Agroforestry: An Appropriate and Sustainable Response to a Changing Climate in Southern Africa?
Abstract
:1. Introduction
2. Background
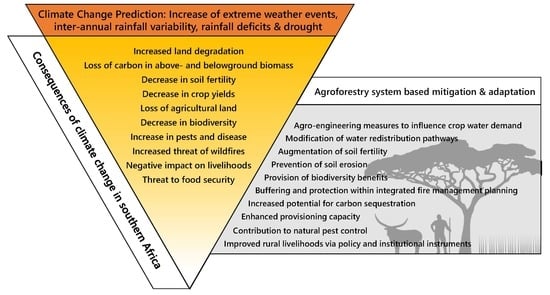
3. Predicted Changing Climate Effects on the Southern African Region
4. Consequences of Predicted Changing Climate Effects for the Southern African Region, Mitigation Offered by Agroforestry Systems
4.1. AFS as an Agro-Engineering Measure to Influence Crop Water Demand
4.2. Modification of Water Fluxes
4.3. Augmentation of Soil Fertility
4.4. Prevention of Soil Erosion and the Degradation of Agricultural Land
4.5. Provision of Biodiversity Benefits
4.6. Contribution to Natural Pest Control
4.7. Buffering and Prevention within Integrated Fire Management Planning
4.8. Increased Potential for Carbon Sequestration
4.9. Enhanced Provisioning Capacity
4.10. Improved Rural Livelihoods via Policy and Institutional Instruments
5. Future Prospects
- (a)
- The proportion of trees decreases at the same linear rate as that of agricultural crop increase. There is no interaction between the two AFS components. The effects of the inter-system competition (competition between the two systems) and the intra-system competition (within the two systems) are equal. Total productivity of this scenario results in an additive effect of the productivities of the individual components. This scenario is unlikely, as the interaction effect between trees and crops is generally proven to provide an influence on growth for one or more components of the system.
- (b)
- The change in component proportion is non-linear. The agricultural crop benefits from the interaction, for example, by means of facilitation or competitive reduction factors. The intra-system competition for the agricultural crop is higher than the inter-system competition with the tree culture; the reverse applies to the tree culture. However, these effects compensate each other so that the net effect of the combination is additive and equal to scenario a.
- (c)
- Interactions between the two land-use systems are incompatible, decreasing proportion of one AFS component results in an opportunistic increase in the other. Intra-system competition is high, leading to an under-yielding scenario. This may be reflected by incompatible species choice or an influence of a biased management of individual components.
- (d)
- Interactions between the two land-use systems are synergistic or mutualistic and non-linear, a combination of components provides an increased yield. Intra-system competition is higher than inter-system competition for both systems. This may result from facilitation, competitive reduction, and/or niche complementarity of both agricultural crops and trees (agricultural crops and trees utilising different soil resources). This leads to over-yielding at the level of the mixture and is the scenario that is most often touted as a benefit of AFS (i.e., increased land equivalent ratio (LER), cf. [220]).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, M.; Lovell, S. Agroforestry—The Next Step in Sustainable and Resilient Agriculture. Sustainability 2016, 8, 574. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.K.R. Climate change mitigation: A low-hanging fruit of agroforestry. In Agroforestry—The Future of Global Land Use; Nair, P.K.R., Garrity, D.P., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2012; pp. 31–67. ISBN 978-94-007-4676-3. [Google Scholar]
- Mbow, C.; van Noordwijk, M.; Luedeling, E.; Neufeldt, H.; Minang, P.A.; Kowero, G. Agroforestry solutions to address food security and climate change challenges in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Lundgrun, B.; Raintree, J.B. Sustained agroforestry. In Agricultural Research for Development: Potentials and Challenges in Asia, Jakarta, Indonesia, 24–29 October 1982; Nestel, B., Ed.; ISNAR: The Hague, The Netherlands, 1983; pp. 37–49. [Google Scholar]
- Somarriba, E. Revisiting the past: An essay on agroforestry definition. Agroforest Syst. 1992, 19, 233–240. [Google Scholar] [CrossRef]
- Nair, P.K.R. An Introduction to Agroforestry; Kluwer Academic Publishers (in cooperation with the International Centre for Research in Agroforestry): Dordrecht, The Netherlands, 1993. [Google Scholar]
- Leakey, R. Definition of agroforestry revisited. Agrofor. Today 1996, 8, 5. [Google Scholar]
- SADC. Consolidated Text of the Treaty of the Southern African Development Community. Available online: https://www.sadc.int/files/5314/4559/5701/Consolidated_Text_of_the_SADC_Treaty_-_scanned_21_October_2015.pdf (accessed on 18 June 2020).
- Syampungani, S.; Chirwa, P.W.; Akinnifesi, F.K.; Ajayi, O.C. The Potential of Using Agroforestry as a Win-Win Solution to Climate Change Mitigation and Adaptation and Meeting Food Security Challenges in Southern Africa. Agric. J. 2010, 5, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Tumushabe, J.T. Climate change, food security and sustainable development in Africa. In The Palgrave Handbook of African Politics, Governance and Development; Oloruntoba, S.O., Falola, T., Eds.; Palgrave Macmillan US: New York, NY, USA, 2018; pp. 853–868. ISBN 978-1-349-95231-1. [Google Scholar]
- WFP. End-of-Season Update for 2018/19 and Overview of the Food Security Situation in 2019/20. Available online: https://docs.wfp.org/api/documents/WFP-0000106747/download/?iframe (accessed on 18 June 2020).
- WFP. Southern Africa: Seasonal Overview and Drought Hotspot Analysis (2019/2020). Available online: https://docs.wfp.org/api/documents/WFP-0000115666/download/?iframe (accessed on 18 June 2020).
- CBD; UNCCD; UNFCCC. The Rio Conventions. Action on Forests; Convention on Biological Diversity (CBD), United Nations Convention to Combat Desertification (UNCCD), United Nations Framework Convention on Climate Change (UNFCCC): Cologne, Germany, 2012; ISBN 92-9219-092-X. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). The State of the World’s Forests, 2018. Forest Pathways to Sustainable Development; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; ISBN 978-92-5-130561-4. [Google Scholar]
- Mbow, C.; van Noordwijk, M.; Prabhu, R.; Simons, T. Knowledge gaps and research needs concerning agroforestry’s contribution to Sustainable Development Goals in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Van Noordwijk, M.; Duguma, L.A.; Dewi, S.; Leimona, B.; Catacutan, D.C.; Lusiana, B.; Öborn, I.; Hairiah, K.; Minang, P.A. SDG synergy between agriculture and forestry in the food, energy, water and income nexus: Reinventing agroforestry? Curr. Opin. Environ. Sustain. 2018, 34, 33–42. [Google Scholar] [CrossRef]
- Van Noordwijk, M.; Duguma, L.A.; Dewi, S.; Leimona, B.; Catacutan, D.C.; Lusiana, B.; Oborn, I.; Hairiah, K.; Minang, P.A.; Ekadinata, A.; et al. Agroforestry into its fifth decade: Local responses to global challenges and goals in the Anthropocene. In Sustainable Development through Trees on Farms: Agroforestry in Its Fifth Decade; van Noordwijk, M., Ed.; World Agroforestry (ICRAF): Bogor, Indonesia, 2019; pp. 397–418. [Google Scholar]
- Rosenstock, T.S.; Dawson, I.K.; Aynekulu, E.; Chomba, S.; Degrande, A.; Fornace, K.; Jamnadass, R.; Kimaro, A.; Kindt, R.; Lamanna, C.; et al. A Planetary Health Perspective on Agroforestry in Sub-Saharan Africa. One Earth 2019, 1, 330–344. [Google Scholar] [CrossRef] [Green Version]
- World Bank. Sustaining Forests. A Development Strategy; The World Bank: Washington, DC, USA, 2004; ISBN 0-8213-5755-7. [Google Scholar]
- Jama, B.; Zeila, A. Agroforestry in the Drylands of Eastern Africa. A Call to Action; ICRAF Working Paper No 1 No. 1; ICRAF: Nairobi, Kenya, 2005. [Google Scholar]
- Nyong, A.P.; Ngankam, T.M.; Felicite, T.L. Enhancement of resilience to climate variability and change through agroforestry practices in smallholder farming systems in Cameroon. Agroforest Syst. 2020, 94, 687–705. [Google Scholar] [CrossRef]
- Pretty, J.; Benton, T.G.; Bharucha, Z.P.; Dicks, L.V.; Flora, C.B.; Godfray, H.C.J.; Goulson, D.; Hartley, S.; Lampkin, N.; Morris, C.; et al. Global assessment of agricultural system redesign for sustainable intensification. Nat. Sustain. 2018, 1, 441–446. [Google Scholar] [CrossRef]
- Reith, E.; Gosling, E.; Knoke, T.; Paul, C. How Much Agroforestry Is Needed to Achieve Multifunctional Landscapes at the Forest Frontier?—Coupling Expert Opinion with Robust Goal Programming. Sustainability 2020, 12, 6077. [Google Scholar] [CrossRef]
- Mwangi, H.M.; Julich, S.; Patil, S.D.; McDonald, M.A.; Feger, K.-H. Modelling the impact of agroforestry on hydrology of Mara River Basin in East Africa. Hydrol. Process. 2016, 30, 3139–3155. [Google Scholar] [CrossRef]
- Kanzler, M.; Böhm, C.; Mirck, J.; Schmitt, D.; Veste, M. Microclimate effects on evaporation and winter wheat (Triticum aestivum L.) yield within a temperate agroforestry system. Agrofor. Syst. 2019, 93, 1821–1841. [Google Scholar] [CrossRef]
- Olsson, L.; Barbosa, H.; Bhadwal, S.; Cowie, A.; Delusca, K.; Flores-Renteria, D.; Hermans, K.; Jobbagy, E.; Kurz, W.; Li, D. Land degradation. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; 2019; pp. 345–436, in press. [Google Scholar]
- Ellison, D.; Morris, C.E.; Locatelli, B.; Sheil, D.; Cohen, J.; Murdiyarso, D.; Gutierrez, V.; van Noordwijk, M.; Creed, I.F.; Pokorny, J.; et al. Trees, forests and water: Cool insights for a hot world. Glob. Environ. Chang. 2017, 43, 51–61. [Google Scholar] [CrossRef]
- Kwesiga, F.; Akinnifesi, F.K.; Mafongoya, P.L.; McDermott, M.H.; Agumya, A. Agroforestry research and development in southern Africa during the 1990s: Review and challenges ahead. Agrofor. Syst. 2003, 59, 173–186. [Google Scholar] [CrossRef]
- CIFOR; ICRAF. CIFOR-ICRAF 2020-2030 Strategy. 2019. Available online: https://www.cifor.org/our-work/cifor-icraf-merger-faq/ (accessed on 9 July 2020).
- Luedeling, E.; Sileshi, G.; Beedy, T.; Dietz, J. Carbon sequestration potential of agroforestry systems in Africa. In Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges; Kumar, B.M., Nair, P.K.R., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 61–83. ISBN 978-94-007-1630-8. [Google Scholar]
- Akinnifesi, F.K.; Chirwa, P.W.; Ajayi, O.C.; Sileshi, G.; Matakala, P.; Kwesiga, F.R.; Harawa, H.; Makumba, W. Contributions of agroforestry research to livelihood of smallholder farmers in Southern Africa: 1. Taking stock of the adaptation, adoption and impact of fertilizer tree options. Agric. J. 2008, 3, 58–75. [Google Scholar]
- Akinnifesi, F.K.; Sileshi, G.; Ajayi, O.C.; Chirwa, P.W.; Kwesiga, F.R.; Harawa, R. Contributions of agroforestry research and development to livelihood of smallholder farmers in Southern Africa: 2. Fruit, medicinal, fuelwood and fodder tree systems. Agric. J. 2008, 3, 76–88. [Google Scholar]
- Akinnifesi, F.K.; Ajayi, O.C.; Sileshi, G.; Chirwa, P.W.; Chianu, J. Fertiliser trees for sustainable food security in the maize-based production systems of East and Southern Africa. A review. Agron. Sustain. Dev. 2010, 30, 615–629. [Google Scholar] [CrossRef]
- Thierfelder, C.; Baudron, F.; Setimela, P.; Nyagumbo, I.; Mupangwa, W.; Mhlanga, B.; Lee, N.; Gérard, B. Complementary practices supporting conservation agriculture in southern Africa. A review. Agron. Sustain. Dev. 2018, 38, 16. [Google Scholar] [CrossRef] [Green Version]
- Boko, M.; Niang, I.; Nyong, A.; Vogel, C.; Githeko, A.; Medany, M.; Osman-Elasha, B.; Tabo, R.; Yanda, P. Africa. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 433–467. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, P.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; ISBN 978-92-9169-143-2. [Google Scholar]
- Engelbrecht, F.A.; McGregor, J.L.; Engelbrecht, C.J. Dynamics of the Conformal-Cubic Atmospheric Model projected climate-change signal over southern Africa. Int. J. Climatol. 2009, 29, 1013–1033. [Google Scholar] [CrossRef]
- James, R.; Washington, R. Changes in African temperature and precipitation associated with degrees of global warming. Clim. Chang. 2013, 117, 859–872. [Google Scholar] [CrossRef]
- New, M.; Hewitson, B.; Stephenson, D.B.; Tsiga, A.; Kruger, A.; Manhique, A.; Gomez, B.; Coelho, C.A.S.; Masisi, D.N.; Kululanga, E.; et al. Evidence of trends in daily climate extremes over southern and west Africa. J. Geophys. Res. 2006, 111, D14102. [Google Scholar] [CrossRef]
- Kusangaya, S.; Warburton, M.L.; van Archer Garderen, E.; Jewitt, G.P.W. Impacts of climate change on water resources in southern Africa: A review. Phys. Chem. Earthparts A/B/C 2014, 67–69, 47–54. [Google Scholar] [CrossRef]
- Niang, I.; Ruppel, O.C.; Abdrabo, M.A.; Essel, A.; Lennard, C.; Padgham, J.; Urquhart, P. Africa. In Climate Change 2014: Impacts, Adaptation and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC, Ed.; Cambridge University Press: New York, NY, USA, 2014; pp. 1199–1265. ISBN 978-1-107-05816-3. [Google Scholar]
- Zhao, T.; Dai, A. The Magnitude and Causes of Global Drought Changes in the Twenty-First Century under a Low–Moderate Emissions Scenario. J. Clim. 2015, 28, 4490–4512. [Google Scholar] [CrossRef]
- Maidment, R.I.; Allan, R.P.; Black, E. Recent observed and simulated changes in precipitation over Africa. Geophys. Res. Lett. 2015, 42, 8155–8164. [Google Scholar] [CrossRef]
- Davis, C.L.; Engelbrecht, F.A.; Tadross, M.; Wolski, P.; Archer, E.R.M. Future climate change over Southern Africa. In South African Risk and Vulnerability Atlas: Understanding the Social & Environmental Implications of Global Change, 2nd ed.; Mambo, J., Faccer, K., Eds.; AFRICAN SUN MeDIA: Stellenbosch, South Africa, 2017; pp. 13–25. [Google Scholar]
- Muthoni, F.K.; Odongo, V.O.; Ochieng, J.; Mugalavai, E.M.; Mourice, S.K.; Hoesche-Zeledon, I.; Mwila, M.; Bekunda, M. Long-term spatial-temporal trends and variability of rainfall over Eastern and Southern Africa. Appl Clim. 2019, 137, 1869–1882. [Google Scholar] [CrossRef] [Green Version]
- Munalula, F.; Seifert, T.; Meincken, M. The Expected Effects of Climate Change on Tree Growth and Wood Quality in Southern Africa. Springer Sci. Rev. 2016, 4, 99–111. [Google Scholar] [CrossRef]
- Zinyengere, N.; Crespo, O.; Hachigonta, S. Crop response to climate change in southern Africa: A comprehensive review. Glob. Planet. Chang. 2013, 111, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Nelson, G.C.; Rosegrant, M.W.; Koo, J.; Robertson, R.D.; Sulser, T.; Zhu, T.; Ringler, C.; Msangi, S.; Palazzo, A.; Batka, M.; et al. Climate Change. Impact on Agriculture and Costs of Adaptation; International Food Policy Research Institute: Washington, DC, USA, 2009; ISBN 0896295354. [Google Scholar]
- Schlenker, W.; Lobell, D.B. Robust negative impacts of climate change on African agriculture. Environ. Res. Lett. 2010, 5, 14010. [Google Scholar] [CrossRef]
- Blanc, E. The Impact of Climate Change on Crop Yields in Sub-Saharan Africa. Am. J. Clim. Chang. 2012, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Akpalu, W.; Rashid, H.M.; Ringler, C. Climate Variability and Maize Yield in South Africa. Results from GME and MELE Methods; IFPRI Discussion Paper 843; IFPRI: Washington, DC, USA, 2008. [Google Scholar]
- Nair, P.K.R. Carbon sequestration studies in agroforestry systems: A reality-check. Agrofor. Syst 2012, 86, 243–253. [Google Scholar] [CrossRef]
- Zomer, R.J.; Neufeldt, H.; Xu, J.; Ahrends, A.; Bossio, D.; Trabucco, A.; van Noordwijk, M.; Wang, M. Global Tree Cover and Biomass Carbon on Agricultural Land: The contribution of agroforestry to global and national carbon budgets. Sci. Rep. 2016, 6, 29987. [Google Scholar] [CrossRef]
- Makate, C.; Makate, M.; Mango, N.; Siziba, S. Increasing resilience of smallholder farmers to climate change through multiple adoption of proven climate-smart agriculture innovations. Lessons from Southern Africa. J. Environ. Manag. 2019, 231, 858–868. [Google Scholar] [CrossRef]
- Littmann, T.; Veste, M. Evapotranspiration, transpiration and dewfall. In Arid Dune Ecosystems: The Nizzana Sands in the Negev Desert; Breckle, S.-W., Yair, A., Veste, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 183–200. ISBN 978-3-540-75498-5. [Google Scholar]
- Cleugh, H.A. Effects of windbreaks on airflow, microclimates and crop yields. Agrofor. Syst. 1998, 41, 55–84. [Google Scholar] [CrossRef]
- Cui, Q.; Feng, Z.; Pfiz, M.; Veste, M.; Küppers, M.; He, K.; Gao, J. Trade-off between shrub plantation and wind-breaking in the arid sandy lands of Ningxia, China. Pak. J. Bot. 2012, 44, 1639–1649. [Google Scholar]
- Wang, H.; Takle, E.S. A numerical simulation of boundary-layer flows near shelterbelts. Bound. Layer Meteorol 1995, 75, 141–173. [Google Scholar] [CrossRef]
- Veste, M.; Littmann, T.; Kunneke, A.; Du Toit, B.; Seifert, T. Windbreaks as part of climate-smart landscapes reduce evapotranspiration in vineyards, Western Cape Province, South Africa. Plant. Soil Environ. 2020, 66, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Campi, P.; Palumbo, A.D.; Mastrorilli, M. Effects of tree windbreak on microclimate and wheat productivity in a Mediterranean environment. Eur. J. Agron. 2009, 30, 220–227. [Google Scholar] [CrossRef]
- Sida, T.S.; Baudron, F.; Kim, H.; Giller, K.E. Climate-smart agroforestry: Faidherbia albida trees buffer wheat against climatic extremes in the Central Rift Valley of Ethiopia. Agric. For. Meteorol. 2018, 248, 339–347. [Google Scholar] [CrossRef]
- Jose, S.; Gillespie, A.R.; Pallardy, S.G. Interspecific interactions in temperate agroforestry. Agrofor. Syst. 2004, 61–62, 237–255. [Google Scholar] [CrossRef]
- Ghezehei, S.B.; Annandale, J.; Everson, C. Optimizing resource distribution and crop productivity in hedgerow intercropping by manipulating tree arrangement. Agrofor. Syst. 2016, 90, 861–873. [Google Scholar] [CrossRef] [Green Version]
- Veste, M.; Ben-Gal, A.; Shani, U. Impact of thermal stress and high vpd on gas exchange and chlorophyll fluorescence of Citrus grandis under desert conditions. Acta Hortic. 2000, 531, 143–150. [Google Scholar] [CrossRef]
- Raveh, E.; Cohen, S.; Raz, T.; Yakir, D.; Grava, A.; Goldschmidt, E.E. Increased growth of young citrus trees under reduced radiation load in a semi-arid climate. J. Exp. Bot. 2003, 54, 365–373. [Google Scholar] [CrossRef]
- Rosskopf, E.; Morhart, C.; Nahm, M. Modelling Shadow Using 3D Tree Models in High Spatial and Temporal Resolution. Remote Sens. 2017, 9, 719. [Google Scholar] [CrossRef] [Green Version]
- Ndoli, A.; Baudron, F.; Schut, A.G.T.; Mukuralinda, A.; Giller, K.E. Disentangling the positive and negative effects of trees on maize performance in smallholdings of Northern Rwanda. Field Crop. Res. 2017, 213, 1–11. [Google Scholar] [CrossRef]
- Sheppard, J.P. Options for Management of High Value Timber within Temperate Agroforestry Systems. Ph.D. Thesis, Albert-Ludwigs-Universität Freiburg i. Br., Freiburg im Breisgau, Germany, 2016. [Google Scholar]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Dalland, A.; Vje, P.I.; Matthews, R.B.; Singh, B.R. The potential of alley cropping in improvement of cultivation systems in the high rainfall areas of Zambia. III. Effects on soil chemical and physical properties. Agrofor. Syst. 1993, 21, 117–132. [Google Scholar] [CrossRef]
- Anderson, S.H.; Udawatta, R.P.; Seobi, T.; Garrett, H.E. Soil water content and infiltration in agroforestry buffer strips. Agrofor. Syst. 2009, 75, 5–16. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Ong, C.K. The redistribution of soil water by tree root systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef]
- Domec, J.-C.; King, J.S.; Noormets, A.; Treasure, E.; Gavazzi, M.J.; Sun, G.; McNulty, S.G. Hydraulic redistribution of soil water by roots affects whole-stand evapotranspiration and net ecosystem carbon exchange. New Phytol. 2010, 187, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.E.; Gyenge, J.; Licata, J.; Schlichter, T.; Bond, B.J. Belowground interactions for water between trees and grasses in a temperate semiarid agroforestry system. Agrofor. Syst. 2008, 74, 185–197. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Su, J. The Soil Water Condition of a Typical Agroforestry System under the Policy of Northwest China. Forests 2018, 9, 730. [Google Scholar] [CrossRef] [Green Version]
- Odhiambo, H.O.; Ong, C.K.; Deans, J.D.; Wilson, J.; Khan, A.A.H.; Sprent, J.I. Roots, soil water and crop yield: Tree crop interactions in a semi-arid agroforestry system in Kenya. Plant. Soil 2001, 235, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Siriri, D.; Wilson, J.; Coe, R.; Tenywa, M.M.; Bekunda, M.A.; Ong, C.K.; Black, C.R. Trees improve water storage and reduce soil evaporation in agroforestry systems on bench terraces in SW Uganda. Agrofor. Syst. 2013, 87, 45–58. [Google Scholar] [CrossRef] [Green Version]
- Chirwa, P.W.; Nair, P.K.R.; Nkedi-Kizza, P. Pattern of soil moisture depletion in alley cropping under semiarid conditions in Zambia. Agrofor. Syst. 1994, 26, 89–99. [Google Scholar] [CrossRef]
- Herberich, M.M.; Gayler, S.; Anand, M.; Tielbörger, K. Hydrological niche segregation of plant functional traits in an individual-based model. Ecol. Model. 2017, 356, 14–24. [Google Scholar] [CrossRef]
- Silvertown, J.; Araya, Y.; Gowing, D.; Cornwell, W. Hydrological niches in terrestrial plant communities: A review. J. Ecol. 2015, 103, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Dawson, T.E. Hydraulic lift and water use by plants: Implications for water balance, performance and plant-plant interactions. Oecologia 1993, 95, 565–574. [Google Scholar] [CrossRef]
- Ludwig, F.; Dawson, T.E.; Kroon, H.; Berendse, F.; Prins, H.H.T. Hydraulic lift in Acacia tortilis trees on an East African savanna. Oecologia 2003, 134, 293–300. [Google Scholar] [CrossRef]
- Hirota, I.; Sakuratani, T.; Sato, T.; Higuchi, H.; Nawata, E. A split-root apparatus for examining the effects of hydraulic lift by trees on the water status of neighbouring crops. Agrofor. Syst. 2004, 60, 181–187. [Google Scholar] [CrossRef]
- Bayala, J.; Prieto, I. Water acquisition, sharing and redistribution by roots: Applications to agroforestry systems. Plant. Soil 2019, 95, 323. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, M.M.; Dawson, T.E.; Richards, J.H. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Schulze, E.-D.; Caldwell, M.M.; Canadell, J.; Mooney, H.A.; Jackson, R.B.; Parson, D.; Scholes, R.; Sala, O.E.; Trimborn, P. Downward flux of water through roots (i.e., inverse hydraulic lift) in dry Kalahari sands. Oecologia 1998, 115, 460–462. [Google Scholar] [CrossRef]
- Smith, D.M.; Jackson, N.A.; Roberts, J.M.; Ong, C.K. Reverse flow of sap in tree roots and downward siphoning of water by Grevillea robusta. Funct. Ecol. 2002, 13, 256–264. [Google Scholar] [CrossRef]
- Hultine, K.R.; Scott, R.L.; Cable, W.L.; Goodrich, D.C.; Williams, D.G. Hydraulic redistribution by a dominant, warm-desert phreatophyte: Seasonal patterns and response to precipitation pulses. Funct. Ecol. 2004, 18, 530–538. [Google Scholar] [CrossRef]
- Burgess, S.S.O. Can hydraulic redistribution put bread on our table? Plant. Soil 2011, 341, 25–29. [Google Scholar] [CrossRef]
- Sekiya, N.; Araki, H.; Yano, K. Applying hydraulic lift in an agroecosystem: Forage plants with shoots removed supply water to neighboring vegetable crops. Plant. Soil 2011, 341, 39–50. [Google Scholar] [CrossRef]
- Phiri, E.; Verplancke, H.; Kwesiga, F.; Mafongoya, P. Water balance and maize yield following improved sesbania fallow in eastern Zambia. Agrofor. Syst. 2003, 59, 197–205. [Google Scholar] [CrossRef]
- Chirwa, P.W.; Ong, C.K.; Maghembe, J.; Black, C.R. Soil water dynamics in cropping systems containing Gliricidia sepium, pigeonpea and maize in southern Malawi. Agrofor. Syst. 2007, 69, 29–43. [Google Scholar] [CrossRef]
- DeBruyne, S.A.; Feldhake, C.M.; Burger, J.A.; Fike, J.H. Tree effects on forage growth and soil water in an Appalachian silvopasture. Agrofor. Syst. 2011, 83, 189–200. [Google Scholar] [CrossRef]
- Everson, C.S.; Dye, P.J.; Gush, M.B.; Everson, T.M. Water use of grasslands, agroforestry systems and indigenous forests. WSA 2011, 37, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Sileshi, G.W.; Mafongoya, P.L.; Akinnifesi, F.K.; Phiri, E.; Chirwa, P.; Beedy, T.; Makumba, W.; Nyamadzawo, G.; Njoloma, J.; Wuta, M.; et al. Agroforestry: Fertilizer trees. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 222–234. ISBN 9780080931395. [Google Scholar]
- Kuyah, S.; Whitney, C.W.; Jonsson, M.; Sileshi, G.W.; Öborn, I.; Muthuri, C.W.; Luedeling, E. Agroforestry delivers a win-win solution for ecosystem services in sub-Saharan Africa. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 47. [Google Scholar] [CrossRef] [Green Version]
- Pavlidis, G.; Tsihrintzis, V.A. Environmental Benefits and Control of Pollution to Surface Water and Groundwater by Agroforestry Systems: A Review. Water Resour. Manag. 2018, 32, 1–29. [Google Scholar] [CrossRef]
- Verchot, L.V.; van Noordwijk, M.; Kandji, S.; Tomich, T.; Ong, C.; Albrecht, A.; Mackensen, J.; Bantilan, C.; Anupama, K.V.; Palm, C. Climate change: Linking adaptation and mitigation through agroforestry. Mitig. Adapt. Strat Glob. Chang. 2007, 12, 901–918. [Google Scholar] [CrossRef]
- Ribeiro-Barros, A.I.; Silva, M.J.; Moura, I.; Ramalho, J.C.; Máguas-Hanson, C.; Ribeiro, N.S. The potential of tree and shrub legumes in agroforestry systems. In Nitrogen in Agriculture—Updates; Amanullah, K., Fahad, S., Eds.; InTech: Rijeka, Croatia, 2018; ISBN 978-953-51-3768-9. [Google Scholar]
- Sierra, J.; Dulormne, M.; Desfontaines, L. Soil nitrogen as affected by Gliricidia sepium in a silvopastoral system in Guadeloupe, French Antilles. Agrofor. Syst. 2002, 54, 87–97. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Mutuo, P.K.; Cadisch, G.; Albrecht, A.; Palm, C.A.; Verchot, L. Potential of agroforestry for carbon sequestration and mitigation of greenhouse gas emissions from soils in the tropics. Nutr. Cycl. Agroecosyst. 2005, 71, 43–54. [Google Scholar] [CrossRef]
- Coulibaly, J.Y.; Chiputwa, B.; Nakelse, T.; Kundhlande, G. Adoption of agroforestry and the impact on household food security among farmers in Malawi. Agric. Syst. 2017, 155, 52–69. [Google Scholar] [CrossRef]
- De Schutter, O. Agroecology, a tool for the realization of the right to food. In Agroecology and Strategies for Climate Change; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–16. ISBN 978-94-007-1904-0. [Google Scholar]
- Akinnifesi, F.K.; Makumba, W.; Kwesiga, F.R. Sustainable Maize Production Using Gliricidia/Maize Intercropping in Southern Malawi. Ex. Agric. 2006, 42, 441–457. [Google Scholar] [CrossRef] [Green Version]
- Beedy, T.L.; Snapp, S.S.; Akinnifesi, F.K.; Sileshi, G.W. Impact of Gliricidia sepium intercropping on soil organic matter fractions in a maize-based cropping system. Agric. Ecosyst. Environ. 2010, 138, 139–146. [Google Scholar] [CrossRef]
- Martinsen, V.; Shitumbanuma, V.; Mulder, J.; Ritz, C.; Cornelissen, G. Effects of hand-hoe tilled conservation farming on soil quality and carbon stocks under on-farm conditions in Zambia. Agric. Ecosyst. Environ. 2017, 241, 168–178. [Google Scholar] [CrossRef]
- Tamene, L.; Le, Q.B. Estimating soil erosion in sub-Saharan Africa based on landscape similarity mapping and using the revised universal soil loss equation (RUSLE). Nutr. Cycl. Agroecosyst. 2015, 102, 17–31. [Google Scholar] [CrossRef]
- Montanarella, L.; Pennock, D.J.; McKenzie, N.; Badraoui, M.; Chude, V.; Baptista, I.; Mamo, T.; Yemefack, M.; Singh Aulakh, M.; Yagi, K.; et al. World’s soils are under threat. SOIL 2016, 2, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Labrière, N.; Locatelli, B.; Laumonier, Y.; Freycon, V.; Bernoux, M. Soil erosion in the humid tropics: A systematic quantitative review. Agric. Ecosyst. Environ. 2015, 203, 127–139. [Google Scholar] [CrossRef]
- Sanchis, M.P.S.; Torri, D.; Borselli, L.; Poesen, J. Climate effects on soil erodibility. Earth Surf. Process. Landf. 2008, 33, 1082–1097. [Google Scholar] [CrossRef]
- Hombegowda, H.C.; van Straaten, O.; Köhler, M.; Hölscher, D. On the rebound: Soil organic carbon stocks can bounce back to near forest levels when agroforests replace agriculture in southern India. SOIL 2016, 2, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Korschens, M.; Schulz, E. Long-term management impacts on soil C, N and physical fertility. Soil Tillage Res. 2006, 91, 39–47. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization Mechanisms of Soil Organic Matter: Implications for C-Saturation of Soils. Plant. Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Haynes, R.J.; Naidu, R. Influence of Lime, Fertilizer and Manure Applications on Soil Organic Matter Content and Soil Physical Conditions: A Review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Schrumpf, M.; Kaiser, K.; Guggenberger, G.; Persson, T.; Kögel-Knabner, I.; Schulze, E.-D. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 2013, 10, 1675–1691. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, S.A.; Bucka, F.B.; Graf-Rosenfellner, M.; Kögel-Knabner, I. Soil microaggregate size composition and organic matter distribution as affected by clay content. Geoderma 2019, 355, 113901. [Google Scholar] [CrossRef]
- Vågen, T.-G.; Lal, R.; Singh, B.R. Soil carbon sequestration in sub-Saharan Africa: A review. Land Degrad. Dev. 2005, 16, 53–71. [Google Scholar] [CrossRef]
- Nair, P.K.R. The coming of age of agroforestry. J. Sci. Food Agric. 2007, 87, 1613–1619. [Google Scholar] [CrossRef]
- Zheng, X. Mechanics of Wind-blown Sand Movements; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-88253-4. [Google Scholar]
- Cheng, H.; Liu, C.; Li, J.; Zou, X.; Liu, B.; Kang, L.; Fang, Y. Wind erosion mass variability with sand bed in a wind tunnel. Soil Tillage Res. 2017, 165, 181–189. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; He, C.; Ding, G. Linking wind erosion to ecosystem services in drylands: A landscape ecological approach. Landsc. Ecol 2017, 32, 2399–2417. [Google Scholar] [CrossRef]
- Wiggs, G.; Holmes, P. Dynamic controls on wind erosion and dust generation on west-central Free State agricultural land, South Africa. Earth Surf. Process. Landf. 2011, 36, 827–838. [Google Scholar] [CrossRef]
- Tully, K.; Sullivan, C.; Weil, R.; Sanchez, P. The State of Soil Degradation in Sub-Saharan Africa: Baselines, Trajectories, and Solutions. Sustainability 2015, 7, 6523–6552. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Nickling, W.; Bergametti, G.; Butler, H.; Chappell, A.; Findlater, P.; Gillies, J.; Ishizuka, M.; Klose, M.; Kok, J.F.; et al. A tribute to Michael R. Raupach for contributions to aeolian fluid dynamics. Aeolian Res. 2015, 19, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Chappell, A.; Webb, N.P. Using albedo to reform wind erosion modelling, mapping and monitoring. Aeolian Res. 2016, 23, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Sterk, G.; Herrmann, L.; Bationo, A. Wind-blown nutrient transport and soil productivity changes in southwest Niger. Land Degrad. Dev. 1996, 7, 325–335. [Google Scholar] [CrossRef]
- Hoffmann, C.; Funk, R.; Li, Y.; Sommer, M. Effect of grazing on wind driven carbon and nitrogen ratios in the grasslands of Inner Mongolia. CATENA 2008, 75, 182–190. [Google Scholar] [CrossRef]
- Ravi, S.; D’Odorico, P.; Breshears, D.D.; Field, J.P.; Goudie, A.S.; Huxman, T.E.; Li, J.; Okin, G.S.; Swap, R.J.; Thomas, A.D.; et al. Aeolian processes and the biosphere. Rev. Geophys. 2011, 49, RG3001. [Google Scholar] [CrossRef] [Green Version]
- Nerger, R.; Funk, R.; Cordsen, E.; Fohrer, N. Application of a modeling approach to designate soil and soil organic carbon loss to wind erosion on long-term monitoring sites (BDF) in Northern Germany. Aeolian Res. 2017, 25, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Wang, S.; Wang, C.; Zhang, G.; Patel, N. Losses of soil organic carbon under wind erosion in China. Glob. Chang. Biol. 2005, 11, 828–840. [Google Scholar] [CrossRef]
- Mercer, G.N. Modelling to determine the optimal porosity of shelterbelts for the capture of agricultural spray drift. Environ. Model. Softw. 2009, 24, 1349–1352. [Google Scholar] [CrossRef]
- Brenner, A.J.; Jarvis, P.G.; van den Beldt, R.J. Windbreak-crop interactions in the Sahel. 1. Dependence of shelter on field conditions. Agric. For. Meteorol. 1995, 75, 215–234. [Google Scholar] [CrossRef]
- Borrelli, J.; Gregory, J.M.; Abtew, W. Wind Barriers: A Reevaluation of Height, Spacing, and Porosity. Trans. ASAE 1989, 32, 2023–2027. [Google Scholar] [CrossRef]
- Schneiders, A.; van Daele, T.; van Landuyt, W.; van Reeth, W. Biodiversity and ecosystem services: Complementary approaches for ecosystem management? Ecol. Indic. 2012, 21, 123–133. [Google Scholar] [CrossRef]
- Folke, C.; Holling, C.S.; Perrings, C. Biological Diversity, Ecosystems, and the Human Scale. Ecol. Appl. 1996, 6, 1018–1024. [Google Scholar] [CrossRef] [Green Version]
- De Cauwer, V.; Knox, N.; Kobue-Lekalake, R.; Lepetu, J.P.; Ompelege, M.; Naidoo, S.; Nott, A.; Parduhn, D.; Sichone, P.; Tshwenyane, S.; et al. Woodland resources and management in southern Africa. In Climate Change and Adaptive Land Management in Southern Africa: Assessments, Changes, Challenges, and Solutions: Product of the First Research Portfolio of SASSCAL 2012-2018; Revermann, R., Krewenka, K.M., Schmiedel, U., Olwoch, J.M., Helmschrot, J., Jürgens, N., Eds.; Klaus Hess Publishers, University of Hamburg: Göttingen, Germany; Windhoek, Namibia; Hamburg, Germany, 2018; pp. 296–308. ISBN 9789991657431. [Google Scholar]
- Chirwa, P.W.; Syampungani, S.; Geldenhuys, C.J. Managing southern African woodlands for biomass production: The potential challenges and opportunities. In Bioenergy from Wood: Sustainable Production in the Tropics; Seifert, T., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 67–87. ISBN 978-94-007-7448-3. [Google Scholar]
- McNicol, I.M.; Ryan, C.M.; Williams, M. How resilient are African woodlands to disturbance from shifting cultivation? Ecol. Appl. 2015, 25, 2320–2336. [Google Scholar] [CrossRef] [PubMed]
- Syampungani, S.; Geldenhuys, C.J.; Chirwa, P.W. Regeneration dynamics of miombo woodland in response to different anthropogenic disturbances: Forest characterisation for sustainable management. Agrofor. Syst. 2016, 90, 563–576. [Google Scholar] [CrossRef]
- Bombelli, A.; Henry, M.; Castaldi, S.; Adu-Bredu, S.; Arneth, A.; de Grandcourt, A.; Grieco, E.; Kutsch, W.L.; Lehsten, V.; Rasile, A.; et al. The Sub-Saharan Africa carbon balance, an overview. Biogeosciences Discuss. 2009, 6, 2085–2123. [Google Scholar] [CrossRef]
- FAO. Trees, Forests and Land Use in Drylands: The First Global Assessment—Full Report; FAO Forestry Paper No. 184; FAO: Rome, Italy, 2019. [Google Scholar]
- Sileshi, G.; Mafongoya, P.L. Variation in macrofaunal communities under contrasting land use systems in eastern Zambia. Appl. Soil Ecol. 2006, 33, 49–60. [Google Scholar] [CrossRef]
- Magoba, R.N.; Samways, M.J. Comparative footprint of alien, agricultural and restored vegetation on surface-active arthropods. Biol. Invasions 2012, 14, 165–177. [Google Scholar] [CrossRef]
- Samways, M.J.; Pryke, J.S. Large-scale ecological networks do work in an ecologically complex biodiversity hotspot. Ambio 2016, 45, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pryke, J.S.; Samways, M.J. Conserving natural heterogeneity is crucial for designing effective ecological networks. Landsc. Ecol. 2015, 30, 595–607. [Google Scholar] [CrossRef]
- Potgieter, L.J.; Richardson, D.M.; Wilson, J.R.U. Casuarina cunninghamiana in the Western Cape, South Africa: Determinants of naturalisation and invasion, and options for management. S. Afr. J. Bot. 2014, 92, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Van Wilgen, B.W.; Measey, J.; Richardson, D.M.; Wilson, J.R.; Zengeya, T.A. Biological Invasions in South Africa; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-32393-6. [Google Scholar]
- Wilson, J.; Tanner, M. Estimation of the impact of alien trees in the Cape Town water crisis using satellite data. In Space Fostering African Societies: Developing the African Continent through Space, Part 1; Froehlich, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–11. ISBN 978-3-030-32930-3. [Google Scholar]
- Gush, M.B.; Dye, P.J. Water-use efficiency within a selection of indigenous and exotic tree species in South Africa as determined using sap flow and biomass measurements. Acta Hortic. 2009, 846, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Allsopp, M.H.; Cherry, M. An Assessment of the Impact on the Bee and Agricultural Industries in the Western Cape of the Clearing of Certain Eucalyptus Species Using Questionnaire Survey Data; Government of the Republic of South Africa, Department of Water Affairs: Pretoria, South Africa, 2004. [Google Scholar]
- Allsopp, M.H.; de Lange, W.J.; Veldtman, R. Valuing insect pollination services with cost of replacement. PLoS ONE 2008, 3, e3128. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- De Lange, W.J.; Veldtman, R.; Allsopp, M.H. Valuation of pollinator forage services provided by Eucalyptus cladocalyx. J. Environ. Manag. 2013, 125, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Glèlè Kakaï, R.; Seifert, T. Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 2016, 6, 7546–7557. [Google Scholar] [CrossRef] [PubMed]
- Mensah, S.; Veldtman, R.; Du Toit, B.; Glèlè Kakaï, R.; Seifert, T. Aboveground Biomass and Carbon in a South African Mistbelt Forest and the Relationships with Tree Species Diversity and Forest Structures. Forests 2016, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Ham, C.; Glèlè Kakaï, R.; Seifert, T. Ecosystem service importance and use vary with socio-environmental factors: A study from household-surveys in local communities of South Africa. Ecosyst. Serv. 2017, 23, 1–8. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Seifert, T. Potential supply of floral resources to managed honey bees in natural mistbelt forests. J. Environ. Manag. 2017, 189, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Mensah, S.; Salako, K.V.; Assogbadjo, A.; Glèlè Kakaï, R.; Sinsin, B.; Seifert, T. Functional trait diversity is a stronger predictor of multifunctionality than dominance: Evidence from an Afromontane forest in South Africa. Ecol. Indic. 2020, 115, 106415. [Google Scholar] [CrossRef]
- Pumariño, L.; Sileshi, G.W.; Gripenberg, S.; Kaartinen, R.; Barrios, E.; Muchane, M.N.; Midega, C.; Jonsson, M. Effects of agroforestry on pest, disease and weed control: A meta-analysis. Basic Appl. Ecol. 2015, 16, 573–582. [Google Scholar] [CrossRef]
- Sow, A.; Seye, D.; Faye, E.; Benoit, L.; Galan, M.; Haran, J.; Brévault, T. Birds and bats contribute to natural regulation of the millet head miner in tree-crop agroforestry systems. Crop. Prot. 2020, 132, 105127. [Google Scholar] [CrossRef]
- Guenat, S.; Kaartinen, R.; Jonsson, M. Shade trees decrease pest abundances on brassica crops in Kenya. Agrofor. Syst. 2019, 93, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.A. Mega-fires, tipping points and ecosystem services: Managing forests and woodlands in an uncertain future. For. Ecol. Manag. 2013, 294, 250–261. [Google Scholar] [CrossRef]
- Du Toit, B.; Malherbe, G.F. A synopsis of silvicultural and management considerations for dryland forestry. South. For. J. For. Sci. 2017, 79, iii–vi. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Nair, V.D.; Kumar, B.M.; Showalter, J.M. Carbon sequestration in agroforestry systems. Adv. Agron. 2010, 108, 237–307. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, H.Y.H.; Bork, E.W.; Carlyle, C.N.; Chang, S.X.; Fortin, J. Carbon accumulation in agroforestry systems is affected by tree species diversity, age and regional climate: A global meta-analysis. Glob. Ecol. Biogeogr. 2020, 99, 1–12. [Google Scholar] [CrossRef]
- Du Toit, B.; Malherbe, G.F.; Kunneke, A.; Seifert, T.; Wessels, C.B. Survival and long-term growth of eucalypts on semi-arid sites in a Mediterranean climate, South Africa. South. For. J. For. Sci. 2017, 79, 235–249. [Google Scholar] [CrossRef]
- Wessels, C.B.; Crafford, P.L.; Du Toit, B.; Grahn, T.; Johansson, M.; Lundqvist, S.O.; Säll, H.; Seifert, T. Variation in physical and mechanical properties from three drought tolerant Eucalyptus species grown on the dry west coast of Southern Africa. Eur. J. Wood Wood Prod. 2016, 74, 563–575. [Google Scholar] [CrossRef]
- Lundqvist, S.-O.; Grahn, T.; Olsson, L.; Seifert, T. Comparison of wood, fibre and vessel properties of drought-tolerant eucalypts in South Africa. South. For. J. For. Sci. 2017, 79, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Phiri, D.; Ackerman, P.; Wessels, B.; Du Toit, B.; Johansson, M.; Säll, H.; Lundqvist, S.-O.; Seifert, T. Biomass equations for selected drought-tolerant eucalypts in South Africa. South. For. J. For. Sci. 2015, 77, 255–262. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). State of the World’s Forests 2011; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-106750-5. [Google Scholar]
- Jindal, R.; Kerr, J.M.; Carter, S. Reducing Poverty Through Carbon Forestry? Impacts of the N’hambita Community Carbon Project in Mozambique. World Dev. 2012, 40, 2123–2135. [Google Scholar] [CrossRef]
- Brouwer, R.; Falcão, M.P. Wood fuel consumption in Maputo, Mozambique. Biomass Bioenergy 2004, 27, 233–245. [Google Scholar] [CrossRef]
- Iiyama, M.; Neufeldt, H.; Dobie, P.; Njenga, M.; Ndegwa, G.; Jamnadass, R. The potential of agroforestry in the provision of sustainable woodfuel in sub-Saharan Africa. Curr. Opin. Environ. Sustain. 2014, 6, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Okello, B.D.; O’Connor, T.G.; Young, T.P. Growth, biomass estimates, and charcoal production of Acacia drepanolobium in Laikipia, Kenya. For. Ecol. Manag. 2001, 142, 143–153. [Google Scholar] [CrossRef]
- Lynd, L.R.; Sow, M.; Chimphango, A.F.; Cortez, L.A.; Brito Cruz, C.H.; Elmissiry, M.; Laser, M.; Mayaki, I.A.; Moraes, M.A.; Nogueira, L.A.; et al. Bioenergy and African transformation. Biotechnol. Biofuels 2015, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handavu, F.; Chirwa, P.W.; Syampungani, S.; Mahamane, L. A review of carbon dynamics and assessment methods in the miombo woodlands. South. For. J. For. Sci. 2017, 79, 95–102. [Google Scholar] [CrossRef]
- Kalaba, F.K.; Quinn, C.H.; Dougill, A.J.; Vinya, R. Floristic composition, species diversity and carbon storage in charcoal and agriculture fallows and management implications in Miombo woodlands of Zambia. For. Ecol. Manag. 2013, 304, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Kachamba, D.; Eid, T.; Gobakken, T. Above- and Belowground Biomass Models for Trees in the Miombo Woodlands of Malawi. Forests 2016, 7, 38. [Google Scholar] [CrossRef] [Green Version]
- Mensah, S.; Veldtman, R.; Seifert, T. Allometric models for height and aboveground biomass of dominant tree species in South African Mistbelt forests. South. For. J. For. Sci. 2017, 79, 19–30. [Google Scholar] [CrossRef]
- Guedes, B.S.; Sitoe, A.A.; Olsson, B.A. Allometric models for managing lowland miombo woodlands of the Beira corridor in Mozambique. Glob. Ecol. Conserv. 2018, 13, e00374. [Google Scholar] [CrossRef]
- Henry, M.; Picard, N.; Trotta, C.; Manlay, R.; Valentini, R.; Bernoux, M.; Saint-André, L. Estimating tree biomass of sub-Saharan African forests: A review of available allometric equations. Silva. Fenn. 2011, 45, 477–569. [Google Scholar] [CrossRef] [Green Version]
- Raumonen, P.; Kaasalainen, M.; Åkerblom, M.; Kaasalainen, S.; Kaartinen, H.; Vastaranta, M.; Holopainen, M.; Disney, M.; Lewis, P. Fast automatic precision tree models from terrestrial laser scanner data. Remote Sens. 2013, 5, 491–520. [Google Scholar] [CrossRef] [Green Version]
- Hackenberg, J.; Morhart, C.; Sheppard, J.P.; Spiecker, H.; Disney, M. Highly Accurate Tree Models Derived from Terrestrial Laser Scan Data: A Method Description. Forests 2014, 5, 1069–1105. [Google Scholar] [CrossRef] [Green Version]
- Disney, M.I.; Boni Vicari, M.; Burt, A.; Calders, K.; Lewis, S.L.; Raumonen, P.; Wilkes, P. Weighing trees with lasers: Advances, challenges and opportunities. Interface Focus 2018, 8, 20170048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, A.; Calders, K.; Bartholomeus, H.; Martius, C.; Raumonen, P.; Herold, M.; Vicari, M.; Sukhdeo, H.; Singh, J.; Goodman, R. Tree Biomass Equations from Terrestrial LiDAR: A Case Study in Guyana. Forests 2019, 10, 527. [Google Scholar] [CrossRef] [Green Version]
- Seidel, D.; Ehbrecht, M.; Dorji, Y.; Jambay, J.; Ammer, C.; Annighöfer, P. Identifying architectural characteristics that determine tree structural complexity. Trees 2019, 33, 911–919. [Google Scholar] [CrossRef]
- Sheppard, J.P.; Morhart, C.; Hackenberg, J.; Spiecker, H. Terrestrial laser scanning as a tool for assessing tree growth. iForest 2017, 10, 172–179. [Google Scholar] [CrossRef]
- Bohn Reckziegel, R.; Kunneke, A.; Christopher, M.; Sheppard, J.P.; Kahle, H.-P. Assessing aboveground carbon sequestration potential of trees in agroforestry systems in Southern Africa using 3D data. In XXV IUFRO World Congress: Forest Research and Cooperation for Sustainable, Proceedings of the XXV IUFRO World Congress: Forest Research and Cooperation for Sustainable, Curitiba, Brazil, 29 September–5 October 2019; de Mattos, P.P., Ed.; Pesquisa Florestal Brasileira: Colombo, Brazil, 2019; p. 605. [Google Scholar]
- Oelkers, E.H.; Cole, D.R. Carbon Dioxide Sequestration A Solution to a Global Problem. Elements 2008, 4, 305–310. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Nair, V.D.; Kumar, B.M.; Haile, S.G. Soil carbon sequestration in tropical agroforestry systems: A feasibility appraisal. Environ. Sci. Policy 2009, 12, 1099–1111. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Mohan Kumar, B.; Nair, V.D. Agroforestry as a strategy for carbon sequestration. J. Plant. Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
- Verchot, L.V.; Dutaur, L.; Shepherd, K.D.; Albrecht, A. Organic matter stabilization in soil aggregates: Understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma 2011, 161, 182–193. [Google Scholar] [CrossRef]
- Dixon, R.K. Agroforestry systems: Sources of sinks of greenhouse gases? Agrofor. Syst. 1995, 31, 99–116. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Nair, V.D. ‘Solid–fluid–gas’: The state of knowledge on carbon-sequestration potential of agroforestry systems in Africa. Curr. Opin. Environ. Sustain. 2014, 6, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- John, B.; Yamashita, T.; Ludwig, B.; Flessa, H. Storage of organic carbon in aggregate and density fractions of silty soils under different types of land use. Geoderma 2005, 128, 63–79. [Google Scholar] [CrossRef]
- Gaiser, T.; Stahr, K.; Bernard, M.; Kang, B.T. Changes in soil organic carbon fractions in a tropical Acrisol as influenced by the addition of different residue materials. Agrofor. Syst. 2012, 86, 185–195. [Google Scholar] [CrossRef]
- Smith, P. How long before a change in soil organic carbon can be detected? Glob. Chang. Biol. 2004, 10, 1878–1883. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Lal, R.; Follett, R.F.; Stewart, B.A.; Kimble, J.M. Soil Carbon Sequestration to Mitigate Climate Change and Advance Food Security. Soil Sci. 2007, 172, 943–956. [Google Scholar] [CrossRef]
- Jensen, J.L.; Schjønning, P.; Watts, C.W.; Christensen, B.T.; Peltre, C.; Munkholm, L.J. Relating soil C and organic matter fractions to soil structural stability. Geoderma 2019, 337, 834–843. [Google Scholar] [CrossRef]
- Kassam, A.; Kueneman, E.; Lott, R.; Friedrich, T.; Lutaladio, N.; Norman, D.; Bwalya, M.; Poisot, A.-S.; Mkomwa, S. The cereal-root crop mixed farming system: A potential bread basket transitioning to sustainable intensification. In Farming Systems and Food Security in Africa: Priorities for Science and Policy under Global Change; Dixon, J., Garrity, D.P., Boffa, J.-M., Williams, T.O., Amede, T., Auricht, C., Lott, R., Mburathi, G.K., Eds.; Routledge: New York, NY, USA, 2020; pp. 214–247. ISBN 978-1-138-96335-1. [Google Scholar]
- Sheppard, J.P.; Chamberlain, J.; Agúndez, D.; Bhattacharya, P.; Chirwa, P.W.; Gontcharov, A.; Sagona, W.C.J.; Shen, H.-l.; Tadesse, W.; Mutke, S. Sustainable Forest Management Beyond the Timber-Oriented Status Quo: Transitioning to Co-production of Timber and Non-wood Forest Products—A Global Perspective. Curr. For. Rep. 2020, 5, 26–46. [Google Scholar] [CrossRef] [Green Version]
- Chirwa, P.W.; Akinnifesi, F.K.; Sileshi, G.; Syampungani, S.; Kalaba, F.K.; Ajayi, O.C. Opportunity for conserving and utilizing agrobiodiversity through agroforestry in Southern Africa. Biodiversity 2008, 9, 45–48. [Google Scholar] [CrossRef]
- Jamnadass, R.H.; Dawson, I.K.; Franzel, S.; Leakey, R.R.B.; Mithöfer, D.; Akinnifesi, F.K.; Tchoundjeu, Z. Improving livelihoods and nutrition in sub-Saharan Africa through the promotion of indigenous and exotic fruit production in smallholders’ agroforestry systems: A review. Int. For. Rev. 2011, 13, 338–354. [Google Scholar] [CrossRef]
- Rasolofoson, R.A.; Hanauer, M.M.; Pappinen, A.; Fisher, B.; Ricketts, T.H. Impacts of forests on children’s diet in rural areas across 27 developing countries. Sci. Adv. 2018, 4, eaat2853. [Google Scholar] [CrossRef] [Green Version]
- Akinnifesi, F.K.; Sileshi, G.; Ajayi, O.C.; Chirwa, P.W.; Mng’omba, S.; Chakeredza, S.; Nyoka, B.I. Domestication and conservation of indigenous Miombo fruit trees for improving rural livelihoods in southern Africa. Biodiversity 2008, 9, 72–74. [Google Scholar] [CrossRef]
- Pretty, J.; Toulmin, C.; Williams, S. Sustainable intensification in African agriculture. Int. J. Agric. Sustain. 2011, 9, 5–24. [Google Scholar] [CrossRef]
- Place, F.; Dewees, P. Policies and incentives for the adoption of improved fallows. Agrofor. Syst. 1999, 47, 323–343. [Google Scholar] [CrossRef]
- Mercer, D.E.; Miller, R.P. Socioeconomic research in agroforestry: Progress, prospects, priorities. Agrofor. Syst. 1997, 38, 177–193. [Google Scholar] [CrossRef]
- Fones-Sundell, M.; Teklehaimanot, Z. Mobilizing Agroforestry Capacity for Development. Final Evaluation of The African Network for Agriculture, Agroforestry and Natural Resources Education (ANAFE) and Zambian Agroforestry Project (ZAP); Swedish International Development Cooperation Agency: Stockholm, Sweden, 2007; ISBN 91-586-8209-0. [Google Scholar]
- Ndlovu, N.P.; Borrass, L. Promises and potentials do not grow trees and crops: A review of institutional and policy research in agroforestry for the Southern African region. 2020; unpublished manuscript. [Google Scholar]
- Garrity, D.P. Agroforestry and the achievement of the Millennium Development Goals. Agrofor. Syst. 2004, 61–62, 5–17. [Google Scholar]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977; ISBN 9780123258502. [Google Scholar]
- Bauhus, J.; Forrester, D.I.; Pretzsch, H. From observations to evidence about effects of mixed-species stands. In Mixed-Species Forests: Ecology and Management; Pretzsch, H., Forrester, D.I., Bauhus, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 27–71. ISBN 978-3-662-54553-9. [Google Scholar]
- Mead, R.; Willey, R.W. The Concept of a ‘Land Equivalent Ratio’ and Advantages in Yields from Intercropping. Exp. Agric. 1980, 16, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Van Ittersum, M.K.; Rabbinge, R. Concepts in production ecology for analysis and quantification of agricultural input-output combinations. Field Crop. Res. 1997, 52, 197–208. [Google Scholar] [CrossRef]
- Dickinson, A.K. Analog forestry: Creating productive landscapes. In Towards Productive Landscapes; Chavez-Tafur, J., Zagt, R.J., Eds.; Tropenbos International: Wageningen, The Netherlands, 2014; pp. 103–110. ISBN 978-90-5113-124-6. [Google Scholar]
- Dixon, J.; Boffa, J.-M.; Williams, T.O.; de Leeuw, J.; Fischer, G.; van Velthuizen, H. Farming and food systems potentials. In Farming Systems and Food Security in Africa: Priorities for Science and Policy under Global Change; Dixon, J., Garrity, D.P., Boffa, J.-M., Williams, T.O., Amede, T., Auricht, C., Lott, R., Mburathi, G.K., Eds.; Routledge: New York, NY, USA, 2020; pp. 535–561. ISBN 978-1-138-96335-1. [Google Scholar]
- Walker, D.H.; Sinclair, F.L.; Thapa, B. Incorporation of indigenous knowledge and perspectives in agroforestry development. Agrofor. Syst. 1995, 30, 235–248. [Google Scholar] [CrossRef]
- Nkomoki, W.; Bavorová, M.; Banout, J. Adoption of sustainable agricultural practices and food security threats: Effects of land tenure in Zambia. Land Use Policy 2018, 78, 532–538. [Google Scholar] [CrossRef]
- Dixon, J.; Garrity, D.; Mburathi, G.K.; Boffa, J.-M.; Amede, T.; Williams, T.O. Ways forward: Strategies for effective science, investments and policies for African farming and food systems. In Farming Systems and Food Security in Africa: Priorities for Science and Policy under Global Change; Dixon, J., Garrity, D.P., Boffa, J.-M., Williams, T.O., Amede, T., Auricht, C., Lott, R., Mburathi, G.K., Eds.; Routledge: New York, NY, USA, 2020; pp. 562–588. ISBN 978-1-138-96335-1. [Google Scholar]
- Jackson, T.; Shenkin, A.; Wellpott, A.; Calders, K.; Origo, N.; Disney, M.; Burt, A.; Raumonen, P.; Gardiner, B.; Herold, M.; et al. Finite element analysis of trees in the wind based on terrestrial laser scanning data. Agric. For. Meteorol. 2019, 265, 137–144. [Google Scholar] [CrossRef]




| Knowledge Gaps Relevant for AFS in Southern Africa | Possible Solutions |
|---|---|
| Ecosystem Services, Provisioning, Carbon Accounting and Integrated Fire Management | |
| Interplay between biodiversity types and land-use strategy | Examination with appropriate methodology, e.g., ecosystem-based management approach. |
| Threats presented by non-native trees | Ecology and distribution of non-native trees. Case studies. |
| AFS influence on pest populations | Observational and experimental exploration of bottom-up and top-down influence of AFS on pest populations, relationships between tree cover, food webs and pest suppression. |
| Crop-pest modelling tools do not account for AFS’ complexity | Data collection on both focused AFS and broad indications for common applications. |
| Benefits of AFS produced NWFP vs. wild harvesting | Case study and experimental explorations of impacts of sustainable wild harvesting and AFS substitution. |
| Extent of carbon sequestration potential in AFS | Landscape level estimations of AFS utilisation, species mixture and applied management, coupled with biomass studies and modelling activities. |
| Allometric functions relevant for trees utilised in AFS | Sampling and development of allometric functions for appropriate species. |
| Minimal data available for carbon storage potential dependent on land management and site conditions | Plot and landscape level modelling, use of LiDAR technology. Empirical studies. Biomass modelling. Long-term observations of carbon dynamics. |
| Low levels of information on fuels and fire behaviour in AFS | Dedicated studies on fuel types and fuel loading and its dynamics over time in AFS. |
| Tree-Crop-Site Interactions | |
| Shelterbelt/tree spatial arrangements within AFS, crop selection and combinations | Measurement of existing systems, modelling and extrapolation (e.g., wind loading of trees; cf. [227]). |
| Competition vs. facilitation (e.g., light and water) | Modelling utilising empirical shading measurements and tree parameters in relation to ecophysiological shade tolerance (i.e., tree-crop water use, 3D-based approach; [67]). |
| Shading effects and benefits provided by AFS | Observational and experimental research into combinations and suitability of crops for usage in AFS. |
| Effects of nitrogen-fixing species | Experimental methodology, ecophysiological measurements. Cultivation of different tree species under different climatic and site conditions [3]. |
| Low data availability on water retention studies and plant-plant soil water flow | Detailed throughflow measurements in combination with plant-plant water transfer (e.g., [85]) with soil/structure type referencing. |
| Evaluation of species combination, soils and management practices for different regions | Disentangling the various influences (case studies in different systems with clear designs regarding species combination, soils and management practices). |
| Low data availability on erosion control and soil conservation in AFS | Experimental quantification of erosion and deposition in relation to land use, soil types, terrain and climate. Research on aggregate formation [53] to assess site-specific erosion risk. |
| Political, Social and Economic Issues | |
| Lack of institutional home, mis-aligned policy agendas | Closer cooperation and communication between government, research and NGOs. |
| Impact of AFS on rural livelihoods | Further scrutiny of impact of AFS on the improvement of food security, employment and resilience issues |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheppard, J.P.; Bohn Reckziegel, R.; Borrass, L.; Chirwa, P.W.; Cuaranhua, C.J.; Hassler, S.K.; Hoffmeister, S.; Kestel, F.; Maier, R.; Mälicke, M.; et al. Agroforestry: An Appropriate and Sustainable Response to a Changing Climate in Southern Africa? Sustainability 2020, 12, 6796. https://doi.org/10.3390/su12176796
Sheppard JP, Bohn Reckziegel R, Borrass L, Chirwa PW, Cuaranhua CJ, Hassler SK, Hoffmeister S, Kestel F, Maier R, Mälicke M, et al. Agroforestry: An Appropriate and Sustainable Response to a Changing Climate in Southern Africa? Sustainability. 2020; 12(17):6796. https://doi.org/10.3390/su12176796
Chicago/Turabian StyleSheppard, Jonathan P., Rafael Bohn Reckziegel, Lars Borrass, Paxie W. Chirwa, Claudio J. Cuaranhua, Sibylle K Hassler, Svenja Hoffmeister, Florian Kestel, Rebekka Maier, Mirko Mälicke, and et al. 2020. "Agroforestry: An Appropriate and Sustainable Response to a Changing Climate in Southern Africa?" Sustainability 12, no. 17: 6796. https://doi.org/10.3390/su12176796