A Compact Raster Lensless Microscope Based on a Microdisplay
Abstract
:1. Introduction
2. Materials and Methods
- Firstly, an UUGL1320 microdisplay (from the Fraunhofer Institute for Organic Electronics, Dresden, Germany [33]), with 720 × 256 OLEDs, 3.6 mm × 1.28 mm area, monochrome green (520–560 nm), diagonal 0.13″, LED size and pitch 5 μm × 5 μm (no spacing between them), providing a brightness of up to 1000 cd/m2. A SPI serial interface with a maximum frame rate of 40 fps was used to switch the LEDs on and off one by one.
- Secondly, a JBD25UMFHDG monochromatic green (520–530 nm) 1920 × 1080 GaN LEDs, 1 μm diameter and 2.5 μm pitch from JBD, Shanghai, China [34] features a 10k dpi resolution with a display area of 4.8 mm × 2.7 mm, providing a luminance of 1.5 Mcd/m2. In this case, the images must be uploaded as in a display through an HDMI interface, i.e., the complete image for every frame.
- For the third light source, a C330TMD-A Mounted Aspheric Lens, ARC: 350–700 nm with f = 3.1 mm and NA = 0.7 (Thorlabs GmbH, Bergkirchen, Germany), was inserted between the first (OLED) microdisplay and the sample, in such a way that the focus plane can be considered as the effective lighting plane. According to Equation (1), this configuration produces an Airy disc of 900–980 nm (owing to the wavelength dispersion). The result of ×10 image reduction is thus a final spot not smaller than 1400–1480 nm with an effective pitch of 500 nm and a FOV for the whole microdisplay of 360 µm × 128 µm.
- Finally, an Oufemar achromatic objective with ×60 for biological microscopy with tube length of 160 mm and NA = 0.85 was tested between the first (OLED) microdisplay and the sample. This configuration produces an Airy disc of 750–810 nm, originating a final spot of around 830–890 nm with an effective pitch of 83 nm and FOV of 60 μm × 21.3 μm.
3. Results and Discussion
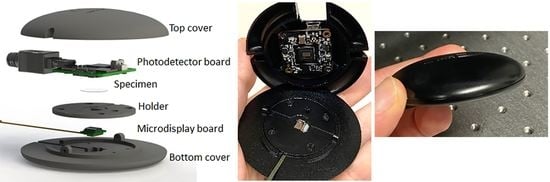
3.1. The Complete Setup
3.2. Static and Dynamic Configurations
3.3. Resolution
3.4. Considerations about the Optical Downscaling Setup
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenbaum, A.; Luo, W.; Su, T.-W.; Göröcs, Z.; Xue, L.; Isikman, S.; Coskun, A.; Mudanyali, O.; Ozcan, A. Imaging without lenses: Achievements and remaining challenges of wide-field on-chip microscopy. Nat. Methods 2012, 9, 889–895. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, J.; Song, P.; Guo, C.; Bian, Z.; Wang, R.; Huang, Y.; Wang, S.; Zhang, H.; Zheng, G. Wide-field, high-resolution lensless on-chip microscopy via near-field blind ptychographic modulation. Lab Chip 2020, 20, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Mielenz, K.D. On the diffraction limit for lensless imaging. J. Res. Natl. Inst. Stand. Technol. 1999, 104, 479–485. [Google Scholar] [CrossRef]
- Repetto, L.; Piano, E.; Pontiggia, C. Lensless digital holographic microscope with light-emitting diode illumination. Opt. Lett. 2004, 29, 1132–1134. [Google Scholar] [CrossRef]
- Corman, R.; Boutu, W.; Campalans, A.; Radicella, P.; Duarte, J.; Kholodtsova, M.; Bally-Cuif, L.; Dray, N.; Harms, F.; Dovillaire, G.; et al. Lensless microscopy platform for single cell and tissue visualization. Biomed. Opt. Express 2020, 11, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, Y.; Feizi, A.; Göröcs, Z.; Ozcan, A. Pixel super-resolution using wavelength scanning. Light Sci. Appl. 2016, 5, e16060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Wang, W.; Liu, C.; Liu, J. Pixel super-resolved lens-free on-chip microscopy based on dual laterally shifting modulation. Appl. Opt. 2020, 59, 3411–3416. [Google Scholar] [CrossRef]
- Cui, X.; Lee, L.; Heng, X.; Zhong, W.; Sternberg, P.; Psaltis, D.; Yang, C. Lensless high-resolution on-chip optofluidic microscopes for Caenorhabditis elegans and cell imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 10670–10675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, D.; Storment, C.W.; Conley, C.A.; Kovacs, G.T.A. A microfluidic shadow imaging system for the study of the nematode Caenorhabditis elegans in space. Sen. Actuators B Chem. 2005, 107, 904–914. [Google Scholar] [CrossRef]
- Yang, C.; Bu, X.; Ma, H.; Zhang, L.; Cao, X.; Yue, T.; Hua, X.; Yan, F. Resolution-Enhanced Lensless Color Shadow Imaging Microscopy Based on Large Field-of-View Submicron-Pixel Imaging Sensors. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition Workshops, Salt Lake City, UT, USA, 18–22 June 2018; Volume 8575473, pp. 2327–2334. [Google Scholar] [CrossRef]
- Faulkner, H.M.L.; Rodenburg, J.M. Movable Aperture Lensless Transmission Microscopy: A Novel Phase Retrieval Algorithm. Phys. Rev. Lett. 2004, 93, 023903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobieranski, A.C.; Inci, F.; Tekin, H.C.; Yuksekkaya, M.; Comunello, E.; Cobra, D.; von Wangenheim, A.; Demirci, U. Portable lensless wide-field microscopy imaging platform based on digital inline holography and multi-frame pixel super-resolution. Light Sci. Appl. 2015, 4, e346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sony Releases Stacked CMOS Image Sensor for Smartphones with Industry’s Highest 48 Effective Megapixels. Available online: https://www.sony.net/SonyInfo/News/Press/201807/18-060E/ (accessed on 1 July 2021).
- Samsung’s Newest 108Mp Mobile Image Sensor with Advanced Features Captures More Details and Produces Sharper Results. Available online: https://news.samsung.com/global/samsungs-newest-108mp-mobile-image-sensor-with-advanced-features-captures-more-details-and-produces-sharper-results (accessed on 1 July 2021).
- Samsung Introduces Industry’s First 0.7 μm-Pixel Mobile Image Sensor. Available online: https://news.samsung.com/global/samsung-introduces-industrys-first-0-7μm-pixel-mobile-image-sensor (accessed on 1 July 2021).
- Sheppard, C.J.R.; Wilson, T. Reciprocity and equivalence in scanning microscopes. J. Opt. Soc. Am. A 1986, 3, 755–756. [Google Scholar] [CrossRef]
- Kermisch, D. Principle of equivalence between scanning and conventional optical imaging systems. J. Opt. Soc. Am. 1977, 67, 1357–1360. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Wilson, T. Image Formation in Scanning Microscopes with Partially Coherent Source and Detector. Opt. Acta: Intern. J. Opt. 1978, 25, 315–325. [Google Scholar] [CrossRef]
- Kirkland, E.J. Advanced Computing in Electron Microscopy, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Franch, N.; Canals, J.; Moro, V.; Vilà, A.; Romano-Rodríguez, A.; Prades, J.D.; Gülink, J.; Bezshlyakh, D.; Waag, A.; Kluczyk-Korch, K.; et al. Nano-illumination microscopy: A technique based on scanning with an array of individually addressable nanoLEDs. Opt. Express 2020, 28, 391497. [Google Scholar] [CrossRef]
- Kluczyk-Korch, K.; Palazzo, D.; Waag, A.; Diéguez, A.; Prades, J.D.; Di Carlo, A.; Auf der Maur, M. Optical design of InGaN/GaN nanoLED arrays on a chip: Toward: Highly resolved illumination. Nanotechnology 2021, 32, 105203. [Google Scholar] [CrossRef]
- Bezshlyakh, D.; Spende, H.; Weimann, T.; Hinze, P.; Bornemann, S.; Gülink, J.; Canals, J.; Prades, J.D.; Dieguez, A.; Waag, A. Directly addressable GaN-based nano-LED arrays: Fabrication and electro-optical characterization. Microsyst. Nanoeng. 2020, 6, 88. [Google Scholar] [CrossRef]
- Huang, Y.; Hsiang, E.L.; Deng, M.Y.; Wu, S. Mini-LED, Micro-LED and OLED displays: Present status and future perspectives. Light Sci. Appl. 2020, 9, 105. [Google Scholar] [CrossRef]
- Kopin OLED. Available online: https://www.kopin.com/oled/ (accessed on 1 July 2021).
- Olightek OLED Microdisplay. Available online: http://www.olightek.com/product/18.html (accessed on 31 October 2020).
- Sony Microdisplay. Available online: https://www.sony-semicon.co.jp/e/products/microdisplay/oled/product.html (accessed on 1 July 2021).
- The Leading Producer of AMOLED Microdisplays. Available online: https://emagin.com/products/ (accessed on 1 July 2021).
- Plessey First Microled Display Module. Available online: http://plesseysemiconductors.com/compound-photonics-plessey-first-microled-display-module/ (accessed on 31 October 2020).
- Jade Bird Display: MicroLED Displays. Available online: https://www.jb-display.com (accessed on 1 July 2021).
- Gibson, G.M.; Johnson, S.D.; Padgett, M.J. Single-pixel imaging 12 years on: A review. Opt. Express 2020, 28, 28190–28208. [Google Scholar] [CrossRef]
- Jauregui-Sánchez, Y.; Clemente, P.; Lancis, J.; Tajahuerce, E. Single-pixel imaging with Fourier filtering: Application to vision through scattering media. Opt. Lett. 2019, 44, 679–682. [Google Scholar] [CrossRef] [Green Version]
- Jauregui-Sánchez, Y.; Clemente, P.; Latorre-Carmona, P.; Lancis, J.; Tajahuerce, E. Single-Pixel Imaging Using Photodiodes. In Advances in Photodetectors—Research and Applications; Chee, K., Ed.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/62718 (accessed on 1 July 2021).
- Ultra-Low-Power OLED Microdisplays. Available online: https://www.fep.fraunhofer.de/en/Geschaeftsfelder/MS/ultra-low-power-oled-mikrodisplays.html (accessed on 1 July 2021).
- Monochromatic 2.5-um Pitch (10K DPI) 1080P. Available online: https://www.jb-display.com/0-22 (accessed on 1 July 2021).
- Wasisto, H.M.; Prades, J.D.; Gülink, J.; Waag, A. Beyond solid-state lighting: Miniaturization, hybrid integration, and applications of GaN nano- and micro-LEDs. Appl. Phys. Rev. 2020, 6, 041315, Correction in 2020, 7, 019901. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.; Liu, Z. Hyperlenses and metalenses for far-field super-resolution imaging. Nat. Commun. 2012, 3, 1205. [Google Scholar] [CrossRef]
- Deng, Y.; Chu, D. Coherence properties of different light sources and their effect on the image sharpness and speckle of holographic displays. Sci. Rep. 2017, 7, 5893. [Google Scholar] [CrossRef]
- Duarte, F.J.; Liao, L.S.; Vaeth, K.M. Coherence characteristics of electrically excited tandem organic light-emitting diodes. Opt. Lett. 2007, 30, 3072–3074. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilà, A.; Moreno, S.; Canals, J.; Diéguez, A. A Compact Raster Lensless Microscope Based on a Microdisplay. Sensors 2021, 21, 5941. https://doi.org/10.3390/s21175941
Vilà A, Moreno S, Canals J, Diéguez A. A Compact Raster Lensless Microscope Based on a Microdisplay. Sensors. 2021; 21(17):5941. https://doi.org/10.3390/s21175941
Chicago/Turabian StyleVilà, Anna, Sergio Moreno, Joan Canals, and Angel Diéguez. 2021. "A Compact Raster Lensless Microscope Based on a Microdisplay" Sensors 21, no. 17: 5941. https://doi.org/10.3390/s21175941