Antioxidants and Oxidative Stress in Children: Influence of Puberty and Metabolically Unhealthy Status
Abstract
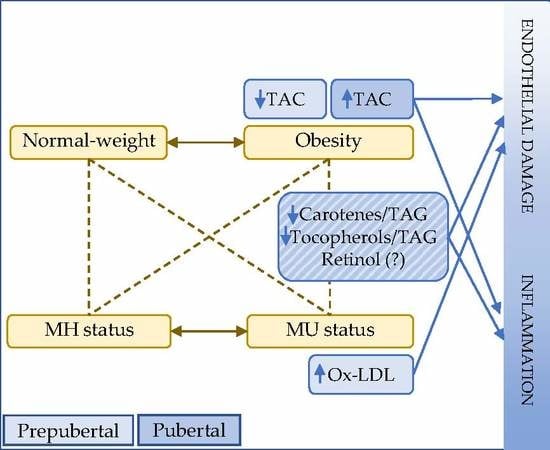
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Clinical and Anthropometric Examination
2.3. Blood Sampling
2.4. Plasma Antioxidants and Ox-LDL Analysis
2.5. Plasma Pro-Inflammatory and Endothelial Damage Biomarkers Analysis
2.6. Metabolic Health Definition
2.7. Dietary Assessment
2.8. Statistical Analysis
3. Results
3.1. Antioxidant and Oxidative Stress Biomarkers in Prepubertal and Pubertal Children According to Metabolic Health and Obesity
3.2. Evaluation of the Dietary Sources of Carotenes and Tocopherols and Their Influence on Carotene/TAG and Tocopherol/TAG Levels
3.3. Exploring the Relationship between Antioxidants and Oxidative Stress Biomarkers and Cardiometabolic, Pro-inflammatory and Endothelial Damage Markers
4. Discussion
4.1. Pubertal Differences in Antioxidant and Oxidative Stress Biomarkers in Children with Obesity or MU Status
4.2. Plasma Retinol Levels Are Decreased in Pubertal Children with MU Status and Correlated with Pro-Inflammatory and Endothelial Damage Biomarkers
4.3. Plasma Carotenes/TAG Levels Are Decreased in Children with Obesity or MU Status and Negatively Correlated with Pro-inflammatory and Endothelial Damage Biomarkers, Insulin and HOMA-IR
4.4. Plasma Tocopherols/TAG Levels Are Decreased in Children with Obesity or MU Status and Negatively Correlated with Pro-inflammatory and Endothelial Damage Biomarkers, Insulin, and HOMA-IR
4.5. Plasma TAC Levels Are Lower in Prepubertal and Higher in Pubertal Children with Obesity, Not Influenced by MU Status and Positively Correlated with MCP-1 Plasma Concentration
4.6. Plasma ox-LDL Levels Are Increased in Prepubertal MU Children and Positively Correlated with PAI-1
4.7. Prevention Strategies
4.8. Limitations and Strenghts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, R.; Dos Santos, T.J.; Ybarra, M.; Atar, M. Children with Metabolically Healthy Obesity: A Review. In Frontiers in Endocrinology; Frontiers Media: Lausanne, Switzerland, 2019. [Google Scholar] [CrossRef]
- Reilly, J.J.; Kelly, J. Long-Term Impact of Overweight and Obesity in Childhood and Adolescence on Morbidity and Premature Mortality in Adulthood: Systematic Review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivistoinen, T.; Hutri-Kähönen, N.; Juonala, M.; Aatola, H.; Kööbi, T.; Lehtimäki, T.; Viikari, J.S.A.; Raitakari, O.T.; Kähönen, M. Metabolic syndrome in childhood and increased arterial stiffness in adulthood—The Cardiovascular Risk in Young Finns Study. Ann. Med. 2011, 43, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.T.; Onuzuruike, A.U.; Magge, S.N. Cardiometabolic risk in obese children. Ann. N. Y. Acad. Sci. 2018, 1411, 166–183. [Google Scholar] [CrossRef]
- Ruperez, A.I.; Olza, J.; Gil-Campos, M.; Leis, R.; Bueno, G.; Aguilera, C.M.M.; Gil, A.; Moreno, L.A.A.; Rupérez, A.I.; Olza, J.; et al. Cardiovascular Risk Biomarkers and Metabolically Unhealthy Status in Prepubertal Children: Comparison of Definitions. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 524–530. [Google Scholar] [CrossRef]
- Kilic, E.; Ozer, O.F.; Erek Toprak, A.; Erman, H.; Torun, E.; Kesgin Ayhan, S.; Caglar, H.G.; Selek, S.; Kocyigit, A. Oxidative Stress Status in Childhood Obesity: A Potential Risk Predictor. Med. Sci. Monit. 2016, 22, 3673–3679. [Google Scholar] [CrossRef] [Green Version]
- Rowicka, G.; Dylag, H.; Ambroszkiewicz, J.; Riahi, A.; Weker, H.; Chelchowska, M. Total Oxidant and Antioxidant Status in Prepubertal Children with Obesity. Oxid. Med. Cell. Longev. 2017, 2017, 989. [Google Scholar] [CrossRef] [Green Version]
- Codoner-Franch, P.; Valls-Belles, V.; Arilla-Codoner, A.; Alonso-Iglesias, E. Oxidant Mechanisms in Childhood Obesity: The Link between Inflammation and Oxidative Stress. Trans. Res. 2011, 158, 369–384. [Google Scholar] [CrossRef]
- Molnar, D.; Decsi, T.; Koletzko, B. Reduced Antioxidant Status in Obese Children with Multimetabolic Syndrome. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1197–1202. [Google Scholar] [CrossRef] [Green Version]
- Montero, D.; Walther, G.; Perez-Martin, A.; Roche, E.; Vinet, A. Endothelial Dysfunction, Inflammation, and Oxidative Stress in Obese Children and Adolescents: Markers and Effect of Lifestyle Intervention. Obes. Rev. 2012, 13, 441–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelsey, M.M.; Pyle, L.; Hilkin, A.; Severn, C.D.; Utzschneider, K.; Van Pelt, R.E.; Nadeau, K.J.; Zeitler, P.S. The impact of obesity on insulin sensitivity and secretion during pubertal progression: A longitudinal study. J. Clin. Endocrinol. Metab. 2020, 105, e2061. [Google Scholar] [CrossRef] [PubMed]
- Perez-Navero, J.L.; Benitez-Sillero, J.D.; Gil-Campos, M.; Guillen-del Castillo, M.; Tasset, I.; Tunez, I. Changes in oxidative stress biomarkers induced by puberty. An. Pediatr. 2009, 70, 424–428. [Google Scholar] [CrossRef]
- Elhadd, T.A.; Khan, F.; Kirk, G.; McLaren, M.; Newton, R.W.; Greene, S.A.; Belch, J.J. Influence of Puberty on Endothelial Dysfunction and Oxidative Stress in Young Patients with Type 1 Diabetes. Diabetes Care 1998, 21, 1990–1996. [Google Scholar] [CrossRef]
- Kim, J.A.; Montagnani, M.; Kwang, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Everitt, A.V.; Hilmer, S.N.; Brand-Miller, J.C.; Jamieson, H.A.; Truswell, A.S.; Sharma, A.P.; Mason, R.S.; Morris, B.J.; Le Couteur, D.G. Dietary Approaches That Delay Age-Related Diseases. Clin. Interv. Aging 2006, 1, 11–31. [Google Scholar] [CrossRef]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.P.; Sinaiko, A.R. Fruit and Vegetable Consumption and Its Relation to Markers of Inflammation and Oxidative Stress in Adolescents. J. Am. Diet Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.; Takkinen, H.M.; Uusitalo, L.; Tapanainen, H.; Ovaskainen, M.L.; Alfthan, G.; Erlund, I.; Ahonen, S.; Akerlund, M.; Toppari, J.; et al. Carotenoid Intake and Serum Concentration in Young Finnish Children and Their Relation with Fruit and Vegetable Consumption. Nutrients 2018, 10, 1533. [Google Scholar] [CrossRef] [Green Version]
- Andersen, C.J.; Fernandez, M.L. Dietary Strategies to Reduce Metabolic Syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 241–254. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Bonet, M.L.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids in Adipose Tissue Biology and Obesity. Subcell. Biochem. 2016, 79, 377–414. [Google Scholar] [CrossRef] [PubMed]
- Sarni, R.O.; Suano de Souza, F.I.; Ramalho, R.A.; Schoeps Dde, O.; Kochi, C.; Catherino, P.; Dias, M.C.; Pessotti, C.F.; Mattoso, L.C.; Colugnat, F.A. Serum Retinol and Total Carotene Concentrations in Obese Pre-School Children. Med. Sci. Monit. 2005, 11, CR510–CR514. [Google Scholar] [PubMed]
- Beydoun, M.A.; Chen, X.; Jha, K.; Beydoun, H.A.; Zonderman, A.B.; Canas, J.A. Carotenoids, Vitamin A, and Their Association with the Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 32–45. [Google Scholar] [CrossRef]
- Monostori, P.; Barath, A.; Fazekas, I.; Hodi, E.; Mate, A.; Farkas, I.; Hracsko, Z.; Varga, I.S.; Sumegi, V.; Gellen, B.; et al. Microvascular Reactivity in Lean, Overweight, and Obese Hypertensive Adolescents. Eur. J. Pediatr. 2010, 169, 1369–1374. [Google Scholar] [CrossRef]
- Silva, I.T.; Mello, A.P.; Sanches, L.B.; Abdalla, D.S.; Damasceno, N.R. Is Plasma Alpha-Tocopherol Associated with Electronegative LDL in Obese Adolescents? J. Nutr. Sci. Vitaminol. 2013, 59, 100–107. [Google Scholar] [CrossRef]
- Garcia, O.P.; Ronquillo, D.; Del Carmen Caamano, M.; Martinez, G.; Camacho, M.; Lopez, V.; Rosado, J.L. Zinc, Iron and Vitamins A, C and E are Associated with Obesity, Inflammation, Lipid Profile and Insulin Resistance in Mexican School-Aged Children. Nutrients 2013, 5, 5012–5030. [Google Scholar] [CrossRef] [Green Version]
- Puchau, B.; Ochoa, M.C.; Zulet, M.A.; Marti, A.; Martinez, J.A.; Members, G. Dietary Total Antioxidant Capacity and Obesity in Children and Adolescents. Int. J. Food Sci. Nutr. 2010, 61, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Matusik, P.; Prokopowicz, Z.; Norek, B.; Olszanecka-Glinianowicz, M.; Chudek, J.; Malecka-Tendera, E. Oxidative/Antioxidative Status in Obese and Sport Trained Children: A Comparative Study. BioMed Res. Int. 2015, 2015, 8. [Google Scholar] [CrossRef]
- Leis, R.; Jurado-Castro, J.M.; Llorente-Cantarero, F.J.; Anguita-Ruiz, A.; Iris-Rupérez, A.; Bedoya-Carpente, J.J.; Vázquez-Cobela, R.; Aguilera, C.M.; Bueno, G.; Gil-Campos, M. Cluster Analysis of Physical Activity Patterns, and Relationship with Sedentary Behavior and Healthy Lifestyles in Prepubertal Children: Genobox Cohort. Nutrients 2020, 12, 1288. [Google Scholar] [CrossRef]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Cole, T.J.; Lobstein, T. Extended International (IOTF) Body Mass Index Cut-Offs for Thinness, Overweight and Obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battino, M.; Leone, L.; Bompadre, S. High-Performance Liquid Chromatography-EC Assay of Mitochondrial Coenzyme Q9, Coenzyme Q9H2, Coenzyme Q10, Coenzyme Q10H2, and Vitamin E with a Simplified on-Line Solid-Phase Extraction. Methods Enzym. 2004, 378, 156–162. [Google Scholar] [CrossRef]
- Olza, J.; Gil-Campos, M.; Leis, R.; Bueno, G.; Aguilera, C.M.; Valle, M.; Canete, R.; Tojo, R.; Moreno, L.A.; Gil, A.; et al. Presence of the Metabolic Syndrome in Obese Children at Prepubertal Age. Ann. Nutr. Metab. 2011, 58, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Anguita-Ruiz, A.; Mendez-Gutierrez, A.; Ruperez, A.I.; Leis, R.; Bueno, G.; Gil-Campos, M.; Tofe, I.; Gomez-Llorente, C.; Moreno, L.A.; Gil, Á.; et al. The protein S100A4 as a novel marker of insulin resistance in prepubertal and pubertal children with obesity. Metabolism 2020, 105, 154187. [Google Scholar] [CrossRef]
- Ziaee, A.; Esmailzadehha, N.; Oveisi, S.; Ghorbani, A.; Ghanei, L. The threshold value of homeostasis model assessment for insulin resistance in Qazvin Metabolic Diseases Study (QMDS): Assessment of metabolic syndrome. J. Res. Health Sci. 2015, 15, 94–100. [Google Scholar]
- AESAN/BEDCA. Spanish Food Composition Database. Available online: http://www.bedca.net (accessed on 28 September 2019).
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Artwohl, M.; Roden, M.; Waldhäusl, W.; Freudenthaler, A.; Baumgartner-Parzer, S.M. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 2004, 18, 146–148. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aliada, A.; Dandona, P. Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects. Diabetes 2003, 52, 2882–2887. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, M.M.; Zeitler, P.S. Insulin Resistance of Puberty. Curr. Diabetes Rep. 2016, 16, 2039–2044. [Google Scholar] [CrossRef]
- Casado, A.; López-Fernández, M.E. Age-correlated changes of the erythrocyte catalase activity in the Spanish population. Gerontology 2003, 49, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Erden-nal, M.; Sunal, E.; Kanbak, G. Age-related changes in the glutathione redox system. Cell Biochem. Funct. 2002, 20, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Van Ommen, B.; Keijer, J.; Heil, S.G.; Kaput, J. Challenging homeostasis to define biomarkers for nutrition related health. Mol. Nutr. Food Res. 2009, 53, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Gunanti, I.R.; Marks, G.C.; Al-Mamun, A.; Long, K.Z. Low Serum Concentrations of Carotenoids and Vitamin E Are Associated with High Adiposity in Mexican-American Children. J. Nutr. 2014, 144, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Farook, V.S.; Reddivari, L.; Mummidi, S.; Puppala, S.; Arya, R.; Lopez-Alvarenga, J.C.; Fowler, S.P.; Chittoor, G.; Resendez, R.G.; Kumar, B.M.; et al. Genetics of Serum Carotenoid Concentrations and Their Correlation with Obesity-Related Traits in Mexican American Children. Am. J. Clin. Nutr. 2017, 106, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Peng, R.; Cao, J.; Kang, Y.; Qu, P.; Liu, Y.; Xiao, X.; Li, T. Serum Vitamin A Status Is Associated with Obesity and the Metabolic Syndrome among School-Age Children in Chongqing, China. Asian Pac. J. Clin. Nutr. 2016, 25, 563–570. [Google Scholar] [CrossRef]
- Suano de Souza, F.I.; Silverio Amancio, O.M.; Saccardo Sarni, R.O.; Sacchi Pitta, T.; Fernandes, A.P.; Affonso Fonseca, F.L.; Hix, S.; Ramalho, R.A. Non-Alcoholic Fatty Liver Disease in Overweight Children and Its Relationship with Retinol Serum Levels. Int. J. Vitam. Nutr. Res. 2008, 78, 27–32. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Shroff, M.R.; Chen, X.; Beydoun, H.A.; Wang, Y.; Zonderman, A.B. Serum Antioxidant Status Is Associated with Metabolic Syndrome among U.S. Adults in Recent National Surveys. J. Nutr. 2011, 141, 903–913. [Google Scholar] [CrossRef] [PubMed]
- De Dios, O.; Navarro, P.; Ortega-Senovilla, H.; Herrero, L.; Gavela-Perez, T.; Soriano-Guillen, L.; Lasuncion, M.A.; Garces, C. Plasma Retinol Levels and High-Sensitivity C-Reactive Protein in Prepubertal Children. Nutrients 2018, 10, 1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morinobu, T.; Murata, T.; Takaya, R.; Tamai, H. Nutritional Status of Beta-Carotene, Alpha-Tocopherol and Retinol in Obese Children. Int. J. Vitam. Nutr. Res. 2002, 72, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Osth, M.; Ost, A.; Kjolhede, P.; Stralfors, P. The Concentration of Beta-Carotene in Human Adipocytes, but Not the Whole-Body Adipocyte Stores, Is Reduced in Obesity. PLoS ONE 2014, 9, e85610. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.S. Comparison of Serum Concentrations of Alpha-Tocopherol and Beta-Carotene in a Cross-Sectional Sample of Obese and Nonobese Children (NHANES III). National Health and Nutrition Examination Survey. J. Pediatr. 1999, 134, 160–165. [Google Scholar] [CrossRef]
- Burrows, T.L.; Warren, J.M.; Colyvas, K.; Garg, M.L.; Collins, C.E. Validation of Overweight Children’s Fruit and Vegetable Intake Using Plasma Carotenoids. Obesity 2009, 17, 162–168. [Google Scholar] [CrossRef]
- Gil, A. Tratado de Nutrición. Bases Fisiológicas y Bioquímicas de la Nutrición, 3rd ed.; Editorial Médica Panamericana: Madrid, Spain, 2017. [Google Scholar]
- Stenzel, A.P.; Carvalho, R.; Jesus, P.; Bull, A.; Pereira, S.; Saboya, C.; Ramalho, A. Serum Antioxidant Associations with Metabolic Characteristics in Metabolically Healthy and Unhealthy Adolescents with Severe Obesity: An Observational Study. Nutrients 2018, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Vehapoglu, A.; Turkmen, S.; Goknar, N.; Ozer, O.F. Reduced Antioxidant Capacity and Increased Subclinical Inflammation Markers in Prepubescent Obese Children and Their Relationship with Nutritional Markers and Metabolic Parameters. Redox Rep. 2016, 21, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.S.; Jacobs, D.R.; Sinaiko, A.R.; Moran, A.; Steffen, L.M.; Steinberger, J. Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr. Diabetes 2010, 11, 552–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okur, I.; Tumer, L.; Ezgu, F.S.; Yesilkaya, E.; Aral, A.; Oktar, S.O.; Bideci, A.; Hasanoglu, A. Oxidized Low-Density Lipoprotein Levels and Carotid Intima-Media Thickness as Markers of Early Atherosclerosis in Prepubertal Obese Children. J. Pediatr. Endocrinol. Metab. 2013, 26, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; De Keyzer, D.; Jacobs, D.R. Oxidized LDL and the metabolic syndrome. Future Lipidol. 2008, 3, 637–649. [Google Scholar] [CrossRef]
- Morell-Azanza, L.; Garcia-Calzon, S.; Rendo-Urteaga, T.; Martin-Calvo, N.; Chueca, M.; Martinez, J.A.; Azcona-Sanjulian, M.C.; Marti, A. Serum Oxidized Low-Density Lipoprotein Levels Are Related to Cardiometabolic Risk and Decreased after a Weight Loss Treatment in Obese Children and Adolescents. Pediatr. Diabetes 2017, 18, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.S.; Bong, C.K.; Hye, K.H.; Hyun, S.L. Oxidized LDL activates PAI-1 transcription through autocrine activation of TGF-β signaling in mesangial cells. Kidney Int. 2005, 67, 1743–1752. [Google Scholar] [CrossRef] [Green Version]
- Guerendiain, M.; Mayneris-Perxachs, J.; Montes, R.; Lopez-Belmonte, G.; Martin-Matillas, M.; Castellote, A.I.; Martin-Bautista, E.; Marti, A.; Martinez, J.A.; Moreno, L.; et al. Relation between Plasma Antioxidant Vitamin Levels, Adiposity and Cardio-Metabolic Profile in Adolescents: Effects of a Multidisciplinary Obesity Programme. Clin. Nutr. 2017, 36, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camarillo-Romero, E.; Dominguez-Garcia, M.V.; Amaya-Chavez, A.; Camarillo-Romero, M.D.S.; Talavera-Piña, J.; Huitron-Bravo, G.; Majluf-Cruz, A. Effects of a physical activity program on markers of endothelial dysfunction, oxidative stress, and metabolic status in adolescents with metabolic syndrome. ISRN Endocrinol. 2012, 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Coronel, J.; Pinos, I.; Amengual, J. Beta-Carotene in Obesity Research: Technical Considerations and Current Status of the Field. Nutrients 2019, 11, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canas, J.A.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef] [Green Version]
- Houston, M.C. The Role of Cellular Micronutrient Analysis, Nutraceuticals, Vitamins, Antioxidants and Minerals in the Prevention and Treatment of Hypertension and Cardiovascular Disease. Ther. Adv. Cardiovasc. Dis. 2010, 4, 165–183. [Google Scholar] [CrossRef]
- Ward, N.C.; Wu, J.H.; Clarke, M.W.; Puddey, I.B.; Burke, V.; Croft, K.D.; Hodgson, J.M. The Effect of Vitamin E on Blood Pressure in Individuals with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Hypertens. 2007, 25, 227–234. [Google Scholar] [CrossRef]
- Arani, K.S.; Ardakani, A.T.; Goudarzi, R.M.; Reza TALARI, H.; Hami, K.; Akbari, H.; Akbari, N. Effect of Vitamin E and Metformin on Fatty Liver Disease in Obese Children Randomized Clinical Trial. Iran. J. Public Health 2014, 43, 1417–1423. [Google Scholar]
- Schwab, S.; Zierer, A.; Schneider, A.; Heier, M.; Koenig, W.; Kastenmuller, G.; Waldenberger, M.; Peters, A.; Thorand, B. Vitamin E Supplementation Is Associated with Lower Levels of C-Reactive Protein Only in Higher Dosages and Combined with Other Antioxidants: The Cooperative Health Research in the Region of Augsburg (KORA) F4 Study. Br. J. Nutr. 2015, 113, 1782–1791. [Google Scholar] [CrossRef] [Green Version]
- Codoner-Franch, P.; Lopez-Jaen, A.B.; De La Mano-Hernandez, A.; Sentandreu, E.; Simo-Jorda, R.; Valls-Belles, V. Oxidative Markers in Children with Severe Obesity Following Low-Calorie Diets Supplemented with Mandarin Juice. Acta Paediatr. 2010, 99, 1841–1846. [Google Scholar] [CrossRef]
- Pennant, M.; Steur, M.; Moore, C.; Butterworth, A.; Johnson, L. Comparative Validity of Vitamin C and Carotenoids as Indicators of Fruit and Vegetable Intake: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2015, 114, 1331–1340. [Google Scholar] [CrossRef] [Green Version]

| All | MH | MU | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Prepubertal | ||||||||||
| Retinol (μg/dL) | 705 | 26.2 | 7.0 | 319 | 25.7 | 6.6 | 342 | 26.6 | 7.5 | 0.456 |
| Carotene/TAG 104 | 705 | 20.31 | 19.77 | 319 | 25.97 | 21.94 | 342 | 14.07 | 12.61 | 0.000 |
| Tocopherols/TAG 103 | 706 | 14.3 | 6.4 | 320 | 16.5 | 6.4 | 342 | 12.0 | 5.6 | 0.000 |
| TAC (mM eq. Trolox) | 781 | 1.95 ** | 0.82 | 359 | 2.06 | 0.80 | 375 | 1.86 ** | 0.81 | 0.336 |
| Ox-LDL (mg/L) | 579 | 1.95 | 1.88 | 288 | 1.86 | 1.90 | 259 | 2.01 | 1.81 | 0.002 |
| Pubertal | ||||||||||
| Retinol (μg/dL) | 279 | 30.9 | 8.6 | 102 | 31.6 | 8.6 | 168 | 30.3 | 8.6 | 0.048 |
| Carotene/TAG 104 | 272 | 14.49 | 13.03 | 102 | 19.03 | 14.58 | 163 | 10.90 | 8.54 | 0.000 |
| Tocopherols/TAG 103 | 277 | 12.9 | 6.1 | 102 | 15.4 | 6.6 | 168 | 11.1 | 5.1 | 0.000 |
| TAC (mM eq. Trolox) | 391 | 2.16 ** | 0.90 | 169 | 2.07 | 0.80 | 209 | 2.25 ** | 0.97 | 0.430 |
| Ox-LDL (mg/L) | 189 | 1.51 | 1.62 | 80 | 1.37 | 1.53 | 104 | 1.60 | 1.71 | 0.414 |
| Prepubertal | Pubertal | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MH | MU | MH | MU | |||||||||||
| n | Mean | SD | n | Mean | SD | p | n | Mean | SD | n | Mean | SD | p | |
| Carotene-rich foods Z-score | 122 | −0.02 | 2.79 | 184 | −0.16 | 2.47 | 0.738 | 122 | −0.51 | 2.08 | 162 | 0.11 | 2.25 | 0.152 |
| Cooked vegetables Z-score | 122 | 0.08 | 1.10 | 184 | −0.14 | 0.92 | 0.238 | 122 | −0.17 | 0.74 | 162 | 0.15 | 1.06 | 0.024 |
| Raw vegetables Z-score | 122 | −0.17 | 1.07 | 184 | −0.11 | 0.91 | 0.968 | 122 | −0.15 | 0.93 | 162 | 0.11 | 0.93 | 0.412 |
| Fruit (no sugar) Z-score | 122 | −0.06 | 1.02 | 184 | 0.02 | 0.97 | 0.785 | 122 | −0.14 | 0.93 | 162 | 0.10 | 1.09 | 0.220 |
| Fruit (sugar) Z-score | 122 | 0.01 | 0.93 | 184 | 0.06 | 1.10 | 0.811 | 122 | −0.16 | 0.66 | 162 | −0.07 | 0.83 | 0.464 |
| Juices Z-score | 122 | 0.11 | 1.02 | 184 | 0.02 | 1.00 | 0.790 | 122 | 0.12 | 1.08 | 162 | −0.18 | 0.91 | 0.115 |
| Tocopherol-rich foods Z-score | 122 | 0.34 | 2.11 | 184 | −0.18 | 1.91 | 0.018 | 122 | 0.04 | 1.84 | 162 | 0.14 | 1.77 | 0.354 |
| Nuts + Seeds Z-score | 122 | 0.14 | 1.06 | 184 | −0.18 | 0.88 | 0.029 | 122 | −0.05 | 1.00 | 162 | 0.13 | 1.04 | 0.120 |
| Vegetable oils Z-score | 122 | 0.10 | 1.00 | 184 | −0.03 | 0.98 | 0.374 | 122 | 0.11 | 1.03 | 162 | 0.07 | 0.93 | 0.946 |
| Mayonnaise Z-score | 122 | 0.10 | 1.11 | 184 | 0.04 | 1.09 | 0.144 | 122 | −0.03 | 0.89 | 162 | −0.05 | 0.91 | 0.898 |
| Retinol (μg/dL) | Carotenes/TAG | Tocopherols/TAG | TAC (mM eq.Trolox) | |||||
|---|---|---|---|---|---|---|---|---|
| n | Corr. | n | Corr. | n | Corr. | n | Corr. | |
| Prepubertal children | ||||||||
| Retinol (μg/dL) | ||||||||
| Carotenes/TAG | 700 | 0.063 | ||||||
| Tocopherols/TAG | 700 | 0.200 *** | 700 | 0.532 *** | ||||
| TAC (mM eq.Trolox) | 574 | −0.006 | 574 | −0.011 | 574 | 0.010 | ||
| ox-LDL (mg/L) | 572 | −0.141 *** | 572 | −0.041 | 572 | −0.130 ** | 575 | −0.003 |
| Pubertal children | ||||||||
| Retinol (μg/dL) | ||||||||
| Carotenes/TAG | 266 | 0.120 | ||||||
| Tocopherols/TAG | 272 | 0.316 *** | 266 | 0.598 *** | ||||
| TAC (mM eq.Trolox) | 182 | −0.011 | 181 | −0.011 | 182 | −0.026 | ||
| ox-LDL (mg/L) | 181 | −0.140 | 180 | −0.261 *** | 181 | −0.187 * | 184 | −0.132 |
| Total sample | ||||||||
| Retinol (μg/dL) | ||||||||
| Carotenes/TAG | 971 | 0.075 * | ||||||
| Tocopherols/TAG | 977 | 0.242 *** | 971 | 0.548 *** | ||||
| TAC (mM eq.Trolox) | 761 | −0.026 | 760 | 0.028 | 761 | 0.022 | ||
| ox-LDL (mg/L) | 758 | −0.125 *** | 757 | −0.085 * | 758 | −0.131 *** | 764 | −0.012 |
| SBP (mm Hg) | DBP (mm Hg) | Glucose (mg/dL) | Insulin (mU/L) | HOMA-IR | Chol (mg/dL) | TAG (mg/dL) | HDL-C (mg/dL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Corr. | n | Corr. | n | Corr. | n | Corr. | n | Corr. | n | Corr. | n | Corr. | n | Corr. | |
| Prepubertal children | ||||||||||||||||
| Retinol (μg/dL) | 665 | 0.014 | 665 | −0.051 | 700 | 0.063 | 673 | 0.075 | 673 | 0.071 | 700 | 0.206 *** | 700 | 0.154 *** | 690 | −0.038 |
| Carotenes/TAG * | 665 | −0.004 | 665 | −0.021 | 700 | −0.046 | 673 | −0.223 *** | 673 | −0.210 *** | 700 | 0.050 | 700 | NA | 690 | 0.288 *** |
| Tocopherols/TAG * | 665 | −0.052 | 665 | −0.062 | 700 | −0.095 * | 673 | −0.223 *** | 673 | −0.213 *** | 700 | 0.030 | 700 | NA | 690 | 0.367 *** |
| TAC (mM eq.Trolox) | 735 | −0.103 ** | 735 | −0.031 | 775 | 0.046 | 740 | −0.003 | 739 | 0.006 | 776 | 0.038 | 776 | 0.055 | 774 | −0.057 |
| ox-LDL (mg/L) * | 544 | 0.056 | 544 | 0.074 | 577 | 0.051 | 555 | −0.014 | 555 | −0.013 | 577 | 0.022 | 577 | 0.062 | 575 | −0.110 ** |
| Pubertal children | ||||||||||||||||
| Retinol (μg/dL) | 266 | 0.023 | 266 | 0.099 | 273 | 0.022 | 270 | 0.050 | 270 | 0.045 | 272 | 0.231 *** | 272 | 0.142 * | 267 | 0.167 ** |
| Carotenes/TAG * | 259 | −0.146 | 259 | −0.050 | 266 | −0.085 | 263 | −0.170 ** | 263 | −0.171 ** | 266 | 0.006 | 266 | NA | 261 | 0.285 *** |
| Tocopherols/TAG * | 265 | 0.015 | 265 | 0.014 | 272 | −0.094 | 269 | −0.237 *** | 269 | −0.239 *** | 272 | 0.039 | 272 | NA | 267 | 0.389 *** |
| TAC (mM eq.Trolox) | 376 | −0.063 | 376 | −0.061 | 385 | −0.023 | 377 | 0.009 | 376 | 0.002 | 386 | 0.026 | 386 | 0.162 ** | 382 | −0.118 * |
| ox-LDL (mg/L) * | 179 | 0.009 | 179 | −0.042 | 184 | 0.187 * | 182 | 0.094 | 182 | 0.118 | 184 | −0.068 | 184 | 0.065 | 183 | −0.038 |
| Total sample | ||||||||||||||||
| Retinol (μg/dL) | 936 | 0.036 | 936 | 0.012 | 978 | 0.071 * | 948 | 0.057 | 948 | 0.055 | 977 | 0.217 *** | 977 | 0.142 *** | 962 | −0.006 |
| Carotenes/TAG * | 929 | −0.059 | 929 | −0.050 | 971 | −0.075 * | 941 | −0.189 *** | 941 | −0.185 *** | 971 | 0.031 | 971 | NA | 956 | 0.285 *** |
| Tocopherols/TAG * | 935 | −0.041 | 935 | −0.053 | 977 | −0.113 *** | 947 | −0.236 *** | 947 | −0.233 *** | 977 | 0.026 | 977 | NA | 962 | 0.357 *** |
| TAC (mM eq.Trolox) | 1116 | −0.087 ** | 1116 | −0.052 | 1165 | 0.014 | 1122 | 0.019 | 1120 | 0.020 | 1167 | 0.025 | 1167 | 0.087 ** | 1161 | −0.086 ** |
| ox-LDL (mg/L) * | 728 | 0.043 | 728 | 0.040 | 766 | 0.073 * | 742 | 0.018 | 742 | 0.025 | 766 | −0.002 | 766 | 0.059 | 763 | −0.104 ** |
| hsCRP (mg/L) | HGF (µg/L) | IL-6 (ng/L) | IL-8 (ng/L) | MCP-1 (ng/L) | TNFα (ng/L) | aPAI-1 (µg/L) | tPAI-1 (µg/L) | MMP-9 (µg/L) | MPO (µg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | Beta | p | |
| Prepubertal Children | ||||||||||||||||||||
| Retinol (μg/dL) | −0.149 | 1.2 × 10−5 | 0.087 | 0.049 | - | - | - | - | 0.233 | 4.2 × 10−8 | 0.121 | 0.004 | 0.162 | 2.9 × 10−5 | 0.103 | 0.011 | - | - | −0.166 | 7.3 × 10−5 |
| Carotenes/TAG | - | - | - | - | - | - | - | - | - | - | - | - | −0.096 | 0.044 | −0.190 | 1.5 × 10−5 | - | - | - | - |
| Tocopherols/TAG | - | - | - | - | - | - | - | - | - | - | 0.140 | 0.001 | −0.087 | 0.059 | - | - | −0.137 | 0.002 | - | - |
| TAC (mM eq.Trolox) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | −0.143 | 7.2 × 10−4 |
| ox-LDL (mg/L) | - | - | - | - | - | - | - | - | - | - | - | - | 0.097 | 0.010 | 0.265 | 6.5 × 10−11 | - | - | - | - |
| Pubertal children | ||||||||||||||||||||
| Retinol (μg/dL) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Carotenes/TAG | - | - | - | - | - | - | - | - | - | - | - | - | 0.093 | 0.003 | - | - | - | - | - | - |
| Tocopherols/TAG | - | - | - | - | - | - | −0.319 | 5.2 × 10−5 | - | - | - | - | - | - | - | - | 0.166 | 0.035 | - | - |
| TAC (mM eq.Trolox) | - | - | - | - | 0.181 | 0.020 | - | - | 0.367 | 8.6 × 10−7 | 0.281 | 6.9 × 10−5 | - | - | −0.218 | 0.002 | - | - | - | - |
| ox-LDL (mg/L) | - | - | 0.162 | 0.036 | - | - | - | - | 0.163 | 0.022 | - | - | - | - | 0.350 | 7.7 × 10−7 | - | - | - | - |
| Total sample | ||||||||||||||||||||
| Retinol (μg/dL) | −0.124 | 1.1 × 10−4 | - | - | - | - | - | - | 0.200 | 1.4 × 10−7 | 0.075 | 0.045 | 0.094 | 0.007 | - | - | - | - | −0.102 | 0.008 |
| Carotenes/TAG | - | - | - | - | - | - | - | - | - | - | −0.093 | 0.036 | −0.162 | 9.2 × 10−6 | −0.162 | 1.7 × 10−5 | - | - | - | - |
| Tocopherols/TAG | - | - | - | - | - | - | −0.285 | 0.043 | - | - | 0.121 | 0.005 | - | - | - | - | - | - | - | - |
| TAC (mM eq.Trolox) | - | - | - | - | - | - | - | - | 0.158 | 7.9 × 10−6 | 0.095 | 0.006 | - | - | −0.098 | 0.004 | - | - | −0.116 | 0.002 |
| ox-LDL (mg/L) | - | - | - | - | - | - | - | - | 0.077 | 0.031 | −0.069 | 0.050 | 0.094 | 0.005 | 0.265 | 3.1 × 10−14 | 0.100 | 0.006 | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupérez, A.I.; Mesa, M.D.; Anguita-Ruiz, A.; González-Gil, E.M.; Vázquez-Cobela, R.; Moreno, L.A.; Gil, Á.; Gil-Campos, M.; Leis, R.; Bueno, G.; et al. Antioxidants and Oxidative Stress in Children: Influence of Puberty and Metabolically Unhealthy Status. Antioxidants 2020, 9, 618. https://doi.org/10.3390/antiox9070618
Rupérez AI, Mesa MD, Anguita-Ruiz A, González-Gil EM, Vázquez-Cobela R, Moreno LA, Gil Á, Gil-Campos M, Leis R, Bueno G, et al. Antioxidants and Oxidative Stress in Children: Influence of Puberty and Metabolically Unhealthy Status. Antioxidants. 2020; 9(7):618. https://doi.org/10.3390/antiox9070618
Chicago/Turabian StyleRupérez, Azahara I., María D. Mesa, Augusto Anguita-Ruiz, Esther M. González-Gil, Rocío Vázquez-Cobela, Luis A. Moreno, Ángel Gil, Mercedes Gil-Campos, Rosaura Leis, Gloria Bueno, and et al. 2020. "Antioxidants and Oxidative Stress in Children: Influence of Puberty and Metabolically Unhealthy Status" Antioxidants 9, no. 7: 618. https://doi.org/10.3390/antiox9070618