3.1. Evolution of Water Composition in Column Tests
The evolution of pH, calcium, and TIC is shown in
Figure 2. A progressive exhaustion of both columns was observed, as indicated by a pH drop in each cell. At the end of the experiment, complete exhaustion was reached only in cells 1 to 3 of column 1 and cell 1 of column 2, which had the same pH as the influent. The effluent pH slowly decreased from approximately 11.6 in both columns to approximately 10.5 in column 1 and 10.8 in column 2. Calcium trends can be explained by a combination of slag dissolution and precipitation of hydroxyapatite and calcite. A high effluent calcium concentration was observed at the beginning of operation due to the high slag initial reactivity. Cell 4 of column 2 had an irregular behavior compared to other cells. Its pH was between 11.0 and 11.5 and its calcium concentration was above 75 mg/L, which were higher than values observed in cells 5 to 9. Such behavior was attributed to a possible dead zone formed around the sampling valve, leading to an artificially longer contact time in this region. A negligible COD removal efficiency of 1% was observed in column 2. Such negligible COD removal can be explained by the high pH and the absence of nutrients in the influent, resulting in a low biological activity.
o-PO
4 curves are not shown as they were already discussed previously [
23], and phosphorus removal performance is not within the scope of the present study. Briefly, the o-PO
4 concentration at the effluent of column 1 was below 0.05 mg/L for the whole duration of the experiment. The o-PO
4 concentration slowly increased in cells 1 to 6 of column 1 following progressive exhaustion.
The slag dissolution and resulting high pH and high calcium concentration are favorable conditions for calcite precipitation. Crystalline calcite observed in X-ray diffractograms and low TIC concentration in cells confirmed the progressive formation of calcite within columns. An example of X-Ray diffractogram is provided in
Figure S2. The TIC concentration was at first around 5 mg/L in all cells, then it slowly increased back to the influent concentration of about 20 to 30 mg/L following pH drop. TIC leaching was observed from cells 1 to 3 of column 2 after day 240.
3.2. Long-Term Loss of Effective Porosity in Steel Slag Filters Fed with Inorganic and Organic Influents
A comparison of tracer test calibration using the PHREEQC double porosity model and the TIS model is shown in
Figure 3. Two TIS calibrations are presented using partial data (t ≤ 11 h or t ≤ 25 h) in the optimization step. Calibrated parameters were
N = 28.2 and
TRHVe = 8.5 h for TIS-11 h;
N = 12.9 and
TRHVe = 9.8 h for TIS-25 h; and
D* = 5 cm,
Dn = 5 × 10
−6 s
−1 and
HRTVe = 9.4 h for the PHREEQC model. The PHREEQC double porosity model resulted in a better calibration than the TIS model. The TIS model can either calibrate the peak or part of the tail, depending on the number of data points considered in the optimization. Adding data to the optimization step slightly improved tail fitting, but peak fitting was shifted. The better accuracy of the PHREEQC double porosity model can be explained by its greater complexity which involves three calibration parameters for effective volume, dispersion, and retardation, compared to the TIS model which involves only two parameters for effective volume and dispersion.
The calibrated hydraulic parameters from tracer tests are shown in
Table 2. The double porosity model accurately reproduced experimental tracer breakthrough curves, as illustrated in
Figures S3 and S4. The mean experimental tracer recovery was 88%. The calibrated dispersivity was between 2.8 and 10 cm, which are typical values for sand to gravel columns [
30]. Dispersivity values in column 2 were higher than those of column 1, which could be attributed to the higher filling of void space in column 2, leading to enhanced dispersion (discussed in the coming section). The dispersivity
D* and the exchange factor
Dn did not show any trend over time. The effective porosity
ne was between 0.267 and 0.422 in column 1, and between 0.184 and 0.401 in column 2. A reduction of effective porosity was observed in both columns, slight in column 1 and high in column 2. The effective porosity was significantly lower than the total porosity (0.49) in both columns, as opposed to other gravel reactors for wetlands where the effective porosity of the clean bed is assumed to be equal to the total porosity [
7]. Slag media is porous and has a highly irregular shape, which results in a stagnant porosity that is not present in natural media filters. Consequently, the effective porosity of a filter made of artificial media should always be determined with tracer tests.
The reduction in effective porosity following column operation is shown in
Figure 4. In this figure, the effective porosity determined by tracer tests is compared with the theoretical reduction in porosity based on the volume occupied by precipitates. Both columns had an influent with a moderate alkalinity of 100 mg CaCO
3/L, resulting in a relatively low theoretical reduction of porosity caused by precipitates (0.05 over 600 d in column 1 and in 0.02 over 400 d in column 2). Experimental points from tracer tests on column 1 roughly followed this calculated porosity reduction trend. In column 2, however, the effective porosity was significantly lower than predicted. These results are supported by visual observations during the experiment. In sampling events, water flowed easily in all sampling valves of column 1, while the water flow was slow in valves of column 2, which was attributed to void clogging. The measurement of water level in column 2 was aborted due to overpressure in the column. Upon dismantling, the slag media from column 1 had the same appearance as fresh slag, while in column 2, slag was cemented in an irregular distribution, in either heavily cemented zones in red, or zones with disintegrated slag grains in black (
Figure S5, Supplemental Materials). In column 2, the presence of a biofilm was suspected due to the observation of a slimy substance in the slag voids of all cells upon dismantling.
The type of influent strongly affected the evolution of clogging and effective porosity in steel slag filters. In the presence of an inorganic influent without suspended solids (column 1), the loss of effective porosity was limited to the loss of the volume occupied by crystals, meaning that the reactive volume was used efficiently. A similar clogging behavior was expected in column 2 due to a similar inorganic composition in the influent (TIC of ~22 mg/L and no suspended solids) and hostile high pH conditions for biological activity. Despite the readily biodegradability of the influent COD, the biofilm establishment and growth on slag surfaces was expected to be strongly inhibited by a high pH in the column, the absence of nutrients in the influent, and the oxygen deficiency. This low biological activity was confirmed by a negligible COD removal efficiency in the column. The loss of effective porosity in column 2 did not follow the expected behavior: the effective porosity was smaller than the porosity predicted by precipitate accumulation. This suggests that in the presence of an organic influent, the progressive formation of precipitates is not sufficient to explain all the loss in porosity; other clogging mechanisms are taking place. The loss of effective porosity in the presence of an organic influent is hypothesized to be due to the formation of biofilm and changes in the slag mineralogical composition, as discussed in the following section on clogging mechanisms.
The high porosity reduction in column 2 led to an inefficient use of the slag reactivity. In a slag filter, the pH drop is due to the combination of the slag media exhaustion and the formation of a precipitate diffusion barrier, both of which affect the OH
− gradient between the slag surface and the bulk void volume [
23]. Fresh slag has a saturation pH of approximately 11.1 and it decreases progressively to pH 9.5 following slag leaching, according to its exhaustion functions [
23]. Therefore, measuring the saturation pH of a disturbed slag sample from an exhausted filter is a way to determine how much slag was efficiently leached during the filter lifetime.
The saturation pH of disturbed slag samples at the inlet of columns is shown in
Table 3. In both columns, cells 1 and 2 were considered exhausted due to their cell pH in the range of 7.5 to 8.3 (first column of the Table). In column 1, the slag saturation pH was approximately 10.0, which is one unit lower than the saturation pH of fresh slag (11.1), meaning that slag was leached efficiently during operation. In column 2, however, the inlet of the column became exhausted even if the slag itself was still reactive, as shown by a measured saturation pH of approximately 11.0. In conclusion, clogging in the first cells of column 2 was such that slag was not contacted efficiently with water.
3.3. Clogging Mechanisms in Steel Slag Filters
Microscopic observations of the slag surface on undisturbed slag samples after dismantling are shown in
Figure 5. In column 1 (inorganic influent), numerous individual crystal seeds of size ~0.1 μm (possibly calcite and hydroxyapatite based on X-ray diffraction measurements) were clustered together in a coral-shaped network. At a larger scale, crystal structures grew according to a spherical shape of size ~30 μm. Crystals in column 1 were well organized in a dense structure with compact void occupancy, and no evidence of biological structures were seen. In column 2 (organic influent), sparse rhombohedral crystals of size ~5 μm (possibly calcite according to X-ray diffraction measurements) coexisted with ~1 μm spheres and a network of spiderweb shaped structures (possibly extracellular polymeric substances, EPS, according to their shapes). The loose crystal organization in column 2 could favor crystal detachment, potentially explaining the TIC leaching observed in cells 1 to 3 after partial exhaustion. Such TIC leaching was not observed in column 1. The presence of a biofilm in column 2 was confirmed by visual observations of a slimy substance in all cells, including cells number 6 to 9 that had a pH above 10 for the whole duration of the experiment. Despite the inhibition potential of alkaline water on bacterial growth [
31] and resulting negligible COD removal, an alkali tolerant biofilm was formed in the steel slag filter over long-term operation. Such biofilm formation is possible due to the absence of wastage or backwashes over more than 400 days of operation. Alkali-tolerant microorganisms are observed in some natural waters [
32] and man-made environments such as district heating systems [
33].
The heterogeneous and unorganized void occupation in column 2 could explain the severe porosity loss observed. We postulate that the formation of crystals in unorganized structures results in the formation of confined voids that are not accessible for water flow, thus accelerating porosity loss. The extent of porosity reduction induced by mineral precipitation in a granular filter is influenced by the presence of biofilm on the media surface. The literature in sedimentary geology has shown that in natural environments, such as thermal springs, the precipitation of carbonate species is highly influenced by the presence of EPS [
32]. EPS serve as effective calcium buffers, preventing seed crystal nucleation even in highly supersaturated conditions, leading to preferential precipitation in mucus-free area [
32]. This phenomenon was observed in column 2, where crystals were dispersed in a heterogeneous distribution (
Figure 5C,D). The effect of biological structures on crystal precipitation and void occupation in alkaline reactive filters has important implications as influents (agricultural runoff, wastewater) almost always contains organic matter at various concentrations. Consequently, studies using inorganic influents are not appropriate to assess the clogging behavior of alkaline reactive filters.
Future research is needed to assess the effect of organic matter and biofilm on mineralogical structures and loss of porosity under realistic scenarios.
Moreover, research is needed on bioclogging in alkaline conditions, especially regarding biokinetic growth parameters needed for estimations of bioclogging [
3].
In column 2, the slag media itself might have contributed to clogging due to dissolution and reprecipitation of some compounds present in slag [
21]. Iron species are sensitive to redox conditions in the influent [
34]; reprecipitation or hydration of iron mineral phases may explain the severe rusty-red cementation observed in column 2.
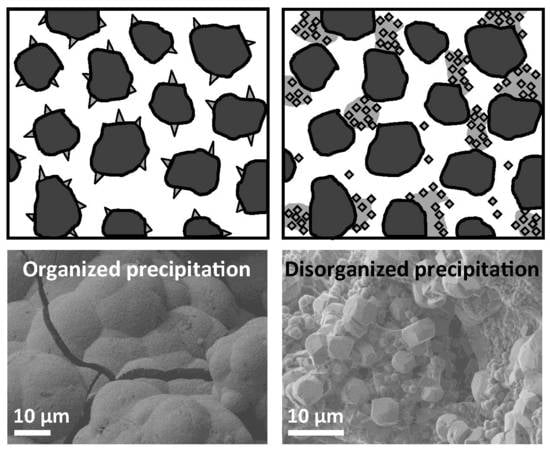
A schematic model of clogging phenomena in AGFs is shown in
Figure 6. Five clogging scenarios are presented with three types of chemical clogging. First, chemical clogging with an organized structure (
Figure 6A) involves compact crystals with minimal impact on the effective porosity, as observed in column 1. Second, chemical clogging with a disorganized structure (
Figure 6B) involves the creation of confined voids that are not occupied by bulk crystals, but are not available for flow. Third, chemical clogging in the presence of a biofilm (
Figure 6E) leads to enhanced occurrence of confined voids, as observed in column 2. The impact of the combination of chemical clogging and biofilm on the porosity loss is higher than the impact of those two separate factors. Even if the alkaline environment of an AGF is a priori unfavorable to bacterial growth, the formation of a biofilm over the long term is possible.
3.4. Effect of Clogging on Pressure Head Build-Up
The evolution of pressure head build-up in column 1 is shown in
Figure 7. The head loss in column 1 was low, below 2.5 cm for the whole duration of the experiment. The head loss was concentrated in cell 1, while no pressure buildup was observed in cells 2 to 8 (the experimental data for all cells is shown in
Figure S6). The pressure head in cell 1 was irregular and it decreased when the influent flow rate was reduced. Interestingly, the pressure head increase observed at approximately 250 d in cell 1 corresponds with its complete exhaustion—its pH was the same as the influent pH. The experimental pressure head build-up is compared with predicted pressure head build-up predicted using the Kozeny–Carman relationship (
Figure 7). In order to calibrate the predicted pressure head with the experimental values, the particle size of slag grains and crystal clusters used in Equation (9) were set at 7 mm and 0.028 mm, respectively.
The Kozeny–Carman relationship predicted a progressive increase of pressure head within the whole column; especially in cells 1, 5, and 2. The predicted pressure head results from the hypothesis behind many predictive equations for hydraulic conductivity: any particle in the granular media blend contributes to the hydraulic conductivity. In the case of column 1, the mass of precipitates is distributed within the whole column, with peaks in cells 1, 5, and 2 (
Figure 8A). The predicted pressure head build-up in cells 2 and 5 is not observed in experimental data, a pressure increase was observed only in cell 1. This illustrates the limitations of traditional models of prediction of hydraulic conductivity of granular media used in applications with non-natural granular blends.
Three main reasons explain these limitations: 1) the Kozeny–Carman relationship is valid only for hydraulic conductivities below 1 × 10
−4 m/s [
35]. In that view, the Kozeny–Carman relationship is not valid in the tested column because the hydraulic conductivity was 0.1 m/s. Note that most of gravel-size man-made filters with uniform grain size are above the applicability range as well, despite the popularity of this relationship in recent works in wetland applications [
3]. 2) The contribution of newly formed precipitates to the hydraulic conductivity reduction is not known. Hydraulic conductivity predictive models are based on a spherical grain assumption with size and specific surface being measured with standard methods in soil sciences and hydrogeology (sieving, sedimentometry, Atterberg limits). These methods are not applicable to the measurement of the specific surface of newly formed precipitates. The concept of particle size must be reviewed for slag filter applications because they are binary media (original media + precipitates). It is technically feasible to measure the size of individual crystals, as illustrated in
Figure 8B. These nm-range measurements cannot be used as inputs to the Kozeny–Carman equations: it would calculate hydraulic conductivity in the range of 1 × 10
−9 m/s or lower, which is not realistic in a gravel bed. 3) Suffossion (internal erosion or migration of fines in the porosity) criteria that are needed to properly measure the hydraulic conductivity of a noncohesive granular media [
35] are not applicable in reactive filters. Newly formed precipitates or biofilm can be either cemented or unstable and transported, which can result in erratic evolution of the hydraulic conductivity. In the case of column 1, an unexplained drop of pressure head was observed at t = 350 d. Though it was not correlated with a maintenance event of piezometers or inlet pipes, such pressure head drop could be attributed to a rearrangement of precipitates within porosity following a slight hydraulic shock. Similarly, the pressure drop observed at t = 517 d should have been proportional to the drop in influent flowrate according to Darcy’s law, but it was not the case.
The crystal structure and connectivity are concepts that could lead to improved predictions of hydraulic conductivity in gravel-size filters with long-term filling of voids. In column 1, the mean calcite crystal size was approximately 150 Å in cells 1 to 3, and was above 2500 Å in cells 6 to 9 (
Figure 8B), which suggests that different precipitation mechanisms took place in the column. The small crystal size in the inlet cells is related to the high calcite supersaturation index, which favors homogeneous precipitation (precipitation by nucleation). The preponderance of homogeneous precipitation in cells 1 to 3 was also observed for hydroxyapatite crystals, as reported previously [
23]. Similarly, the large crystal sizes observed in cells 6 to 9 could be related to the prevalence of heterogeneous precipitation (precipitation by crystal growth) in low supersaturation index conditions. We postulate that homogeneous precipitation leads to a high crystal connectivity that impacts hydraulic conductivity, while heterogeneous precipitation has little impact on the crystal connectivity, because crystals grow on existing grains. Therefore, crystal aggregates that contribute to the determination of the media specific surface are small and large in the case of homogeneous and heterogeneous precipitation, respectively. The high supersaturation index that favors heterogeneous precipitation in the close inlet region might explain that a pressure head build-up was observed only in cell 1.
While column 1 showed how the supersaturation index affects the calcite crystal size and the development of pressure head in inorganic water, column 2 shows how the presence of organic matter affects the precipitation of calcite. Indeed, the calcite crystal size distribution within column (
Figure 8B) does not reflect the progression from a high supersaturation index (inlet) to a low supersaturation index (outlet). However, this erratic distribution agrees with the variety and unorganized structure of crystals observed in SEM (
Figure 5C,D). Crystal aggregates in column 2 certainly contributed to clogging and development of pressure head, though it was not possible to quantify pressure head build up for column 2. Overall, there is a need to develop new methods or criteria to describe crystal aggregates and their connectivity in AGFs, in both inorganic and organic water conditions. Existing methods for the measurement of specific surface in cohesive soils (correlations with Atterberg limits, BET specific surface [
35]) could possibly be adapted for this purpose.
3.6. Implications for Alkaline Granular Filters Hydraulic Models
The findings of the manuscript regarding evolution of porosity, development of head losses, and clogging control are useful input for alkaline granular filter models, which could lead to improved design tools. Given the important effect of clogging on the loss of effective porosity, slag reactivity, and pressure head buildup, we recommend a mechanistic approach for hydraulic modelling of AGFs, using hydraulic models based on the advection-diffusion-reaction equation [
28], as opposed to the TIS model [
7]. Models using the advection-diffusion-reaction equation are based on intrinsic hydrogeological properties of the media (
ne,
D*), which allow a mechanistic approach for modelling of phenomena taking place in AGFs.
Advantages inherent to these models include first, the possible calculation of the loss of effective porosity at each iteration of the simulation based on the mass precipitated. The TIS model, however, does not offer a mechanistic approach to calculate the evolution of hydraulic parameters, even if it was successfully used to calibrate tracer tests of full-scale AGFs [
13]. Indeed, there is no direct mathematical relationship between the TIS hydraulic parameters (
N,
HRTVe) and the porosity reduction caused by the volume occupied by precipitates.
Second, models based on the advection–diffusion–reaction equation can be adapted to predict pressure head build up in filters. Actual clogging models, however, must be improved before providing satisfactory predictions of hydraulic conductivity and pressure build up in gravel-size AGFs. First, biofilm growth kinetic constants in alkaline conditions must be assessed before being used in a clogging model [
3]. Second, the results of this study showed that in a gravel-size AGFs made of steel slag, the development of pressure head is not directly related to the amount of precipitation, as illustrated by the absence of pressure head build up in the zones where precipitation was most important (column 1, cell 5,
Figure 7 and
Figure 8). Further research is needed to understand the impact of crystal structures on hydraulic conductivity and pressure head. Actual predictive models from particle size distribution are not suitable for AGFs.
Third, models based on the advection–diffusion–reaction equation can be complexified with useful features related to clogging. For example, the definition of new crystal structure parameters should lead to improved predictions of pressure within the filter. Crystal structure is affected by the supersaturation level [
36] and by water velocity [
37]. In such scenarios, the model must allow the calculation of local water velocity and local saturation index. In particular, local velocities in 2D flows subject to progressive clogging are complex [
3]. The calculation of local velocities is not straightforward in models based on the TIS hypothesis.
In summary, we recommend dedicating research efforts in the development of hydraulic models for AGFs using a model based on the advection–diffusion–reaction equation. Three important upgrades are recommended:
- (1)
evolution of porosity (affecting the available contact time),
- (2)
evolution of hydraulic conductivity (affecting pressure build up), and
- (3)
study of mechanisms affecting the precipitate structure (refining the first two points).
Such features are important steps towards the development of accurate reactive filter longevity prediction tools.