sci. 2019, 70, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Qin, H.; Wang, Q.; Lu, Z.; Yao, X.; Yu, Z.; Chen, X.; Zhang, L.; Lu, J. Optimizing analysis of coal property using laser-induced breakdown and near-infrared reflectance spectroscopies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118492. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Fontes, L.M.; Pasquini, C. Comparing laser induced breakdown spectroscopy, near infrared spectroscopy, and their integration for simultaneous multi-elemental determination of micro- and macronutrients in vegetable samples. Anal. Chim. Acta 2019, 1062, 28–36. [Google Scholar] [CrossRef]
- Xu, F.; Hao, Z.; Huang, L.; Liu, M.; Chen, T.; Chen, J.; Zhang, L.; Zhou, H.; Yao, M. Comparative identification of citrus huanglongbing by analyzing leaves using laser-induced breakdown spectroscopy and near-infrared spectroscopy. Appl. Phys. B 2020, 126, 43. [Google Scholar] [CrossRef]
- Soares, A.A.; Moldrup, P.; Minh, L.N.; Vendelboe, A.L.; Schjonning, P.; De Jonge, L.W. Sorption of phenanthrene on agricultural soils. Water Air Soil Pollut. 2013, 224, 1519. [Google Scholar] [CrossRef] [Green Version]
- Vendelboe, A.L.; Moldrup, P.; Heckrath, G.; Jin, Y.; de Jonge, L.W. Colloid and Phosphorus Leaching From Undisturbed Soil Cores Sampled Along a Natural Clay Gradient. Soil Sci. 2011, 176, 399–406. [Google Scholar] [CrossRef]
- Heckrath, G.; Djurhuus, J.; Quine, T.A.; Van Oost, K.; Govers, G.; Zhang, Y. Tillage erosion and its effect on soil properties and crop yield in Denmark. J. Environ. Qual. 2005, 34, 312–324. [Google Scholar] [PubMed]
- Hermansen, C.; Knadel, M.; Moldrup, P.; Greve, M.H.; Gislum, R.; de Jonge, L.W. Visible–near-infrared spectroscopy can predict the clay/organic carbon and mineral. fines/organic carbon ratios. Soil Sci. Soc. Am. J. 2016, 80, 1486–1495. [Google Scholar] [CrossRef]
- Sissingh, H.A. Analytical technique of the Pw method, used for the assessment of the phosphate status of arable soils in the Netherlands. Plant Soil 1971, 34, 483–486. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954.
- Rubæk, G.; Kristensen, K. Protocol for Bicarbonate Extraction of Inorganic Phosphate from Agricultural Soils; Aarhus University: Aarhus, Denmark, 2017; ISBN 9788793398870. [Google Scholar]
- International Organization for Standardization. Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method 2004, ISO 6878; ISO: Geneva, Switzerland.
- Schoumans, O.F. Determination of the degree of phosphate saturation in non-calcareous soils. In Methods of Phosphorus Analysis for Soils, Sediments, Residuals and Waters; Pierzynski, G.M., Ed.; Coop. Ser. Bull. 396, Publ. SERA-IEG-17; North Carolina State University: Raleigh, NC, USA, 2000; pp. 31–34. [Google Scholar]
- Katuwal, S.; Knadel, M.; Moldrup, P.; Norgaard, T.; Greve, M.H.; de Jonge, L.W. Visible–Near-Infrared Spectroscopy can predict Mass Transport of Dissolved Chemicals through Intact Soil. Sci. Rep. 2018, 8, 11188. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Boelens, H.F.M. Baseline Correction with Asymmetric Least Squares Smoothing; Anal. Chem.: Leiden, The Netherlands, 2005. [Google Scholar]
- Martens, H.; Stark, E. Extended multiplicative signal correction and spectral interference subtraction: New preprocessing methods for near infrared spectroscopy. J. Pharm. Biomed. Anal. 1991, 9, 625–635. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 15 January 2020).
- Andersen, C.M.; Bro, R. Variable selection in regression—A tutorial. J. Chemom. 2010, 24, 728–737. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Matlab Matlab: 2019 Version R2019b; The MathWorks Inc.: Natick, MA, USA, 2019.
- Kucheryavskiy, S. mdatools–R package for chemometrics. Chemom. Intell. Lab. Syst. 2020, 198, 103937. [Google Scholar] [CrossRef]
- Gowen, A.A.; Downey, G.; Esquerre, C.; O’Donnell, C.P. Preventing over-fitting in PLS calibration models of near-infrared (NIR) spectroscopy data using regression coefficients. J. Chemom. 2011, 25, 375–381. [Google Scholar] [CrossRef]
- Rodionova, O.Y.; Pomerantsev, A.L. Detection of outliers in projection-based modeling. Anal. Chem. 2020, 92, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Wuenscher, R.; Unterfrauner, H.; Peticzka, R.; Zehetner, F. A comparison of 14 soil phosphorus extraction methods applied to 50 agricultural soils from Central Europe. Plant Soil Environ. 2015, 61, 86–96. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, G.; Ji, H.; Xiong, X. Accurate quantitative determination of heavy metals in oily soil by laser induced breakdown spectroscopy (LIBS) combined with interval partial least squares (IPLS). Anal. Methods 2019, 11, 3657–3664. [Google Scholar] [CrossRef]
- Liu, J.; Sun, T.; Gan, L. Detection of Fenthion Content by LIBS Combined with Internal Standard and CARS Variable Selection Method. Chin. J. Lumin. 2018, 39, 737–744. [Google Scholar]
- Nespeca, M.G.; Vieira, A.L.; Júnior, D.S.; Neto, J.A.G.; Ferreira, E.C. Detection and quantification of adulterants in honey by LIBS. Food Chem. 2020, 311, 125886. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Gupta, B.B.; Rho, S.; Liu, Q.; Hou, H.; Jing, D.; Shen, W. Variable Selection and Optimization in Rapid Detection of Soybean Straw Biomass Based on CARS. IEEE Access 2018, 6, 5290–5299. [Google Scholar] [CrossRef]
- Huang, L.; Meng, L.; Yang, L.; Wang, J.; Li, S.; He, Y.; Wu, D. A novel method to extract important features from laser induced breakdown spectroscopy data: Application to determine heavy metals in mulberries. J. Anal. At. Spectrom. 2019, 34, 460–468. [Google Scholar] [CrossRef]
- Fu, X.; Duan, F.J.; Huang, T.T.; Ma, L.; Jiang, J.J.; Li, Y.C. A fast variable selection method for quantitative analysis of soils using laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2017. [Google Scholar] [CrossRef]
- Van der Zee, S.E.A.T.M.; Van Riemsdijk, W.H. Transport of reactive solute in spatially variable soil systems. Water Resour. Res. 1987, 23, 2059–2069. [Google Scholar] [CrossRef]
- Rauschenbach, I.; Lazic, V.; Pavlov, S.G.; Hübers, H.-W.; Jessberger, E.K. Laser induced breakdown spectroscopy on soils and rocks: Influence of the sample temperature, moisture and roughness. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1205–1215. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Lv, Y.; Liu, F.; Peng, J.; Shen, T.; Zhao, Y.; Tang, Y.; Luo, S. Quantitative analysis of nutrient elements in soil using single and double-pulse laser-induced breakdown spectroscopy. Sensors 2018, 18, 1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIST Standard Reference Database; NIST: Gaithersburg, MD, USA, October 2018.
- Vieira, A.L.; Silva, T.V.; de Sousa, F.S.I.; Senesi, G.S.; Júnior, D.S.; Ferreira, E.C.; Neto, J.A.G. Determinations of phosphorus in fertilizers by spark discharge-assisted laser-induced breakdown spectroscopy. Microchem. J. 2018. [Google Scholar] [CrossRef] [Green Version]
- Vohland, M.; Ludwig, M.; Thiele-Bruhn, S.; Ludwig, B. Determination of soil properties with visible to near- and mid-infrared spectroscopy: Effects of spectral variable selection. Geoderma 2014, 223–225, 88–96. [Google Scholar] [CrossRef]
- Hermansen, C.; Knadel, M.; Moldrup, P.; Greve, M.H.; Karup, D.; de Jonge, L.W. Complete Soil Texture is Accurately Predicted by Visible Near-Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2017, 81, 758–769. [Google Scholar] [CrossRef]
- Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Andriamananjara, A.; Rabenarivo, M.; Asai, H.; Rakotoson, T.; Razafimbelo, T. Laboratory visible and near-infrared spectroscopy with genetic algorithm-based partial least squares regression for assessing the soil phosphorus content of upland and lowland rice fields in Madagascar. Remote Sens. 2019, 11, 506. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil phosphorus availability. Cienc. e Agrotecnologia 2016, 40, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Sherman, D.M.; Waite, T.D. Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am. Mineral. 1985, 70, 1262–1269. [Google Scholar]
- Scheinost, A.C.; Chavernas, A.; Barrón, V.; Torrent, J. Use and Limitations of Second-Derivative Diffuse Reflectance Spectroscopy in the Visible to Near-Infrared Range to Identify and Quantify Fe Oxide Minerals in Soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Fouad, Y.; Walter, C. Using a digital camera to measure soil organic carbon and iron contents. Biosyst. Eng. 2008, 100, 149–159. [Google Scholar] [CrossRef]
- Ben-Dor, E.A. Quantitative Remote Sensing of Soil Properties; Academic Press: Cambridge, MA, USA, 2002; Volume 75, pp. 173–243. ISBN 0065-2113. [Google Scholar]
- Daniel, K.W.; Tripathi, N.K.; Honda, K. Artificial neural network analysis of laboratory and in situ spectra for the estimation of macronutrients in soils of Lop Buri (Thailand). Soil Res. 2003, 41, 47–59. [Google Scholar] [CrossRef]
- Ramaroson, V.H.; Becquer, T.; Sá, S.O.; Razafimahatratra, H.; Delarivière, J.L.; Blavet, D.; Vendrame, P.R.S.; Rabeharisoa, L.; Rakotondrazafy, A.F.M. Mineralogical analysis of ferralitic soils in Madagascar using NIR spectroscopy. CATENA 2018, 168, 102–109. [Google Scholar] [CrossRef]
- Bricklemyer, R.S.; Brown, D.J.; Turk, P.J.; Clegg, S. Comparing vis-NIRS, LIBS, and Combined vis-NIRS-LIBS for Intact Soil Core Soil Carbon Measurement. Soil Sci. Soc. Am. J. 2018, 82, 1482–1496. [Google Scholar] [CrossRef]







| Soil Property 1 | Mean | Max | Min | SD 2 | CV 3 | Skewness | Kurtosis | Q1 4 | Q3 5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Clay | g·100g−1 | 12.8 | 42.2 | 3.3 | 7.1 | 0.55 | 1.81 | 4.29 | 8.2 | 15.2 |
| Silt | 22.5 | 45.9 | 4.5 | 10.5 | 0.47 | 0.02 | −1.34 | 12.3 | 32.4 | |
| Sand | 64.7 | 91.2 | 22.8 | 16.1 | 0.25 | −0.43 | −0.49 | 53.4 | 78.9 | |
| SOC | 2.2 | 6.6 | 0.9 | 1.2 | 0.55 | 1.58 | 1.62 | 1.4 | 2.4 | |
| Pwater | mg·kg−1 | 8.8 | 23.1 | 1.3 | 5.1 | 0.58 | 0.67 | −0.62 | 4.5 | 12.8 |
| Polsen | 33 | 79 | 6 | 18 | 0.53 | 0.73 | −0.13 | 21 | 44 | |
| Pox | 390 | 991 | 121 | 142 | 0.36 | 1.09 | 1.83 | 293 | 496 | |
| TP | 581 | 1157 | 275 | 141 | 0.24 | 0.72 | 1.06 | 483 | 663 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Esteva, S.; Knadel, M.; Kucheryavskiy, S.; de Jonge, L.W.; Rubæk, G.H.; Hermansen, C.; Heckrath, G. Combining Laser-Induced Breakdown Spectroscopy (LIBS) and Visible Near-Infrared Spectroscopy (Vis-NIRS) for Soil Phosphorus Determination. Sensors 2020, 20, 5419. https://doi.org/10.3390/s20185419
Sánchez-Esteva S, Knadel M, Kucheryavskiy S, de Jonge LW, Rubæk GH, Hermansen C, Heckrath G. Combining Laser-Induced Breakdown Spectroscopy (LIBS) and Visible Near-Infrared Spectroscopy (Vis-NIRS) for Soil Phosphorus Determination. Sensors. 2020; 20(18):5419. https://doi.org/10.3390/s20185419
Chicago/Turabian StyleSánchez-Esteva, Sara, Maria Knadel, Sergey Kucheryavskiy, Lis W. de Jonge, Gitte H. Rubæk, Cecilie Hermansen, and Goswin Heckrath. 2020. "Combining Laser-Induced Breakdown Spectroscopy (LIBS) and Visible Near-Infrared Spectroscopy (Vis-NIRS) for Soil Phosphorus Determination" Sensors 20, no. 18: 5419. https://doi.org/10.3390/s20185419