Biofilm Production by Critical Antibiotic-Resistant Pathogens from an Equine Wound
Abstract
:Simple Summary
Abstract
1. Introduction
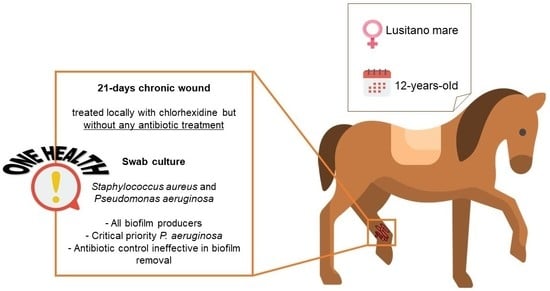
2. Detailed Case Description
2.1. Sample Collection and Processing
2.2. Bacterial Isolates and Culture Conditions
2.3. Minimum Inhibitory Concentration (MIC) Determination
2.4. Biofilm Formation and Classification
2.5. Biofilm Control
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowden, A.; Boynova, P.; Brennan, M.L.; England, G.C.W.; Mair, T.S.; Furness, W.A.; Freeman, S.L.; Burford, J.H. Retrospective Case Series to Identify the Most Common Conditions Seen “out-of-Hours” by First-Opinion Equine Veterinary Practitioners. Vet. Rec. 2020, 187, 404. [Google Scholar] [CrossRef] [PubMed]
- Wilmink, J.M.; Ladefoged, S.; Jongbloets, A.; Vernooij, J.C.M. The Evaluation of the Effect of Probiotics on the Healing of Equine Distal Limb Wounds. PLoS ONE 2020, 15, e0236761. [Google Scholar] [CrossRef] [PubMed]
- da Fontoura Pereira, R.C.; De La Côrte, F.D.; Brass, K.E.; da Silva Azevedo, M.; Gallio, M.; Cantarelli, C.; Dau, S.L.; Cezar, A.S.; Inkelmann, M.A. Evaluation of Three Methods of Platelet-Rich Plasma for Treatment of Equine Distal Limb Skin Wounds. J. Equine Vet. Sci. 2019, 72, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.E.; Al-Abbadi, H.A.; El-Daharawy, M.H.; Ahmed, M.F. Enhancement of Chronic Wound Healing with Maltodextrin/Ascorbic Acid Gel: A Clinical Evaluation of Distal Limb Wounds in Horses. J. Anim. Sci. Technol. 2022, 64, 997–1007. [Google Scholar] [CrossRef]
- Knottenbelt, D.C. Equine Wound Management: Are There Significant Differences in Healing at Different Sites on the Body? Vet. Dermatol. 1997, 8, 273–290. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in Chronic Wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Duerden, B.I. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [Green Version]
- Westgate, S.J.; Percival, S.L.; Knottenbelt, D.C.; Clegg, P.D.; Cochrane, C.A. Microbiology of Equine Wounds and Evidence of Bacterial Biofilms. Vet. Microbiol. 2011, 150, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, K.; Woods, E.; Welsby, S.; Percival, S.L.; Cochrane, C.A. Biofilm Evidence and the Microbial Diversity of Horse Wounds. Can. J. Microbiol. 2009, 55, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, L.L.; Hermans, K.; Haspeslagh, M.; Chiers, K.; Pint, E.; Boyen, F.; Martens, A.M. A Quantitative Swab Is a Good Non-Invasive Alternative to a Quantitative Biopsy for Quantifying Bacterial Load in Wounds Healing by Second Intention in Horses. Vet. J. 2017, 225, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mah, T.F. Biofilm-Specific Antibiotic Resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fèvre, E.M.; Woolhouse, M.E.J.; Van Bunnik, B.A.D. Are Food Animals Responsible for Transfer of Antimicrobial-Resistant Escherichia coli or Their Resistance Determinants to Human Populations? A Systematic Review. Foodborne Pathog. Dis. 2018, 15, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Belas, A.; Menezes, J.; Gama, L.T.; Pomba, C. Sharing of Clinically Important Antimicrobial Resistance Genes by Companion Animals and Their Human Household Members. Microb. Drug Resist. 2020, 26, 1174–1185. [Google Scholar] [CrossRef]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial Usage and Resistance in Companion Animals: A Cross-Sectional Study in Three European Countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, Y.; Irekeola, A.A.; Engku Nur Syafirah, E.A.R.; Yusof, W.; Huey, L.L.; Muhammad, S.L.; Harun, A.; Yean, C.Y.; Zaidah, A.R. Prevalence of Vancomycin-Resistant Enterococcus (VRE) in Companion Animals: The First Meta-Analysis and Systematic Review. Antibiotics 2021, 10, 138. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Simões, L.; Simões, M.; Vieira, M. Influence of the Diversity of Bacterial Isolates from Drinking Water on Resistance of Biofilms to Disinfection. Appl. Environ. Microbiol. 2010, 76, 6673–6679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, L.; Simões, M.; Vieira, M. Biofilm Interactions between Distinct Bacterial Genera Isolated from Drinking Water. Appl. Environ. Microbiol. 2007, 73, 6192–6200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monte, J.; Abreu, A.C.; Borges, A.; Simões, L.C.; Simões, M. Antimicrobial Activity of Selected Phytochemicals against Escherichia coli and Staphylococcus aureus and Their Biofilms. Pathogens 2014, 3, 473–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, M.; Borges, A.; Teodósio, J.; Araújo, P.; Mergulhão, F.; Melo, L.; Simões, M. The Effects of Ferulic and Salicylic Acids on Bacillus cereus and Pseudomonas fluorescens Single- and Dual-Species Biofilms. Int. Biodeterior. Biodegrad. 2014, 86, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Lux, C.N. Wound Healing in Animals: A Review of Physiology and Clinical Evaluation. Vet. Dermatol. 2022, 33, 91-e27. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Kali, A.; Stephen, S.; Umadevi, S. Laboratory Evaluation of Phenotypic Detection Methods of Methicillin-Resistant Staphylococcus aureus. Biomed. J. 2014, 37, 411–414. [Google Scholar] [CrossRef]
- Bugg, T.D.H. Bacterial Peptidoglycan Biosynthesis and Its Inhibition. Compr. Nat. Prod. Chem. 1999, 3, 241–294. [Google Scholar] [CrossRef]
- EMA. Categorisation of Antibiotics Used in Animals Promotes Responsible Use to Protect Public and Animal Health; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Meletis, G. Carbapenem Resistance: Overview of the Problem and Future Perspectives. Ther. Adv. Infect. Dis. 2016, 3, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of Increased Resistance to Chlorhexidine and Cross-Resistance to Colistin Following Exposure of Klebsiella pneumoniae Clinical Isolates to Chlorhexidine. Antimicrob. Agents Chemother. 2016, 61, e01162-16. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Lu, R.; Chen, Y.; Qiu, J.; Deng, C.; Tan, Q. Study of Cross-Resistance Mediated by Antibiotics, Chlorhexidine and Rhizoma Coptidis in Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2016, 7, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Laumen, J.G.E.; Van Dijck, C.; Manoharan-Basil, S.S.; Abdellati, S.; De Baetselier, I.; Cuylaerts, V.; De Block, T.; Van den Bossche, D.; Xavier, B.B.; Malhotra-Kumar, S.; et al. Sub-Inhibitory Concentrations of Chlorhexidine Induce Resistance to Chlorhexidine and Decrease Antibiotic Susceptibility in Neisseria gonorrhoeae. Front. Microbiol. 2021, 12, 776909. [Google Scholar] [CrossRef] [PubMed]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The Role of Biofilms as Environmental Reservoirs of Antibiotic Resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Lima, Â.; Sousa, L.G.V.; Rosca, A.S.; Muzny, C.A.; Cerca, N. Crystal Violet Staining Alone Is Not Adequate to Assess Synergism or Antagonism in Multi-Species Biofilms of Bacteria Associated with Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2022, 11, 795797. [Google Scholar] [CrossRef]
- EMA Gentamicin. Available online: https://www.ema.europa.eu/en/medicines/veterinary/referrals/gentamicin (accessed on 11 February 2023).
- Van Laar, T.A.; Chen, T.; You, T.; Leung, K.P. Sublethal Concentrations of Carbapenems Alter Cell Morphology and Genomic Expression of Klebsiella pneumoniae Biofilms. Antimicrob. Agents Chemother. 2015, 59, 1707–1717. [Google Scholar] [CrossRef] [Green Version]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC Versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, U.F.; Igwenagu, E.; Mu’azu, A.; Aliyu, S.; Umar, M.I. Intrigues of Biofilm: A Perspective in Veterinary Medicine. Vet. World 2016, 9, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]


| Pseudomonas aeruginosa (F09MH/BHI) | Staphylococcus aureus (F09Mannitol) | Staphylococcus aureus (BPA1) | Staphylococcus aureus (BPA2) | |
|---|---|---|---|---|
| Ertapenem | R n.s. | |||
| Imipenem | R MIC16 µg/mL | |||
| Amikacin | S MIC = 4 µg/mL | |||
| Gentamicin | I MIC = 4 µg/mL | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL |
| Enrofloxacin | R MIC µg/mL | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL |
| Marbofloxacin | R MIC 4 µg/mL | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL |
| Kanamycin | S MIC 4 µg/mL | S MIC 4 µg/mL | S MIC 4 µg/mL | |
| Neomycin | S MIC 2 µg/mL | S MIC 2 µg/mL | S MIC 2 µg/mL | |
| Pradofloxacin | S MIC 0.12 µg/mL | S MIC 0.12 µg/mL | S MIC 0.12 µg/mL | |
| Erythromycin | S MIC 0.25 µg/mL | S MIC 0.25 µg/mL | S MIC 0.25 µg/mL | |
| Clindamycin | S MIC = 0.25 µg/mL | S MIC = 0.25 µg/mL | S MIC = 0.25 µg/mL | |
| Doxycycline | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL | S MIC 0.5 µg/mL | |
| Tetracycline | S MIC 1 µg/mL | S MIC 1 µg/mL | S MIC 1 µg/mL | |
| Nitrofurantoin | S MIC 16 µg/mL | S MIC 16 µg/mL | S MIC 16 µg/mL | |
| Chloramphenicol | S MIC 4 µg/mL | S MIC 4 µg/mL | S MIC 4 µg/mL | |
| Trimethoprim/sulfamethoxazole | S MIC 10 µg/mL | S MIC 10 µg/mL | S MIC 10 µg/mL | |
| Cefoxitin screening test | Negative | Negative | Positive | |
| Oxacillin | n.s. MIC = 1 µg/mL | n.s. MIC = 0.25 µg/mL | n.s. MIC 0.25 µg/mL |
| Pseudomonas aeruginosa (F09MH/BHI) | Staphylococcus aureus (F09Mannitol) | Staphylococcus aureus (BPA1) | Staphylococcus aureus (BPA2) | |
|---|---|---|---|---|
| 24 h | Moderate | Moderate | Moderate | Moderate |
| 48 h | Strong | Moderate | Moderate | Moderate |
| Pseudomonas aeruginosa (F09MH/BHI) | Staphylococcus aureus (F09Mannitol) | Staphylococcus aureus (BPA1) | Staphylococcus aureus (BPA2) | |
|---|---|---|---|---|
| MIC | High | Moderate | High | High |
| 10MIC | High | High | High | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, A.C.; Sousa, M.; Pinto, A.R.; Cotovio, M.; Simões, M.; Saavedra, M.J. Biofilm Production by Critical Antibiotic-Resistant Pathogens from an Equine Wound. Animals 2023, 13, 1342. https://doi.org/10.3390/ani13081342
Afonso AC, Sousa M, Pinto AR, Cotovio M, Simões M, Saavedra MJ. Biofilm Production by Critical Antibiotic-Resistant Pathogens from an Equine Wound. Animals. 2023; 13(8):1342. https://doi.org/10.3390/ani13081342
Chicago/Turabian StyleAfonso, Ana C., Mariana Sousa, Ana Rita Pinto, Mário Cotovio, Manuel Simões, and Maria José Saavedra. 2023. "Biofilm Production by Critical Antibiotic-Resistant Pathogens from an Equine Wound" Animals 13, no. 8: 1342. https://doi.org/10.3390/ani13081342