Ethanol Sensing Properties and First Principles Study of Au Supported on Mesoporous ZnO Derived from Metal Organic Framework ZIF-8
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of ZIF-8 Materials
2.3. Preparation of Au Supported ZnO Materials
2.4. Gas Sensing Measurements
2.5. Material Characterization
3. Results and Discussion
3.1. Morphology and Structure of ZIF-8 Derived Au/ZnO Samples
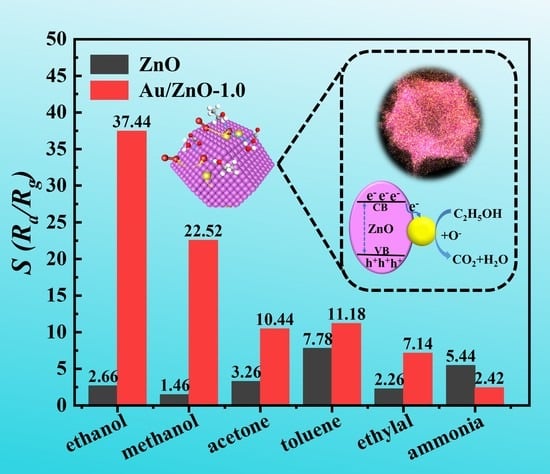
3.2. Au/ZnO Samples for C2H5OH Sensing Performance Testing and Analysis
3.3. Gas Sensing Mechanism of the Au/ZnO Sensors
3.4. Adsorption Characteristics of the Au/ZnO Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Liu, J.; Liu, Q.; Chen, R.; Zhang, H.; Yu, J.; Song, D.; Li, J.; Zhang, M.; Wang, J. Core-shell structure of ZnO/Co3O4 composites derived from bimetallic-organic frameworks with superior sensing performance for ethanol gas. Appl. Surf. Sci. 2019, 475, 700–709. [Google Scholar] [CrossRef]
- Cao, P.; Yang, Z.; Navale, S.T.; Han, S.; Liu, X.; Liu, W.; Lu, Y.; Stadler, F.J.; Zhu, D. Ethanol sensing behavior of Pd-nanoparticles decorated ZnO-nanorod based chemiresistive gas sensors. Sens. Actuators B 2019, 298, 126850. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, S.; Tang, P.; Feng, Y.; Li, D. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ion. 2021, 360, 115544. [Google Scholar] [CrossRef]
- Yan, Z.; Tao, C.; Bai, Y.; Liu, S. Adsorption of nitrogen based gas molecules on noble metal functionalized carbon nitride nanosheets: A theoretical investigation. Comput. Theor. Chem. 2021, 1194, 112950. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Mishra, A.; Bhatt, G.; Bhattacharya, S. Enhanced He gas detection by V2O5-noble metal (Au, Ag, and Pd) nanocomposite with temperature dependent n- to p-type transition. Mater. Sci. Semicond. Process. 2021, 123, 105528. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Zhang, L.; Chen, J.; Zhou, Q.; Zhang, H.; Nie, L.; Dong, Z.; Zhang, Z.A.; Wang, Z.; et al. Construction of a low-temperature, highly sensitive H2S sensor based on surfaces and interfaces reaction triggered by Au-doped hierarchical structured composites. Chem. Phys. Lett. 2021, 763, 138188. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, D.; Yao, A.; Gao, Y.; Asadi, H. A computational study on the Pd-decorated ZnO nanocluster for H2 gas sensing: A comparison with experimental results. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 124, 114237. [Google Scholar] [CrossRef]
- Hsueh, T.-J.; Wu, S.-S. Highly sensitive Co3O4 nanoparticles/MEMS NO2 gas sensor with the adsorption of the Au nanoparticles. Sens. Actuators B Chem. 2021, 329, 129201. [Google Scholar] [CrossRef]
- Cui, W.; Kang, X.; Zhang, X.; Zheng, Z.; Cui, X. Facile synthesis of porous cubic microstructure of Co3O4 from ZIF-67 pyrolysis and its Au doped structure for enhanced acetone gas-sensing. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 113, 165–171. [Google Scholar] [CrossRef]
- Kohl, D. The role of noble metals in the chemistry of solid-state gas sensors. Sens. Actuators B Chem. 1990, 1, 158–165. [Google Scholar] [CrossRef]
- Koo, W.T.; Qiao, S.; Ogata, A.F.; Jha, G.; Jang, J.S.; Chen, V.T.; Kim, I.D.; Penner, R.M. Accelerating palladium nanowire H2 sensors using engineered nanofiltration. ACS Nano 2017, 11, 9276–9285. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.-J.; Peng, C.-H.; Chen, W.-S. A transparent ZnO nanowire MEMS gas sensor prepared by an ITO micro-heater. Sens. Actuators B Chem. 2020, 304, 127319. [Google Scholar] [CrossRef]
- Zhou, X.; Lin, X.; Yang, S.; Zhu, S.; Chen, X.; Dong, B.; Bai, X.; Wen, X.; Geyu, L.; Song, H. Highly dispersed Metal–Organic-Framework-Derived Pt nanoparticles on three-dimensional macroporous ZnO for trace-level H2S sensing. Sens. Actuators B Chem. 2020, 309, 127802. [Google Scholar] [CrossRef]
- Wang, J.; Han, G.; Wang, L.; Du, L.; Chen, G.; Gao, Y.; Ma, Y.; Du, C.; Cheng, X.; Zuo, P.; et al. ZIF-8 with ferrocene encapsulated: A promising precursor to single-atom Fe embedded nitrogen-doped carbon as highly efficient catalyst for oxygen electroreduction. Small 2018, 14, 1704282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Meng, X.; Zhang, Z.; Mu, S. High response methane sensor based on Au-modified hierarchical porous nanosheets-assembled ZnO microspheres. Mater. Chem. Phys. 2020, 250, 123027. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, L.; Lu, Y.; Fan, Y.; Guo, S.; Adimi, S.; Liu, D.; Ruan, S. Metal-organic frameworks-derived hierarchical ZnO structures as efficient sensing materials for formaldehyde detection. Chin. Chem. Lett. 2020, 31, 2071–2076. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, R.; Zhu, Z.; Wang, S. Au nanoparticles decorated ZnS hollow spheres for highly improved gas sensor performances. Sens. Actuators B Chem. 2017, 245, 112–121. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wang, L.; Yang, T.; Guo, X.; Wu, S.; Zhang, S.; Wang, S. A simple one-pot strategy for the synthesis of ternary reduced graphite oxide/SnO2/Au hybrid nanomaterials. Carbon 2011, 49, 3538–3543. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Wu, S.; Xu, M.; Guo, X.; Wang, S. Au nanoparticle-decorated porous SnO2 hollow spheres: A new model for a chemical sensor. J. Mater. Chem. 2010, 20, 6453–6459. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Chen, C.; Lin, C. Investigation of humidity sensor based on Au modified ZnO nanosheets via hydrothermal method and first principle. Sens. Actuators B Chem. 2019, 287, 526–534. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Ma, Q.; Fang, Y.; Wang, J.; Che, Q.; Wang, G.; Yang, P. Novel construction of morphology-tunable C-N/SnO2/ZnO/Au microspheres with ultrasensitivity and high selectivity for triethylamine under various temperature detections. ACS Appl. Mater. Interfaces 2019, 11, 8601–8611. [Google Scholar] [CrossRef]
- Yang, T.H.; Huang, L.D.; Harn, Y.W.; Lin, C.C.; Chang, J.K.; Wu, C.I.; Wu, J.M. High density unaggregated Au nanoparticles on ZnO nanorod arrays function as efficient and recyclable photocatalysts for environmental purification. Small 2013, 9, 3169–3182. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B 2018, 244, 1021–1064. [Google Scholar] [CrossRef]
- Shingange, K.; Tshabalala, Z.P.; Ntwaeaborwa, O.M.; Motaung, D.E.; Mhlongo, G.H. Highly selective NH3 gas sensor based on Au loaded ZnO nanostructures prepared using microwave-assisted method. J. Colloid Interface Sci. 2016, 479, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Li, Q.; Lei, X.; Jian, S.; Song, H. Au modified three-dimensional In2O3 inverse opals: Synthesis and improved performance for acetone sensing toward diagnosis of diabetes. Nanoscale 2015, 7, 13051–13060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Sun, J.; Xu, L.; Zhou, Q.; Chen, X.; Zhu, S.; Dong, B.; Lu, G.; Song, H. Au@ZnO functionalized three–dimensional macroporous WO3: A application of selective H2S gas sensor for exhaled breath biomarker detection. Sens. Actuators B 2020, 324, 128725. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, A.; Wang, Y.; Bian, L.; Yang, Z.; Bian, Y.; Gong, Y.; Wu, X.; Han, N.; Chen, Y. Crystal defect dependent gas sensing mechanism of the single ZnO nanowire sensors. ACS Sens. 2018, 3, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhao, J.; Shi, S.; Lin, H.; Mao, Z.; Zhang, F.; Qu, F. Boosting ppb-level triethylamine sensing of ZnO: Adjusting proportions of electron donor defects. J. Mater. Chem. C 2020, 8, 6734–6742. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, T.; Wang, L.; Zhang, T. Metal-organic frameworks-derived hierarchical Co3O4 structures as efficient sensing materials for acetone detection. ACS Appl. Mater. Interfaces 2018, 10, 9765–9773. [Google Scholar] [CrossRef]
- Lu, H.-B.; Liao, L.; Li, H.; Wang, D.-F.; Tian, Y.; Li, J.-C.; Fu, Q.; Zhu, B.-P.; Wu, Y. Hollow MgO nanotube arrays by using ZnO nanorods as templates. Eur. J. Inorg. Chem. 2008, 2008, 2727–2732. [Google Scholar] [CrossRef]
- Wang, W.; Dahl, M.; Yin, Y. Hollow nanocrystals through the nanoscale kirkendall effect. Chem. Mater. 2012, 25, 1179–1189. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Liu, H.; Chen, J.; Tang, W.; Chen, Y. Hierarchical hollow ZnO cubes constructed using self-sacrificial ZIF-8 frameworks and their enhanced benzene gas-sensing properties. New J. Chem. 2015, 39, 7060–7065. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Tian, F.; Liang, H.; Wang, K.; Zhao, X.; Lu, Z.; Jiang, K.; Yang, L.; Lou, X. From the surface reaction control to gas-diffusion control: The synthesis of hierarchical porous SnO2 microspheres and their gas-sensing mechanism. J. Phys. Chem. C 2015, 119, 15963–15976. [Google Scholar] [CrossRef]
- Xiong, H.; Fu, J.; Li, J.; Ali, R.; Wang, H.; Liu, Y.; Su, H.; Li, Y.; Lau, W.-M.; Mahmood, N.; et al. Strain-regulated sensing properties of α-Fe2O3 nano-cylinders with atomic carbon layers for ethanol detection. J. Mater. Sci. Technol. 2021, 68, 132–139. [Google Scholar] [CrossRef]
- Yu, Z.; Gao, J.; Xu, L.; Liu, T.; Liu, Y.; Wang, X.; Suo, H.; Zhao, C. Fabrication of lettuce-like ZnO gas sensor with enhanced H2S gas sensitivity. Crystals 2020, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Shen, Y.; Hao, F.; Kang, C.; Meng, F. P-n junctions based on CuO-decorated ZnO nanowires for ethanol sensing application. Appl. Surf. Sci. 2021, 538, 148140. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Hao, W.; Sun, L.; Cao, E. Ethanol sensing characteristics of Au and Cl-comodified ZnO nanoparticles. Mater. Lett. 2020, 279, 128505. [Google Scholar] [CrossRef]
- Mei, H.; Zhou, S.; Lu, M.; Cheng, L. Tetrapod-like ZnO/ZnFe2O4 based heterostructure for enhanced ethanol detection. J. Alloys Compd. 2020, 840, 155583. [Google Scholar] [CrossRef]
- Jaballah, S.; Benamara, M.; Dahman, H.; Ly, A.; Mir, L. Effect of Mg-doping ZnO nanoparticles on detection of low ethanol concentrations. Mater. Chem. Phys. 2020, 255, 123643. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Yi, G.; Zhou, L.; Cao, J.; Sun, G.; Chen, Z.; Bala, H.; Zhang, Z. Hydrothermal synthesis of Ag modified ZnO nanorods and their enhanced ethanol-sensing properties. Mater. Sci. Semicond. Process. 2017, 75, 327–333. [Google Scholar] [CrossRef]
- Saaedi, A.; Yousefi, R. Improvement of gas-sensing performance of ZnO nanorods by group-I elements doping. J. Appl. Phys. 2017, 122, 224505. [Google Scholar] [CrossRef]
- Yousefi, H.R.; Hashemi, B.; Mirzaei, A.; Roshan, H.; Sheikhi, M.H. Effect of Ag on the ZnO nanoparticles properties as an ethanol vapor sensor. Mater. Sci. Semicond. Process. 2020, 117, 105172. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B Chem. 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Sundaram, K.B.; Khan, A. Work function determination of zinc oxide films. J. Vac. Sci. Technol. 1997, 15, 428–430. [Google Scholar] [CrossRef]
- Lee, J.-S.; Katoch, A.; Kim, J.-H.; Kim, S.S. Effect of Au nanoparticle size on the gas-sensing performance of p-CuO nanowires. Sens. Actuators B Chem. 2016, 222, 307–314. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xu, P.; Zhu, Y.; Chen, X.; Yu, W. Decoration of ZnO nanowires with Pt nanoparticles and their improved gas sensing and photocatalytic performance. Nanotechnology 2010, 21, 285501. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Liu, J.; Xiong, Y.; Zheng, J.; Liu, Y.; Tang, Z. Core-shell noble-metal@metal-organic-framework nanoparticles with highly selective sensing property. Angew. Chem. 2013, 125, 3829–3833. [Google Scholar] [CrossRef]
- Fu, F.; Wang, C.; Wang, Q.; Martinez-Villacorta, A.M.; Escobar, A.; Chong, H.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E. Highly selective and sharp volcano-type synergistic Ni2Pt@ZIF-8-catalyzed hydrogen evolution from ammonia borane hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.-H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of Au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Rai, P.; Majhi, S.M.; Yu, Y.-T.; Lee, J.-H. Noble metal@metal oxide semiconductor core@shell nano-architectures as a new platform for gas sensor applications. RSC Adv. 2015, 5, 76229–76248. [Google Scholar] [CrossRef]
- Velmathi, G.; Mohan, S.; Henry, R. Analysis of factors for improving functionality of tin oxide gas sensor. IETE Tech. Rev. 2015, 33, 122–129. [Google Scholar] [CrossRef]
- Li, J.-H.; Wu, J.; Yu, Y.-X. DFT exploration of sensor performances of two-dimensional WO3 to ten small gases in terms of work function and band gap changes and I-V responses. Appl. Surf. Sci. 2021, 546, 149104. [Google Scholar] [CrossRef]
- Fu, J.; Ali, R.; Mu, C.; Liu, Y.; Mahmood, N.; Lau, W.-M.; Jian, X. Large-scale preparation of 2D VSe2 through a defect-engineering approach for efficient hydrogen evolution reaction. Chem. Eng. J. 2021, 411, 128494. [Google Scholar] [CrossRef]
- Liangruksa, M.; Sukpoonprom, P.; Junkaew, A.; Photaram, W.; Siriwong, C. Gas sensing properties of palladium-modified zinc oxide nanofilms: A DFT study. Appl. Surf. Sci. 2021, 544, 148868. [Google Scholar] [CrossRef]
- Ni, Z.; Bao, S.; Gong, X.-Q. A DFT study of the CO adsorption and oxidation at ZnO surfaces and its implication for CO detection. Chin. Chem. Lett. 2020, 31, 1674–1679. [Google Scholar] [CrossRef]










| Materials | Concentration (ppm) | Temperature (°C) | Response (Ra/Rg) | LOD (ppm) | Ref. |
|---|---|---|---|---|---|
| CuO/ZnO nanowires | 100 | 300 | 28 | 1.06 | [37] |
| Au,Cl-ZnO nanoparticles | 100 | 220 | 19.64 | 19.64 | [38] |
| T-ZnO/ZnFe2O4 Tetrapods | 100 | 300 | 13.95 | 1 | [39] |
| Mg doped ZnO | 5 | 300 | 80 | 1 | [40] |
| Ag/ZnO nanorods | 100 | 360 | 36.6 | 50 | [41] |
| K-doped ZnO nanorods | 100 | 300 | 45.2 | 2 | [42] |
| Ag-ZnO | 50 | 325 | 32.5 | 50 | [43] |
| ZnO nanorods | 100 | 300 | 44.9 | 10 | [44] |
| Au/ZnO | 100 | 250 | 37.74 | 1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Zhang, L.; Wang, W.; Yu, F. Ethanol Sensing Properties and First Principles Study of Au Supported on Mesoporous ZnO Derived from Metal Organic Framework ZIF-8. Sensors 2021, 21, 4352. https://doi.org/10.3390/s21134352
Kang Y, Zhang L, Wang W, Yu F. Ethanol Sensing Properties and First Principles Study of Au Supported on Mesoporous ZnO Derived from Metal Organic Framework ZIF-8. Sensors. 2021; 21(13):4352. https://doi.org/10.3390/s21134352
Chicago/Turabian StyleKang, Yanli, Lu Zhang, Wenhao Wang, and Feng Yu. 2021. "Ethanol Sensing Properties and First Principles Study of Au Supported on Mesoporous ZnO Derived from Metal Organic Framework ZIF-8" Sensors 21, no. 13: 4352. https://doi.org/10.3390/s21134352