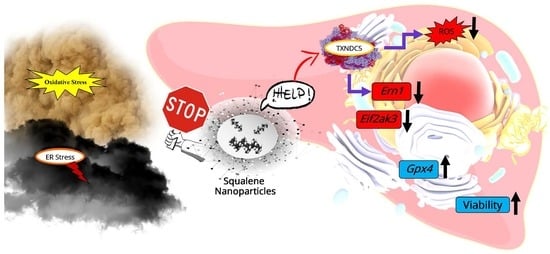
Squalene Loaded Nanoparticles Effectively Protect Hepatic AML12 Cell Lines against Oxidative and Endoplasmic Reticulum Stress in a TXNDC5-Dependent Way
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PLGA-Based Squalene-Loaded Nanoparticles
2.2. Physicochemical Characterization of the Nanoparticles
2.3. PLGA Encapsulation Efficiency Experiment
2.4. Squalene Extraction
2.5. AML12 Cell Culture
2.6. Characterization of Cell Morphology in Presence of PLGA-Squalene Nanoparticles
2.7. Generation of a Stable TXNDC5 Knockout AML12 Cell Line
2.8. RNA Extraction
2.9. Quantitative Real-Time PCR (RT-qPCR)
2.10. Western Blot
2.11. Cell Viability Assay
2.12. Reactive Oxygen Species Assay
2.13. Statistical Analysis
3. Results
3.1. Synthesis and Physicochemical Characterization of Squalene Loaded PLGA Nanoparticles
3.2. Influence of Several PLGA NPs Concentrations on the Morphology of AMl12 Cell Line
3.3. AML12 Cellular Uptake of Squalene
3.4. Squalene Based PLGA NPs Could Effectively Protect against Oxidative Stress
3.5. Effect of Squalene Protection on Oxidative Stress Response In Vitro by Gpx4 Induction
3.6. Squalene Enhanced the Cell Viability of Mouse Hepatocytes in Harsh ER Stress
3.7. Squalene Protects Thapsigargin-Induced ER Stress by Ern1 and Eif2ak3 Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLGA | Poly lactic-co-glycolic acid |
| NPs | Nanoparticles |
| ROS | Reactive oxygen species |
| AML12 | Alpha mouse liver cell line |
| WT | Wild-type |
| KO | Knock-out |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| MDA-MB-231 | M.D. Anderson-metastatic breast 231 |
| ER | Endoplasmic reticulum |
| UPR | Unfolded protein response |
| HL-60 | Human leukemic cell line 60 |
| PDI | Protein disulfide isomerase |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| HPLC | High performance liquid chromatography |
| MTT | 3-(4 5-dimethylthiazol- 2-yl)-2 5-diphenyltetrazolium bromide |
| DCFH-DA | 2,7-dichlorofluorescein diacetate |
| DCF | Dichlorofluorescein |
| Ppib | Peptidylprolyl isomerase B |
| Tbp | TATA box binding protein |
| Gpx4 | Glutathione peroxidase 4 |
| Eif2ak3 | Eukaryotic translation initiation factor 2 alpha kinase 3 |
| Atf6 | Activating transcription factor 6 |
| Ern1 | Endoplasmic reticulum (ER) to nucleus signaling 1 |
| Txndc5 | Thioredoxin domain containing 5 |
References
- Guasch-Ferré, M.; Willett, W. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Shannon, O.M.; Ashor, A.W.; Scialo, F.; Saretzki, G.; Martin-Ruiz, C.; Lara, J.; Matu, J.; Griffiths, A.; Robinson, N.; Lillà, L. Mediterranean diet and the hallmarks of ageing. Eur. J. Clin. Nutr. 2021, 75, 1–17. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, Y.; Willett, W.C.; Sun, Q.; Sampson, L.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Hu, F.B. Consumption of olive oil and risk of total and cause-specific mortality among US adults. J. Am. Coll. Cardiol. 2022, 79, 101–112. [Google Scholar] [CrossRef]
- Martínez-Beamonte, R.; Sánchez-Marco, J.; Felices, M.J.; Barranquero, C.; Gascón, S.; Arnal, C.; Burillo, J.C.; Lasheras, R.; Busto, R.; Lasunción, M.A. Dietary squalene modifies plasma lipoproteins and hepatic cholesterol metabolism in rabbits. Food Funct. 2021, 12, 8141–8153. [Google Scholar] [CrossRef]
- Hu, F.B. The Mediterranean diet and mortality-olive oil and beyond. N. Engl. J. Med. 2003, 348, 2595–2596. [Google Scholar] [CrossRef] [Green Version]
- Gabás-Rivera, C.; Barranquero, C.; Martínez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Osada, J. Dietary squalene increases high density lipoprotein-cholesterol and paraoxonase 1 and decreases oxidative stress in mice. PLoS ONE 2014, 9, e104224. [Google Scholar] [CrossRef] [PubMed]
- Martakos, I.; Kostakis, M.; Dasenaki, M.; Pentogennis, M.; Thomaidis, N. Simultaneous determination of pigments, tocopherols, and squalene in Greek olive oils: A study of the influence of cultivation and oil-production parameters. Foods 2020, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Lou-Bonafonte, J.M.; Martínez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J.; Arnal, C.; Osada, J. Current insights into the biological action of squalene. Mol. Nutr. Food Res. 2018, 62, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Gaforio, J.J.; Sánchez-Quesada, C.; López-Biedma, A.; del Carmen Ramírez-Tortose, M.; Warleta, F. Molecular Aspects of Squalene and Implications for Olive Oil and the Mediterranean Diet. In The Mediterranean Diet; Elsevier: Amsterdam, The Netherlands, 2015; pp. 281–290. [Google Scholar]
- Katdare, M.; Singhal, H.; Newmark, H.; Osborne, M.P.; Telang, N.T. Prevention of mammary preneoplastic transformation by naturally-occurring tumor inhibitors. Cancer Lett. 1997, 111, 141–147. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; Gutiérrez-Santiago, F.; Rodríguez-García, C.; Gaforio, J.J. Synergistic Effect of Squalene and Hydroxytyrosol on Highly Invasive MDA-MB-231 Breast Cancer Cells. Nutrients 2022, 14, 255. [Google Scholar] [CrossRef]
- Murakoshi, M.; Nishino, H.; Tokuda, H.; Iwashima, A.; Okuzumi, J.; Kitano, H.; Iwasaki, R. Inhibition by squalene of the tumor-promoting activity of 12-O-Tetradecanoylphorbol-13-acetate in mouse-skin carcinogenesis. Int. J. Cancer 1992, 52, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S.; Ponte, B.M.; Boonme, P.; Silva, A.M.; Souto, E.B. Nanoencapsulation of polyphenols for protective effect against colon–rectal cancer. Biotechnol. Adv. 2013, 31, 514–523. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Garduno-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Development and validation of a high-performance liquid chromatography method for the quantification of ursolic/oleanic acids mixture isolated from Plumeria obtusa. J. Chromatogr. B 2015, 983, 111–116. [Google Scholar] [CrossRef]
- Silva, A.M.; Alvarado, H.L.; Abrego, G.; Martins-Gomes, C.; Garduño-Ramirez, M.L.; García, M.L.; Calpena, A.C.; Souto, E.B. In vitro cytotoxicity of oleanolic/ursolic acids-loaded in PLGA nanoparticles in different cell lines. Pharmaceutics 2019, 11, 362. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and atherosclerosis. Nat. Med. 2010, 16, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Watkins, S.M.; Hotamisligil, G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [Green Version]
- Oliván, S.; Martínez-Beamonte, R.; Calvo, A.C.; Surra, J.C.; Manzano, R.; Arnal, C.; Osta, R.; Osada, J. Extra virgin olive oil intake delays the development of amyotrophic lateral sclerosis associated with reduced reticulum stress and autophagy in muscle of SOD1G93A mice. J. Nutr. Biochem. 2014, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Torres, A.; Barceló-Batllori, S.; Martínez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Arnal, C.; Guillén, N.; Acín, S.; Osada, J. Proteomics and gene expression analyses of squalene-supplemented mice identify microsomal thioredoxin domain-containing protein 5 changes associated with hepatic steatosis. J. Proteom. 2012, 77, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative stress in Alzheimer’s disease: Why did antioxidant therapy fail? Oxidative Med. Cell. Longev. 2014, 2014, 427318. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, Y.; Zhao, H.; Zhou, Y. Dietary squalene supplementation alleviates diquat-induced oxidative stress and liver damage of broiler chickens. Poult. Sci. 2021, 100, 100919. [Google Scholar] [CrossRef]
- Albano, E.; Mottaran, E.; Occhino, G.; Reale, E.; Vidali, M. role of oxidative stress in the progression of non-alcoholic steatosis. Aliment. Pharmacol. Ther. 2005, 22, 71–73. [Google Scholar] [CrossRef]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene targets pro-and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Func. Food 2015, 14, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, T.; Harashima, M.; Kurogi, K.; Suiko, M.; Liu, M.-C.; Sakakibara, Y. A novel procedure for the assessment of the antioxidant capacity of food components. Anal. Biochem. 2016, 507, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warleta, F.; Campos, M.; Allouche, Y.; Sánchez-Quesada, C.; Ruiz-Mora, J.; Beltrán, G.; Gaforio, J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010, 48, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabás-Rivera, C.; Jurado-Ruiz, E.; Sánchez-Ortiz, A.; Romanos, E.; Martínez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Arnal, C.; Rodríguez-Yoldi, M.J.; Andrés-Lacueva, C. Dietary Squalene Induces Cytochromes Cyp2b10 and Cyp2c55 Independently of Sex, Dose, and Diet in Several Mouse Models. Mol. Nutr. Food Res. 2020, 64, 2000354. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Huminiecki, L.; Moore, J.W.; Boyle, J.J.; Poulsom, R.; Creamer, D.; Barker, J.; Bicknell, R. EndoPDI, a novel protein-disulfide isomerase-like protein that is preferentially expressed in endothelial cells acts as a stress survival factor. J. Biol. Chem. 2003, 278, 47079–47088. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hou, Y.; Li, N.; Wu, K.; Zhai, J. The influence of TXNDC5 gene on gastric cancer cell. J. Cancer Res. Clin. Oncol. 2010, 136, 1497–1505. [Google Scholar] [CrossRef]
- Horna-Terrón, E.; Pradilla-Dieste, A.; Sánchez-de-Diego, C.; Osada, J. TXNDC5, a newly discovered disulfide isomerase with a key role in cell physiology and pathology. Int. J. Mol. Sci. 2014, 15, 23501–23518. [Google Scholar] [CrossRef] [Green Version]
- Edman, J.C.; Ellis, L.; Blacher, R.W.; Roth, R.A.; Rutter, W.J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature 1985, 317, 267–270. [Google Scholar] [CrossRef]
- Freedman, R.B.; Hirst, T.R.; Tuite, M.F. Protein disulphide isomerase: Building bridges in protein folding. Trends Biochem. Sci. 1994, 19, 331–336. [Google Scholar] [CrossRef]
- Lacoma, A.; Usón, L.; Mendoza, G.; Sebastián, V.; Garcia-Garcia, E.; Muriel-Moreno, B.; Domínguez, J.; Arruebo, M.; Prat, C. Novel intracellular antibiotic delivery system against Staphylococcus aureus: Cloxacillin-loaded poly (d,l-lactide-co-glycolide) acid nanoparticles. Nanomedicine 2020, 15, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Larrea, A.; Rodriguez-Fernandez, P.; Alfaro, S.; Gracia, B.; Lucía, A.; Usón, L.; Gomez, A.-C.; Mendoza, G.; Lacoma, A. Matryoshka-type gastro-resistant microparticles for the oral treatment of Mycobacterium tuberculosis. Nanomedicine 2019, 14, 707–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Beamonte, R.; Alda, O.; Sanclemente, T.; Felices, M.J.; Escusol, S.; Arnal, C.; Herrera-Marcos, L.V.; Gascón, S.; Surra, J.C.; Osada, J. Hepatic subcellular distribution of squalene changes according to the experimental setting. J. Physiol. Biochem. 2018, 74, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Wang, H.; Gao, C.; Shen, H.; Fa, X.e. Drug loaded gold nano-particulates for therapeutics of myocardial infarction in rat model. J. Biomater. Tissue Eng. 2018, 8, 197–205. [Google Scholar] [CrossRef]
- Kumar, L.R.; Chatterjee, N.; Tejpal, C.; Vishnu, K.; Anas, K.; Asha, K.; Anandan, R.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Z.; Zhang, Y.; Feng, Z.; Gu, P.; Huang, Y.; Liu, J.; Wu, Y.; Wang, D. Lentinan PLGA-stabilized pickering emulsion for the enhanced vaccination. Int. J. Pharm. 2022, 611, 121348. [Google Scholar] [CrossRef]
- Hu, Y.; Hoerle, R.; Ehrich, M.; Zhang, C. Engineering the lipid layer of lipid–PLGA hybrid nanoparticles for enhanced in vitro cellular uptake and improved stability. Acta Biomater. 2015, 28, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Alejo, T.; Uson, L.; Landa, G.; Prieto, M.; Yus Argón, C.; Garcia-Salinas, S.; de Miguel, R.; Rodríguez-Largo, A.; Irusta, S.; Sebastian, V. Nanogels with High Loading of Anesthetic Nanocrystals for Extended Duration of Sciatic Nerve Block. ACS Appl. Mater. Interfaces 2021, 13, 17220–17235. [Google Scholar] [CrossRef]
- Garms, B.C.; Poli, H.; Baggley, D.; Han, F.Y.; Whittaker, A.K.; Anitha, A.; Grøndahl, L. Evaluating the effect of synthesis, isolation, and characterisation variables on reported particle size and dispersity of drug loaded PLGA nanoparticles. Mater. Adv. 2021, 2, 5657–5671. [Google Scholar] [CrossRef]
- Wu, D.; Xu, Z.; Li, Z.; Yuan, W.; Wang, H.-Q.; Xie, X. Reduction and temperature dually-triggered size-shrinkage and drug release of micelles for synergistic photothermal-chemotherapy of cancer. Eur. Polym. J. 2021, 154, 110535. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 369–400. [Google Scholar]
- Alejo, T.; Andreu, V.; Mendoza, G.; Sebastian, V.; Arruebo, M. Controlled release of bupivacaine using hybrid thermoresponsive nanoparticles activated via photothermal heating. J. Colloid Interface Sci. 2018, 523, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmaële, D.; Gref, R.; Couvreur, P. Squalenoylation: A generic platform for nanoparticular drug delivery. J. Control. Release 2012, 161, 609–618. [Google Scholar] [CrossRef]
- Arias, J.L.; Reddy, L.H.; Othman, M.; Gillet, B.; Desmaele, D.; Zouhiri, F.; Dosio, F.; Gref, R.; Couvreur, P. Squalene based nanocomposites: A new platform for the design of multifunctional pharmaceutical theragnostics. ACS Nano 2011, 5, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Sahoo, S.K.; Prabha, S.; Bargar, T.; Labhasetwar, V. Fluorescence and electron microscopy probes for cellular and tissue uptake of poly (D,L-lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2003, 262, 1–11. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Panyam, J.; Zhou, W.Z.; Prabha, S.; Sahoo, S.K.; Labhasetwar, V. Rapid endo-lysosomal escape of poly (DL-lactide-coglycolide) nanoparticles: Implications for drug and gene delivery. FASEB J. 2002, 16, 1217–1226. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin-and fish oil-loaded spongosome and cubosome nanoparticles with neuroprotective potential against H2O2-induced oxidative stress in differentiated human SH-SY5Y cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Kohno, Y.; Egawa, Y.; Itoh, S.; Nagaoka, S.-i.; Takahashi, M.; Mukai, K. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1995, 1256, 52–56. [Google Scholar] [CrossRef]
- Motawi, T.M.; Sadik, N.A.; Refaat, A. Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: An experimental study on rat myocardium, testicles and urinary bladder. Food Chem. Toxicol. 2010, 48, 2326–2336. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Yogeeta, S.K.; Subashini, R.; Devaki, T. Attenuation of cyclophosphamide induced toxicity by squalene in experimental rats. Chem. Biol. Interact. 2006, 160, 252–260. [Google Scholar] [CrossRef]
- Farvin, K.S.; Anandan, R.; Kumar, S.H.S.; Shiny, K.; Sankar, T.; Thankappan, T. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar]
- Farvin, K.S.; Kumar, S.H.S.; Anandan, R.; Mathew, S.; Sankar, T.; Nair, P.V. Supplementation of squalene attenuates experimentally induced myocardial infarction in rats. Food Chem. 2007, 105, 1390–1395. [Google Scholar] [CrossRef]
- Skorkowska-Telichowska, K.; Hasiewicz-Derkacz, K.; Gębarowski, T.; Kulma, A.; Moreira, H.; Kostyn, K.; Gębczak, K.; Szyjka, A.; Wojtasik, W.; Gąsiorowski, K. Emulsions made of oils from seeds of GM flax protect V79 cells against oxidative stress. Oxidative Med. Cell. Longev. 2016, 2016, 7510759. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.; Cook, K.L.; Hu, R.; Facey, C.O.; Tavassoly, I.; Schwartz, J.L.; Baumann, W.T.; Tyson, J.J.; Xuan, J.; Wang, Y. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012, 72, 1321–1331. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-A.; Groenendyk, J.; Michalak, M. Endoplasmic reticulum stress associated responses in cancer. Biochim. Et Biophys. Acta Mol. Cell Res. 2014, 1843, 2143–2149. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-O.; Jin, U.-H.; Kang, J.H.; Kim, S.B.; Guthrie, A.S.; Sreevalsan, S.; Lee, J.-S.; Safe, S. The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Mol. Cancer Res. 2014, 12, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Gu, M.-X.; Fu, Y.; Sun, X.-L.; Ding, Y.-Z.; Li, C.-H.; Pang, W.; Pan, S.; Zhu, Y. Proteomic analysis of endothelial lipid rafts reveals a novel role of statins in antioxidation. J. Proteome Res. 2012, 11, 2365–2373. [Google Scholar] [CrossRef]
- Kojima, R.; Okumura, M.; Masui, S.; Kanemura, S.; Inoue, M.; Saiki, M.; Yamaguchi, H.; Hikima, T.; Suzuki, M.; Akiyama, S. Radically different thioredoxin domain arrangement of ERp46, an efficient disulfide bond introducer of the mammalian PDI family. Structure 2014, 22, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Angeli, J.P.F.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, M.; Yu, B.; Mao, X.; Zheng, P.; He, J.; Chen, D. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 2012, 6, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bermudez, J.; Baudrier, L.; Bayraktar, E.C.; Shen, Y.; La, K.; Guarecuco, R.; Yucel, B.; Fiore, D.; Tavora, B.; Freinkman, E. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature 2019, 567, 118–122. [Google Scholar] [CrossRef]
- Jessop, C.E.; Watkins, R.H.; Simmons, J.J.; Tasab, M.; Bulleid, N.J. Protein disulphide isomerase family members show distinct substrate specificity: P5 is targeted to BiP client proteins. J. Cell Sci. 2009, 122, 4287–4295. [Google Scholar] [CrossRef] [Green Version]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Shamu, C.E.; Walter, P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996, 15, 3028–3039. [Google Scholar] [CrossRef]
- Hassler, J.; Cao, S.S.; Kaufman, R.J. IRE1, a double-edged sword in pre-miRNA slicing and cell death. Dev. Cell 2012, 23, 921–923. [Google Scholar] [CrossRef] [Green Version]
- Schuck, S.; Prinz, W.A.; Thorn, K.S.; Voss, C.; Walter, P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 2009, 187, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef] [Green Version]
- Chawsheen, H.A.; Jiang, H.; Ying, Q.; Ding, N.; Thapa, P.; Wei, Q. The redox regulator sulfiredoxin forms a complex with thioredoxin domain–containing 5 protein in response to ER stress in lung cancer cells. J. Biol. Chem. 2019, 294, 8991–9006. [Google Scholar] [CrossRef]
- Shih, Y.-C.; Chen, C.-L.; Zhang, Y.; Mellor, R.L.; Kanter, E.M.; Fang, Y.; Wang, H.-C.; Hung, C.-T.; Nong, J.-Y.; Chen, H.-J. Endoplasmic reticulum protein TXNDC5 augments myocardial fibrosis by facilitating extracellular matrix protein folding and redox-sensitive cardiac fibroblast activation. Circ. Res. 2018, 122, 1052–1068. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Jhao, P.-Y.; Hung, C.-T.; Wu, Y.-F.; Lin, S.-J.; Chiang, W.-C.; Lin, S.-L.; Yang, K.-C. Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGF-β signaling in kidney fibroblasts. J. Clin. Investig. 2021, 131, e143645. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-T.; Su, T.-H.; Chen, Y.-T.; Wu, Y.-F.; Chen, Y.-T.; Lin, S.-J.; Lin, S.-L.; Yang, K.-C. Targeting ER protein TXNDC5 in hepatic stellate cell mitigates liver fibrosis by repressing non-canonical TGFβ signalling. Gut 2021, 2021, 325065. [Google Scholar] [CrossRef] [PubMed]









| Squalene Initial Volume | Squalene Concentration (µM) | Squalene Encapsulation (%) | Squalene/PLGA (w/w) |
|---|---|---|---|
| 100 µL | 7472 ± 530 | 35.8 ± 3.8 | 0.614 |
| 75 µL | 5417 ± 474 | 34.6 ± 3.0 | 0.445 |
| 50 µL | 8122 ± 735 | 77.8 ± 5.1 | 0.667 |
| 25 µL | 4842 ± 671 | 92.7 ± 12.9 | 0.398 |
| Polymer | Diameter (nm) | Dispersion | Zeta Potential (mV) |
|---|---|---|---|
| PLGA NPs with squalene | 259 ± 107 | 0.173 | −36.2 ± 0.3 |
| PLGA NPs without squalene | 129 ± 37 | 0.083 | −46.8 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidooki, S.H.; Alejo, T.; Sánchez-Marco, J.; Martínez-Beamonte, R.; Abuobeid, R.; Burillo, J.C.; Lasheras, R.; Sebastian, V.; Rodríguez-Yoldi, M.J.; Arruebo, M.; et al. Squalene Loaded Nanoparticles Effectively Protect Hepatic AML12 Cell Lines against Oxidative and Endoplasmic Reticulum Stress in a TXNDC5-Dependent Way. Antioxidants 2022, 11, 581. https://doi.org/10.3390/antiox11030581
Bidooki SH, Alejo T, Sánchez-Marco J, Martínez-Beamonte R, Abuobeid R, Burillo JC, Lasheras R, Sebastian V, Rodríguez-Yoldi MJ, Arruebo M, et al. Squalene Loaded Nanoparticles Effectively Protect Hepatic AML12 Cell Lines against Oxidative and Endoplasmic Reticulum Stress in a TXNDC5-Dependent Way. Antioxidants. 2022; 11(3):581. https://doi.org/10.3390/antiox11030581
Chicago/Turabian StyleBidooki, Seyed Hesamoddin, Teresa Alejo, Javier Sánchez-Marco, Roberto Martínez-Beamonte, Roubi Abuobeid, Juan Carlos Burillo, Roberto Lasheras, Victor Sebastian, María J. Rodríguez-Yoldi, Manuel Arruebo, and et al. 2022. "Squalene Loaded Nanoparticles Effectively Protect Hepatic AML12 Cell Lines against Oxidative and Endoplasmic Reticulum Stress in a TXNDC5-Dependent Way" Antioxidants 11, no. 3: 581. https://doi.org/10.3390/antiox11030581