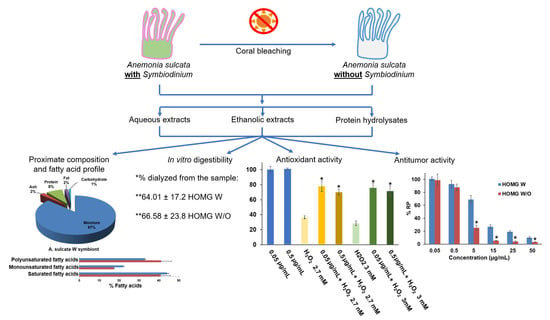
Anemonia sulcata and Its Symbiont Symbiodinium as a Source of Anti-Tumor and Anti-Oxidant Compounds for Colon Cancer Therapy: A Preliminary In Vitro Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Cell Lines and Animals
2.2. Proximate Composition and Fatty Acid Profile
2.3. Anemonia sulcata Crude Extract Preparation W and W/O the Symbiont
2.4. Ethanolic Extraction of Anemonia sulcata W and W/O the Symbiont
2.5. Anemone Protein Hydrolysates
2.6. Mass Spectrophotometry Analysis
2.7. In Vitro Digestibility of the Crude Extracts
2.8. Antioxidant Activity of the Samples
2.8.1. Quantification of Total Polyphenols
2.8.2. Free Radicals’ Uptake/Retention (ABTS)
2.8.3. Antioxidant Activity in Cultured Cells
2.9. Antiproliferative Activity in CRC Cells
2.10. Cell Cycle and Apoptosis Assay
2.11. Wound Healing Assay
2.12. Multicellular Tumor Spheroids Antitumor Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition and Fatty Acid Profile
3.2. Anemone Protein Hydrolysates
3.3. Mass Spectrophotometry Analysis
3.4. In Vitro Digestibility
3.5. Antioxidant Activity of the Samples
3.5.1. Quantification of Total Polyphenols
3.5.2. Assessment of Free Radicals’ Uptake/Retention
3.5.3. Antioxidant Activity in Cultured Cells
3.6. Antitumor Activity
3.6.1. Antiproliferative Effect in Cultured Cells
3.6.2. Cell Cycle Assay
3.6.3. Wound Healing Assay
3.6.4. Multicellular Tumor Spheroids Antitumor Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| W/O | without |
| CRC | colorectal cancer |
| MNPs | marine natural products |
| MDR | multidrug resistance |
| HMEC | Human Microvascular Endothelial Cells |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| DMSO | dimethyl sulfoxide |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| CCK-8 | Cell Counting Kit-8 |
| ATCC | American Type Culture Collection |
| CIC-UGR | Centre for Scientific Instrumentation of the University of Granada |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| PH | protein hydrolysate |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| HOMG | crude homogenates |
| UPLC | ultra-performance liquid chromatography |
| QTOF | quadrupole-time-of-flight |
| MS | mass fragments |
| RT | retention times |
| GAE | gallic acid equivalent |
| %RP | relative proliferation |
| ETOH | ethanolic extracts |
| IC50 | half-maximal inhibitory concentration |
| MTS | multicellular tumor spheroids |
| SD | standard deviation |
| PUFA | polyunsaturated fatty acids |
| SFA | saturated fatty acids |
| EPA | 5,8,11,14,17-eicosapentaenoic acid |
| DHA | cis-4,7,10,13,16,19-docosahexaenoic acid |
| MW | molecular weight |
| DIAL | dialyzed |
| RET | retained |
| ROS | reactive oxygen species |
| FITC | fluorescein |
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Nweze, J.A.; Mbaoji, F.N.; Huang, G.; Li, Y.; Yang, L.; Zhang, Y.; Huang, S.; Pan, L.; Yang, D. Antibiotics development and the potentials of marine-derived compounds to stem the tide of multidrug-resistant pathogenic Bacteria, Fungi, and Protozoa. Mar. Drugs 2020, 18, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.J. Natural Products from Marine Fungi. Mar. Drugs 2020, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.R.M.; Bezerra, W.P.; Souto, J.T. Marine alkaloids with anti-inflammatory activity: Current knowledge and future perspectives. Mar. Drugs 2020, 18, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stien, D. Marine microbial diversity as a source of bioactive natural products. Mar. Drugs 2020, 18, 215. [Google Scholar] [CrossRef]
- Etman, S.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Fucoidan, a natural biopolymer in cancer combating: From edible algae to nanocarrier tailoring. Int. J. Biol. Macromol. 2020, 147, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Hwang, P.-A. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin. Transl. Med. 2019, 8, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. World Health Organization Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 17 June 2019).
- Ruiz-Torres, V.; Rodríguez-Pérez, C.; Herranz-López, M.; Martín-García, B.; Gómez-Caravaca, A.-M.; Arráez-Román, D.; Segura-Carretero, A.; Barrajón-Catalán, E.; Micol, V. Marine invertebrate extracts induce colon cancer cell death via ROS-mediated DNA oxidative damage and mitochondrial impairment. Biomolecules 2019, 9, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Enriching Cancer Pharmacology with drugs of marine origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.S.; Adki, K.M. Marine Natural products for multi-targeted cancer treatment: A future insight. Biomed. Pharmacother. 2018, 105, 233–245. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-derived bioactive compounds with anti-lung cancer potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef] [Green Version]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Othman, E.M.; Reimer, A.; Grüne, M.; Kozjak-Pavlovic, V.; Stopper, H.; Hentschel, U.; Abdelmohsen, U.R. Ageloline a, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces Sp. SBT345. Tetrahedron Lett. 2016, 57, 2786–2789. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Chapter 26—Seaweed as a source of novel nutraceuticals: Sulfated polysaccharides and peptides. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Medicinal Foods; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 325–337. [Google Scholar]
- Jin, Q.; Yu, H.; Li, P. The evaluation and utilization of marine-derived bioactive compounds with anti-obesity effect. Curr. Med. Chem. 2018, 25, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef] [PubMed]
- Lewis Ames, C. Medusa: A review of an ancient cnidarian body form. Results Probl. Cell Differ. 2018, 65, 105–136. [Google Scholar] [PubMed]
- Pejin, B.; Mojovic, M.; Savic, A.G. Novel and highly potent antitumour natural products from cnidarians of marine origin. Nat. Prod. Res. 2014, 28, 2237–2244. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860–1886. [Google Scholar] [CrossRef]
- Ruppert, E.E.; Barnes, R.D. Invertebrate Zoology, 6th ed.; Saunders College Publishing: Orlando, FL, USA, 1993; p. 1056. [Google Scholar]
- Silva, T.C.; de Andrade, P.B.; Paiva-Martins, F.; Valentão, P.; Pereira, D.M. In vitro anti-inflammatory and cytotoxic effects of aqueous extracts from the edible sea anemones Anemonia sulcata and Actinia equina. Int. J. Mol. Sci. 2017, 18, 653. [Google Scholar] [CrossRef] [Green Version]
- Frazão, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: An overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef] [Green Version]
- Schweitz, H.; Vincent, J.P.; Barhanin, J.; Frelin, C.; Linden, G.; Hugues, M.; Lazdunski, M. Purification and pharmacological properties of eight sea anemone toxins from Anemonia sulcata, Anthopleura xanthogrammica, Stoichactis giganteus, and Actinodendron plumosum. Biochemistry 1981, 20, 5245–5252. [Google Scholar] [CrossRef]
- Smith, H.G. The significance of the relationship between actinians and zooxanthellae. J. Exp. Biol. 1939, 16, 334–345. [Google Scholar]
- Revel, J.; Massi, L.; Mehiri, M.; Boutoute, M.; Mayzaud, P.; Capron, L.; Sabourault, C. Differential distribution of lipids in epidermis, gastrodermis and hosted symbiodinium in the sea anemone Anemonia viridis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Bulati, M.; Longo, A.; Masullo, T.; Vlah, S.; Bennici, C.; Bonura, A.; Salamone, M.; Tagliavia, M.; Nicosia, A.; Mazzola, S.; et al. Partially purified extracts of sea anemone Anemonia viridis affect the growth and viability of selected tumour cell lines. BioMed Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Loret, E.P.; Luis, J.; Nuccio, C.; Villard, C.; Mansuelle, P.; Lebrun, R.; Villard, P.H. A low molecular weight protein from the sea anemone Anemonia viridis with an anti-angiogenic activity. Mar. Drugs 2018, 16, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merle, P.-L.; Sabourault, C.; Richier, S.; Allemand, D.; Furla, P. Catalase characterization and implication in bleaching of a symbiotic sea anemone. Free Radic. Biol. Med. 2007, 42, 236–246. [Google Scholar] [CrossRef]
- Pey, A.; Zamoum, T.; Christen, R.; Merle, P.-L.; Furla, P. Characterization of glutathione peroxidase diversity in the symbiotic sea anemone Anemonia viridis. Biochimie 2017, 132, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; Kapravelou, G.; Donaire, A.; Lopez-Chaves, C.; Arrebola, F.; Galisteo, M.; Cantarero, S.; Aranda, P.; Porres, J.M.; López-Jurado, M. Effects of a combined intervention with a lentil protein hydrolysate and a mixed training protocol on the lipid metabolism and hepatic markers of NAFLD in Zucker rats. Food Funct. 2018, 9, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cañizares, F.J.; Galisteo, M.; Porres, J.M. Improvement of the antioxidant and hypolipidaemic effects of cowpea flours (Vigna Unguiculata) by fermentation: Results of in vitro and in vivo experiments. J. Sci. Food Agric. 2015, 95, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Porres, J.M.; Aranda, P.; López-Jurado, M.; Urbano, G. Nutritional potential of raw and free alpha-galactosides lupin (Lupinus albus Var. multolupa) seed flours. Effect of phytase treatment on nitrogen and mineral dialyzability. J. Agric. Food Chem. 2005, 53, 3088–3094. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. (Lond.) 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkondjock, A.; Shatenstein, B.; Maisonneuve, P.; Ghadirian, P. Specific Fatty acids and human colorectal cancer: An overview. Cancer Detect. Prev. 2003, 27, 55–66. [Google Scholar] [CrossRef]
- Sellem, L.; Srour, B.; Guéraud, F.; Pierre, F.; Kesse-Guyot, E.; Fiolet, T.; Lavalette, C.; Egnell, M.; Latino-Martel, P.; Fassier, P.; et al. Saturated, mono- and polyunsaturated fatty acid intake and cancer risk: Results from the French prospective cohort Nutrinet-Santé. Eur. J. Nutr. 2019, 58, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Irún, P.; Lanas, A.; Piazuelo, E. Omega-3 polyunsaturated fatty acids and their bioactive metabolites in gastrointestinal malignancies related to unresolved inflammation. A review. Front. Pharmacol. 2019, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Losantos, R.; Churio, M.S.; Sampedro, D. Computational exploration of the photoprotective potential of gadusol. ChemistryOpen 2015, 4, 155–160. [Google Scholar] [CrossRef]
- D’Auria, M.V.; Riccio, R.; Minale, L. Ophioxanthin, a new marine carototenoid sulphate from the ophiuroid Ophioderma longicaudum. Tetrahedron Lett. 1985, 26, 1871–1872. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [Green Version]
- Dark, G.G.; Hill, S.A.; Prise, V.E.; Tozer, G.M.; Pettit, G.R.; Chaplin, D.J. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997, 57, 1829–1834. [Google Scholar] [PubMed]
- Shi, Y.-W.; Yuan, W.; Wang, X.; Gong, J.; Zhu, S.-X.; Chai, L.-L.; Qi, J.-L.; Qin, Y.-Y.; Gao, Y.; Zhou, Y.-L.; et al. Combretastatin A-4 efficiently inhibits angiogenesis and induces neuronal apoptosis in zebrafish. Sci. Rep. 2016, 6, 30189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A Review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, H.-M.; Chen, B.-A. Anticancer activity and underlying mechanism of neogambogic acid. Chin. J. Nat. Med. 2018, 16, 641–643. [Google Scholar] [CrossRef]
- Vieira-Júnior, G.M.; Dutra, L.A.; Ferreira, P.M.P.; de Moraes, M.O.; Costa Lotufo, L.V.; Pessoa, C.D.Ó.; Torres, R.B.; Boralle, N.; Bolzani, V.d.S.; Cavalheiro, A.J. Cytotoxic clerodane diterpenes from Casearia rupestris. J. Nat. Prod. 2011, 74, 776–781. [Google Scholar] [CrossRef]
- Horiuchi, T.; Fujiki, H.; Suganuma, M.; Hakii, H.; Nakayasu, M.; Hitotsuyanagi, Y.; Aimi, N.; Sakai, S.; Endo, Y.; Shudo, K. Studies on olivoretins indicate a requirement for a free hydroxyl group for teleocidin B activity. Gan 1984, 75, 837–840. [Google Scholar] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Effect of simulated gastrointestinal digestion and fermentation on polyphenolic content and bioactivity of brown seaweed phlorotannin-rich extracts. Mol. Nutr. Food Res. 2017, 61, 1700223. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.-E. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Zeng, F.; Qi, J.; Qin, W.; Tan, C.; Luo, Q.; Wu, D.; Zhang, Q.; Lin, D.; et al. Functional components, antioxidant activity and hypoglycemic ability following simulated gastro-intestinal digestion of pigments from walnut brown shell and green husk. Antioxidants 2019, 8, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef] [PubMed]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Jones, R. Impacts of light limitation on corals and crustose coralline algae. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberty, S.; Furla, P.; Plumier, J.-C. Differential antioxidant response between two symbiodinium species from contrasting environments. Plant Cell Environ. 2016, 39, 2713–2724. [Google Scholar] [CrossRef]
- Wietheger, A.; Starzak, D.E.; Gould, K.S.; Davy, S.K. Differential ROS generation in response to stress in Symbiodinium spp. Biol. Bull. 2018, 234, 11–21. [Google Scholar] [CrossRef]
- Hoepner, C.M.; Abbott, C.A.; Burke da Silva, K. The ecological importance of toxicity: Sea anemones maintain toxic defence when bleached. Toxins 2019, 11, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezanpour, M.; da Silva, K.B.; Sanderson, B.J.S. The effect of sea anemone (H. magnifica) venom on two human breast cancer lines: Death by apoptosis. Cytotechnology 2014, 66, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Ramezanpour, M.; da Silva, K.B.; Sanderson, B.J.S. Venom present in sea anemone (Heteractis magnifica) induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria-mediated pathway. Biotechnol. Lett. 2014, 36, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Xu, H.; Chen, X.; Qiao, G.; Farooqi, A.A.; Tian, Y.; Yuan, R.; Liu, X.; Li, C.; Li, X.; et al. CS5931, A novel marine polypeptide, inhibits migration and invasion of cancer cells via interacting with enolase 1. Recent Patents Anticancer Drug Discov. 2018, 13, 360–367. [Google Scholar] [CrossRef]
- Mudit, M.; El Sayed, K.A. Cancer control potential of marine natural product scaffolds through inhibition of tumor cell migration and invasion. Drug Discov. Today 2016, 21, 1745–1760. [Google Scholar] [CrossRef]
- Fraser, S.P.; Salvador, V.; Manning, E.A.; Mizal, J.; Altun, S.; Raza, M.; Berridge, R.J.; Djamgoz, M.B.A. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: I. lateral motility. J. Cell. Physiol. 2003, 195, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Riffle, S.; Pandey, R.N.; Albert, M.; Hegde, R.S. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer 2017, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Kundu, B.; Silva-Correia, J.; Kundu, S.C.; Oliveira, J.M.; Reis, R.L.; Correlo, V.M. Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol. Ther. 2018, 184, 201–211. [Google Scholar] [CrossRef] [PubMed]








| HOMG W | |||||||||
| RT | PPM | [M-H]- | MF | Compound | MS Fragments | Biological Activity | Ref. | ||
| 0.98 | 2 | 203.056 | C8H12O6 | * Gadusol | 153.0464 | 1,369,942 | 119.042 | Antioxidant | [41] |
| 1.22 | −2.2 | 315.1225 | C18H20O5 | Combretastatin A-4 | 159.0089 | 151.0379 | 120.0161 | Antitumor | [44,45] |
| 2.73 | 4 | 455.2217 | C23H37O7P | * 1-Arachidonoylglycerone 3-phosphate (arachidonic acid -ARA derived) | 267.0494 | 119.0465 | 112.9926 | Unknown | [46] |
| 11.8 | −2.2 | 645.3082 | C38H46O9 | Neogambogic acid | 554.2553 | 553.3141 | 267.0923 | Antitumor | [47] |
| 12.59 | −1 | 599.3214 | C34H48O9 | Casearupestrin A, B | 555.2526 | 373.0900 | 313.1264 | Antitumor | [48] |
| 14.58 | 6 | 603.4449 | C40H60O4 | * Ophioxanthol (carotenoid) | 347.2022 | 327.2592 | 301.2049 | Antioxidant | [42] |
| HOMG W/O | |||||||||
| RT | PPM | [M-H]- | MF | Compound | MS Fragments | Biological Activity | Ref. | ||
| 1.02 | 0 | 203.0556 | C8H12O6 | * Gadusol | 173.0172 | 119.9808 | 112.9946 | Antioxidant | [41] |
| 3.92 | −9.5 | 483.2489 | C32H36O4 | 2-(4-Cyclohexylphenyl)-2-oxoethyl 4-{[4-(2-methyl-2-propanyl)phenoxy] methyl}benzoate | 239.0783 | 119.0519 | 112.995 | Unknown | - |
| 11.44 | −0.9 | 645.3058 | C38H46O9 | Neogambogic acid | 554.2521 | 433.0973 | 239.0723 | Antitumor | [47] |
| 14.33 | −3.7 | 599.3198 | C34H48O9 | Casearupestrin A, B | 554.2556 | 415.2043 | 347.2144 | Antitumor | [48] |
| 14.09 | −9.9 | 464.3231 | C29H43N3O2 | Olivoretin A, B, C, E | 377.2533 | 347.2082 | 267.0982 | Inactive | [49] |
| 14.58 | 2.3 | 603.4427 | C40H60O4 | * Ophioxanthol (carotenoid) | 348.2070 | 301.2073 | 179.0856 | Antioxidant | [42] |
| 15.63 | 3.1 | 835.5445 | C43H80O15 | 3-{[6-O-(α-D-Galactopyranosyl)-β-D-galactopyranosyl]oxy}-1,2-propanediyl ditetradecanoate | 554.255 | 553.3211 | 347.2074 | Unknown | - |
| W Symbiont | W/O Symbiont | |
|---|---|---|
| HOMG | 13.37 ± 0.59 | 13.77 ± 0.13 |
| EXTOH | 12.55 ± 0.19 | 9.4 ± 0.21 |
| PH | 11.23 ± 0.19 | 9.31 ± 0.52 |
| CONTROL DIAL | 5.75 ± 0.45 | 5.87 ± 0.6 |
| CONTROL RET | 4.96 ± 0.27 | 6.17 ± 0.53 |
| HOMG DIAL | 7.26 ± 0.8 | 9.19 ± 0.95 |
| HOMG RET | 6.44 ± 0.3 | 7.75 ± 0.38 |
| W Symbiont | W/O Symbiont | |
|---|---|---|
| HOMG | 4.71 ± 0.13 | 5.21 ± 0.04 |
| EXTOH | 1.97 ± 0.04 | 4.4 ± 0.17 |
| PH | 9.81 ± 0.9 | 4.53 ± 0.2 |
| CONTROL DIAL | 7.73 ± 0.2 | 8.66 ± 0.42 |
| CONTROL RET | 4.08 ± 0.43 | 6.59 ± 0.69 |
| HOMG DIAL | 0 ± 0.51 | 1.52 ± 0.5 |
| HOMG RET | 1.81 ± 0.11 | 0.83 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabeza, L.; Peña, M.; Martínez, R.; Mesas, C.; Galisteo, M.; Perazzoli, G.; Prados, J.; Porres, J.M.; Melguizo, C. Anemonia sulcata and Its Symbiont Symbiodinium as a Source of Anti-Tumor and Anti-Oxidant Compounds for Colon Cancer Therapy: A Preliminary In Vitro Study. Biology 2021, 10, 134. https://doi.org/10.3390/biology10020134
Cabeza L, Peña M, Martínez R, Mesas C, Galisteo M, Perazzoli G, Prados J, Porres JM, Melguizo C. Anemonia sulcata and Its Symbiont Symbiodinium as a Source of Anti-Tumor and Anti-Oxidant Compounds for Colon Cancer Therapy: A Preliminary In Vitro Study. Biology. 2021; 10(2):134. https://doi.org/10.3390/biology10020134
Chicago/Turabian StyleCabeza, Laura, Mercedes Peña, Rosario Martínez, Cristina Mesas, Milagros Galisteo, Gloria Perazzoli, Jose Prados, Jesús M. Porres, and Consolación Melguizo. 2021. "Anemonia sulcata and Its Symbiont Symbiodinium as a Source of Anti-Tumor and Anti-Oxidant Compounds for Colon Cancer Therapy: A Preliminary In Vitro Study" Biology 10, no. 2: 134. https://doi.org/10.3390/biology10020134