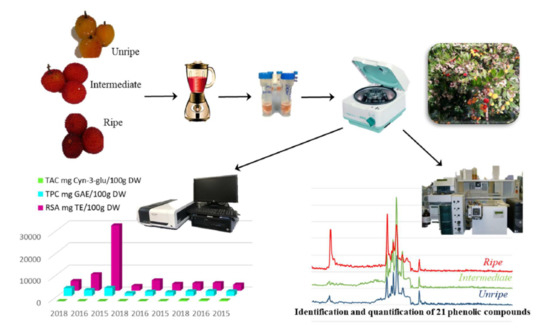
Study of the Phenolic Compound Profile of Arbutus unedo L. Fruits at Different Ripening Stages by HPLC-TQ-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Standard Solutions
2.2. Samples
2.3. Conditions Used for the Optimization of the Extraction Procedure
2.3.1. Total Phenolic Compounds (TPC)
2.3.2. Free Radical DPPH• Scavenging Activity (RSA)
2.3.3. Total Anthocyanin Compounds (TAC)
2.4. Sample Preparation
2.5. Chromatographic Analysis by HPLC-TQ-MS/MS and Analytical Parameters
2.6. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Extraction Procedure of Phenolic Compounds
3.2. TPC, RSA and TAC of Strawberry Tree Berries at Different Ripening Stages, Harvested in Different Years and at Different Geographical Locations
3.3. Analysis of Phenolic Compounds by HPLC-TQ-MS/MS
3.3.1. Development of the HPLC-TQ-MS/MS Method
3.3.2. Profile of Phenolic Compounds at Different Ripening Stages
3.4. Comparison of the Phenolic Composition Profile with Other Fruits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pawlowska, A.M.; de Leo, M.; Braca, A. Phenolics of Arbutus unedo L. (Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agr. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; del Castillo, M.D.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, M.; Damiani, F.; Gabrielli, L.; Cossignani, L.; Blasi, F.; Marini, F.; Montesano, D.; Maurizi, A.; Ventura, F.; Bosi, A.; et al. Characterization of triacylglycerols in arbutus unedo L. seeds. Ital. J. Food Sci. 2008, 20, 49–56. [Google Scholar]
- Ayaz, F.A.; Kucukislamoglu, M.; Reunanen, M. Sugar, non-volatile and phenolic acids composition of strawberry tree (Arbutus unedo L. var. ellipsoidea) fruits. J. Food Compos. Anal. 2000, 13, 171–177. [Google Scholar] [CrossRef]
- Barros, N.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özcan, M.M.; Hacıseferoğulları, H. The strawberry (Arbutus unedo L.) fruits: Chemical composition, physical properties and mineral contents. J. Food Eng. 2007, 78, 1022–1028. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.A.; Barreiro, M.F.; Rodrigues, A.; Curran, T.P.; Barros, L.; Ferreira, I.C.F.R. Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crop. Prod. 2017, 95, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, R.; Barros, L.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of different enriched phenolic extracts of wild fruits from Northeastern Portugal: A comparative study. Plant Food Hum. Nutr. 2014, 69, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Bnouham, M.; Merhfour, F.Z.; Legssyer, A.; Mekhfi, H.; Maallem, S.; Ziyyat, A. Antihyperglycemic activity of Arbutus unedo, Ammoides pusilla and Thymelaea hirsuta. Pharmazie 2007, 62, 630–632. [Google Scholar] [CrossRef]
- Ziyyat, A.; Boussairi, E. Cardiovascular effects of Arbutus unedo L. in spontaneously hypertensive rats. Phytother. Res. 1998, 12, 110–113. [Google Scholar] [CrossRef]
- El Haouari, M.; López, J.J.; Mekhfi, H.; Rosado, J.A.; Salido, G.M. Antiaggregant effects of Arbutus unedo extracts in human platelets. J. Ethnopharmacol. 2007, 113, 325–331. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M.; Mekhfi, H. Anti-platelet aggregation effects of extracts from Arbutus unedo leaves. Plant Sci. Today 2017, 4, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Mekhfi, H.; ElHaouari, M.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A. Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation. Phytother. Res. 2006, 20, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS analysis and characterization of polyphenols in food. Trac Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Câmara, J.S.; Sierra, I. Two novel strategies in food sample preparation for the analysis of dietary polyphenols: Micro-extraction techniques and new silica-based sorbent materials. Trends Food Sci. Technol. 2020, 98, 167–180. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. The role of phenolic compounds in the fight against cancer- a review. Anti Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Metaanalysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Alonso, M.; Rimbach, G.; Sasai, M.; Nakahara, M.; Matsugo, S.; Uchida, Y.; Rivas-Gonzalo, J.C.; De Pascual-Teresa, S. Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins. Mol. Nutr. Food Res. 2005, 49, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, M.; De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Evaluation of the antioxidant properties of fruits. Food Chem. 2004, 84, 13–18. [Google Scholar] [CrossRef]
- Oliveira, I.; Baptista, P.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J.A. Influence of strawberry tree (Arbutus unedo L.) fruit ripening stage on chemical composition and antioxidant activity. Food Res. Int. 2011, 44, 1401–1407. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.-M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; EEUU: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R.E. Anthocyanins. Characterization and Measurement with UV-Visible Spectroscopy. In Current Protocols in Food Analytical Chemistry; (F1.2.1–F1.2.13); Wrolstad, R.E., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Sharma, P. Cinnamic acid derivatives: A new chapter of various pharmacological activities. J. Chem. Pharm. Res. 2011, 3, 403–423. [Google Scholar]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S. Flavan-3-Ols and proanthocyanidins. In Handbook of Analysis of Active Compounds in Functional Foods; Nollet, N.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Rzeppa, S.; Von Bargen, C.; Bittner, K.; Humpf, H.U. Analysis of Flavan-3-ols and Procyanidins in Food Samples by Reversed Phase High-Performance Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry (RP-HPLC-ESI-MS/MS). J. Agric. Food Chem. 2011, 59, 10594–10603. [Google Scholar] [CrossRef] [PubMed]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of phenolic compounds and antioxidant activity in passion fruit pulp (Passiflora spp.) using a modified QuEChERS method and UHPLC-MS/MS. LWT Food Sci. Technol. 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Gruz, J.; Novak, O.; Strnad, M. Separation, characterization, and quantitation of phenolic acids in a little-known blueberry (Vaccinium arctostaphylos L.) fruit by HPLC-MS. J. Agric. Food Chem. 2005, 53, 8116–8122. [Google Scholar] [CrossRef]



| Harvest Year | Ripening Stage | Moisture (%) | TPC mg GAE/100g DW | RSA mg TE/100g DW | TAC mg Cyn-3-glu/100g DW |
|---|---|---|---|---|---|
| 2018 | Unripe | 71.9 ± 0.5 b | 3473 ± 77 e | 4281 ± 23 cd | 22 ± 7 a |
| Intermediate | 72.5 ± 0.8 b | 1309 ± 99 a | 2258 ± 212 a | 76 ± 8 a | |
| Ripe | 72.9 ± 0.3 b | 2206 ± 129 c | 3568 ± 166 b,c | 380 ± 53 b | |
| 2016 | Unripe | 74.3 ± 0.2 b,c | 2671 ± 335 d | 7476 ± 419 e | 19 ± 7 a |
| Intermediate | 76.1 ± 0.9 c,d | 2018 ± 60 b,c | 4800 ± 161 d | 69 ± 2 a | |
| Ripe | 78 ± 1 d | 2164 ± 176 c | 3446 ± 604 b,c | 348 ± 25 b | |
| 2015 | Unripe | 67.2 ± 0.6 a | 3612 ± 21 e | 29531 ± 288 f | 26 ± 3 a |
| Intermediate | 72 ± 1 b | 1705 ± 23 b | 3093 ± 104 a,b | 84 ± 22 a | |
| Ripe | 72 ± 1 b | 2071 ± 146 b,c | 2719 ± 4 a,b | 345 ± 72 b |
| Origin | Harvest year | Moisture (%) | TPC mg GAE/100g DW | RSA mg TE/100g DW | TAC mg Cyn-3-glu/100g DW |
|---|---|---|---|---|---|
| Móstoles | 2018 | 72.5 ± 0.8 b | 1309 ± 99 a | 2258 ± 212 a | 76 ± 8 ab |
| Losar de la Vera | 2018 | 69.6 ± 0.5 a | 1965 ± 96 b | 3256 ± 281 b | 91 ± 27 b |
| Móstoles | 2016 | 76.1 ± 0.9 c | 2018 ± 60 b | 4800 ± 161 c | 69 ± 2 ab |
| Madrid | 2016 | 77.1 ± 0.9 c | 1943 ± 62 b | 4730 ± 151 c | 72 ± 8 ab |
| Manzanares el Real | 2016 | 71.0 ± 0.5 ab | 1895 ± 25 b | 2338 ± 82 a | 50 ± 12 a |
| Class | Analyte | Rt (min) | Molecular Ion; Product Ions (Coll. Energy, eV) (m/z) | Linear Range (mg L−1) | Linearity; R2 | LOD (mg L−1) | LOQ (mg L−1) |
|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acids | 4-hydroxybenzoic acid | 10.6 | 136.8; 92.9*(16V) | 0.025–10.0 | y = 2.39E8x + 1.71E6; 0.999 | 0.008 | 0.025 |
| Gallic acid | 8.8 | 168.9; 124.7*(14V)/166.7(10V) | 0.05–10.0 | y = 1.90E8 x − 1.16E7; 0.995 | 0.015 | 0.050 | |
| Protocatechuic acid | 8.8 | 152.7; 106.7(26V)/108.7*(14V)/151.6(5V) | 0.025–10.0 | y = 2.27E8x – 7.78E6; 0.999 | 0.008 | 0.025 | |
| Syringic acid | 9.5 | 197.0; 195.7(8V)/180.7*(16V)/158.2(14V)/121.7 (16V) | 0.1–10.0 | y = 6.26E6x – 3.21E5; 0.999 | 0.030 | 0.100 | |
| Vanillic acid | 9.5 | 167.0; 165.7(5V)/151.7*(14V)/122.8(12V) | 0.25–10.0 | y = 8.61E6x – 8.81E5; 0.999 | 0.075 | 0.250 | |
| Hydroxycinnamic acids | p-coumaric acid | 9.9 | 163.0; 160.8(10V)/118.8*(16V)/92.4(32V) | 0.01–10.0 | y = 3.62E8x + 3.08E7; 0.998 | 0.003 | 0.010 |
| Chlorogenic acid | 8.9 | 353.1; 190.7*(16V)/189.5(20V) | 0.025–10.0 | y = 1.43E8x – 7.52E6; 0.999 | 0.008 | 0.025 | |
| Caffeic acid | 9.4 | 178.9; 178.6(8V)/134.7*(16V) | 0.05–10.0 | y = 2.94E8x + 4.86E7; 0.997 | 0.015 | 0.050 | |
| Caftaric acid | 8.8 | 310.9; 178.8(14V)/148.8*(12V)/134.6(26V) | 0.1–10.0 | y = 9.96E7x – 3.64E7; 0.997 | 0.030 | 0.100 | |
| Flavonols | Quercetin | 12.5 | 300.9; 178.6(18.0V)/150.6*(22V) | 0.1–10.0 | y = 2.04E8x + 2.01E7; 0.998 | 0.030 | 0.100 |
| Quercetin 3-β-glucoside | 10.1 | 463.1; 301.0(20V)/299.7*(26V)/270.9(45V) | 0.025–10.0 | y = 3.85E7x – 5.71E6; 0.998 | 0.008 | 0.025 | |
| Rutin | 10.7 | 609.5; 300.1*(34V)/271.3(45V)/150.2(40V) | 0.025–10.0 | y = 5.13E7x + 3.82E6; 0.997 | 0.008 | 0.025 | |
| Kaempferol | 13.4 | 284.9; 283.7(14V)/226.3*(30V) | 0.01–10.0 | y = 3.21E8x + 7.22E6; 0.999 | 0.003 | 0.010 | |
| Flavanols | (+) Catechin | 9.3 | 288.9; 244.7(12V)/108.8*(22V) | 0.1–10.0 | y = 2.14E7x + 9.58E4; 0.999 | 0.030 | 0.100 |
| (−)-Epigallocatechin gallate | 9.6 | 457.1; 168.5(18V)/124.4*(36V) | 0.1–10.0 | y = 6.11E7x – 1.06E7; 0.999 | 0.030 | 0.100 | |
| Epigallocatechin | 8.9 | 304.9; 178.7(14V)/218.4(16V)/166.8(16V)/164.6*(20V) | 0.25–10.0 | y = 6.20E6x – 1.26E6; 0.999 | 0.075 | 0.250 | |
| Procyanidin B2 | 9.3 | 577.2; 425.2(16V)/407.2(22V)/288.8*(24V) | 5.0–200.0 | y = 4.59E6x – 5.26E6; 0.999 | 1.500 | 5.000 | |
| Flavanones | Naringin | 10.5 | 579.1; 270.8(36V)/151.0*(45V) | 0.01–10.0 | y = 2.76E7x – 7.45E5; 0.999 | 0.003 | 0.010 |
| Flavones | Vitexin | 10.0 | 431.1; 429.9(12V)/311.1*(22V)/282.8(34V) | 0.05–10.0 | y = 6.48E6x – 5.88E5; 0.999 | 0.015 | 0.050 |
| Stilbenes | trans-Resveratrol | 11.4; 13.1 (cis) | 226.9; 184.8(12V)/142.8*(32V) | 0.25–10.0 | y = 1.83E7x – 8.94E5; 0.999 | 0.075 | 0.250 |
| trans-Piceid | 10.7; 11.4 (cis) | 390.0; 227.4*(20V) | 0.1–10.0 | y = 1.18E7x – 9.17E4; 0.999 | 0.030 | 0.100 |
| Class | Analyte ([M-H]−1/MS 2,‡) | mg 100 g−1 DW | ||
|---|---|---|---|---|
| Unripe | Intermediate | Ripe | ||
| Hydroxybenzoic acids | 4-hydroxybenzoic acid (136.8/92.9) | 0.50 ± 0.03 a | 0.34 ± 0.02 b | 0.37 ± 0.02 b |
| Gallic acid (168.9/124.7) | 1.4 ± 0.1 a | 2.1 ± 0.2 b | 4.7 ± 0.4 c | |
| Protocatechuic acid (152.7/108.7) | X | X | X | |
| Syringic acid (197.0/180.7) | ✓ | ✓ | 0.63 ± 0.04 | |
| Vanillic acid (167.0/151.7) | X | X | X | |
| Hydroxycinnamic acids | p-coumaric acid (163.0/118.8) | ✓ | ✓ | ✓ |
| Chlorogenic acid (353.1/190.7) | ✓ | ✓ | 0.676 ± 0.005 | |
| Caffeic acid (178.9/134.7) | X | X | X | |
| Caftaric acid (310.9/148.8) | ✓ | ✓ | ✓ | |
| Flavonols | Quercetin (300.9/150.6) | 0.79 ± 0.01 a | 0.84 ± 0.01 a | 0.84 ± 0.01 a |
| Quercetin 3-β-glucoside (463.1/299.7) | 2.3 ± 0.2 a | 1.7 ± 0.1 b | 2.6 ± 0.2 a | |
| Rutin (609.5/300.1) | 0.43 ± 0.03 a | 0.54 ± 0.04 b | 0.57 ± 0.04 b | |
| Kaempferol (284.9/226.3) | 0.74 ± 0.08 a | 0.39 ± 0.04 b | 0.73 ± 0.09 a | |
| Flavanols | (+) Catequin (288.9/108.8) | 149 ± 19 a | 29 ± 4 b | 28 ± 5 b |
| (-)-Epigallocatechin gallate (457.1/124.4) | 1.83 ± 0.04 | ✓ | ✓ | |
| Epigallocatechin (304.9/164.6) | 26 ± 2 a | 10 ± 1 b | 11 ± 1 b | |
| Procyanidin B2 (577.2/288.8) | 49 ± 2 a | 41 ± 2 b | 36 ± 2 c | |
| Flavanones | Naringin (579.1/151.0) | ✓ | 0.35 ± 0.04 | ✓ |
| Flavones | Vitexin (431.1/311.1) | 0.911 ± 0.004 a | 0.980 ± 0.004 a | 0.895 ± 0.005 a |
| Stilbenes | trans-Resveratrol (226.9/142.8) | ✓ | ✓ | ✓ |
| trans-Piceid (390.0/227.4) | ✓ | ✓ | ✓ | |
| Fruit | Hydroxybenzoic acid | Hydroxycinnamic acids | Flavonols | Flavanols | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GA | 4-HbA | SA | ChA | Q | K | R | C | Ec | PB2 | ||
| Apple | - | - | - | - | - | - | - | 8.5 ± 0.7 | 47 ± 1 | 70 ± 4 | [36] |
| Apricot | - | - | - | - | - | - | - | 21.12 ± 0.06 | 39 ± 2 | 26 ± 2 | [36] |
| Peach | - | - | - | - | - | - | - | 14.4 ± 0.8 | 0.70 ± 0.05 | 0.45 ± 0.01 | [36] |
| Pear | - | - | - | - | - | - | - | 3.64 ± 0.05 | 38 ± 1 | 16 ± 1 | [36] |
| Passion fruit | - | - | - | - | - | - | - | 0.06 ± 0.01 | 4.0 ± 0.4 | 0.15 ± 0.01 | [36] |
| - | 0.044 ± 0.004 | - | 0.07 ± 0.01 | 0.149 ± 0.002 | - | 0.08 ± 0.01 | - | - | - | [37] | |
| Blackberry | - | - | - | - | - | - | - | 6.7 ± 0.6 | 152 ± 2 | 37 ± 2 | [36] |
| Blueberry | - | - | - | - | - | - | - | 5.1 ± 0.2 | 21.7 ± 0.9 | 21 ± 1 | [36] |
| - Pink-lemonade | 0.54 ± 0.03 | 7 ± 1 | - | 331 ± 3 | 1.54 ± 0.02 | - | - | 26.0 ± 0.5 | - | 6.6 ± 0.3 | [38] |
| - Pink-Champagne | 0.33 ± 0.01 | 3.7 ± 0.2 | - | 149 ± 2 | 0.56 ± 0.01 | - | - | 13.1 ± 0.7 | - | 5.4 ± 0.1 | [38] |
| - Florida Rose | 0.28 ± 0.03 | 6.3 ± 0.4 | - | 270 ± 3 | 0.75 ± 0.03 | 44 ± 3 | - | 20.3 ± 0.2 | - | 9.9 ± 0.4 | [38] |
| - US 2117 | 0.26 ± 0.01 | 5.1 ± 0.6 | - | 259 ± 4 | 1.31 ± 0.01 | 27.1 ± 0.7 | - | 20.4 ± 0.6 | - | 5.10 ± 0.05 | [38] |
| - US 2211 | 0.01 ± 0.01 | 5.2 ± 0.2 | - | 208.8 ± 2.6 | 0.78 ± 0.05 | 7.95 ± 0.04 | - | 17.9 ± 0.4 | - | 3.0 ± 0.1 | [38] |
| - US 2235 | 0.15 ± 0.02 | 3.9 ± 0.1 | - | 302 ± 3 | 0.57 ± 0.03 | 47 ± 4 | - | 13.93 ± 0.07 | - | 2.51 ± 0.01 | [38] |
| - Lowbush | 0.35 ± 0.05 | 7 ± 1 | - | 415 ± 8 | 1.22 ± 0.04 | - | - | 11.8 ± 0.3 | - | 7.2 ± 0.4 | [38] |
| - V. arctostaphylos L. | 0.05 ± 0.01 | 0.49 ± 0.01 | 0.90 ± 0.02 | - | - | - | - | - | - | - | [39] |
| Cranberry | - | - | - | - | - | - | - | 4.06 ± 0.08 | 32 ± 1 | 25 ± 1 | [36] |
| Currant | - | - | - | - | - | - | - | 8.5 ± 0.5 | 3.13 ± 0.04 | 0.78 ± 0.02 | [36] |
| Gooseberry | - | - | - | - | - | - | - | 18 ± 1 | 4.23 ± 0.08 | 2.5 ± 0.1 | [36] |
| Raspberry | - | - | - | - | - | - | - | 4.7 ± 0.3 | 52 ± 2 | - | [36] |
| Strawberry | - | - | - | - | - | - | - | 39 ± 2 | 1.27 ± 0.05 | - | [36] |
| Cherry | - | - | - | - | - | - | - | 35 ± 2 | 36.6 ± 0.6 | 20.4 ± 0.4 | [36] |
| Plum | - | - | - | - | - | - | - | 17 ± 1 | 26.2 ± 0.9 | 29 ± 2 | [36] |
| Pomegranate | - | - | - | - | - | - | - | 10.0 ± 0.4 | 0.51 ± 0.03 | 0.23 ± 0.02 | [36] |
| Grape | - | - | - | - | - | - | - | 9.7 ± 0.6 | 2.02 ± 0.09 | 0.80 ± 0.02 | [36] |
| Unripe SWT | 1.4 ± 0.1 | 0.50 ± 0.03 | - | - | 0.79 ± 0.01 | 0.74 ± 0.08 | 0.43 ± 0.03 | 149 ± 19 | - | 49 ± 2 | This work |
| Intermediate SWT | 2.1 ± 0.2 a | 0.34 ± 0.02 | - | - | 0.84 ± 0.01 | 0.39 ± 0.04 | 0.54 ± 0.04 | 29 ± 4 | - | 41 ± 2 | This work |
| Ripe SWT | 4.7 ± 0.4 b | 0.37 ± 0.02 | 0.63 ± 0.04 | 0.676 ± 0.005 | 0.84 ± 0.01 | 0.73 ± 0.09 | 0.57 ± 0.04 | 28 ± 5 | - | 36 ± 2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izcara, S.; Morante-Zarcero, S.; Casado, N.; Sierra, I. Study of the Phenolic Compound Profile of Arbutus unedo L. Fruits at Different Ripening Stages by HPLC-TQ-MS/MS. Appl. Sci. 2021, 11, 11616. https://doi.org/10.3390/app112411616
Izcara S, Morante-Zarcero S, Casado N, Sierra I. Study of the Phenolic Compound Profile of Arbutus unedo L. Fruits at Different Ripening Stages by HPLC-TQ-MS/MS. Applied Sciences. 2021; 11(24):11616. https://doi.org/10.3390/app112411616
Chicago/Turabian StyleIzcara, Sergio, Sonia Morante-Zarcero, Natalia Casado, and Isabel Sierra. 2021. "Study of the Phenolic Compound Profile of Arbutus unedo L. Fruits at Different Ripening Stages by HPLC-TQ-MS/MS" Applied Sciences 11, no. 24: 11616. https://doi.org/10.3390/app112411616