Assessment of PDE4 Inhibitor-Induced Hypothermia as a Correlate of Nausea in Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Duration of Ketamine/Xylazine Anesthesia
2.4. Measurement of Core body Temperature
2.5. Data and Statistical Analysis
3. Results
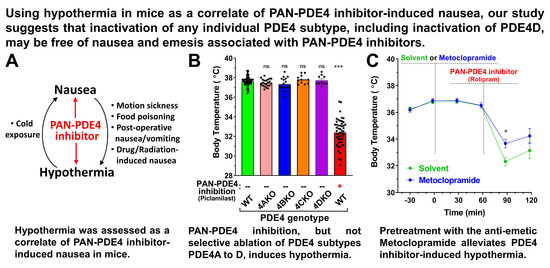
3.1. Selective Ablation of PDE4D, but Not Ablation of PDE4A, PDE4B, or PDE4C Mimics the Effect of PAN-PDE4 Inhibitors to Shorten the Duration of Ketamine/Xylazine-Induced Anesthesia in Mice
3.2. Treatment with PAN-PDE4 Inhibitors Induces Hypothermia in Mice
3.3. PDE4 Inhibitor-Induced Hypothermia Results from the Concurrent Inhibition of Multiple PDE4 Subtypes
3.4. Role of α2-Adrenoceptor Signaling in Body Temperature Regulation
3.5. Treatment with the Antiemetic Metoclopramide Alleviates PAN-PDE4 Inhibitor-Induced Hypothermia in Mice
4. Discussion
4.1. Assessing Hypothermia as a Correlate of PDE4 Inhibitor-Induced Nausea and Emesis in Mice: Comparison of First- and Second-Generation PDE4 Inhibitors
4.2. Role of Individual PDE4 Subtypes in Mediating the Side Effects of PAN-PDE4 Inhibitors: Comparison of Distinct Correlates of Emetic Potential in Mice
4.3. Variable Responses of PDE4D-KO Mice to Hypothermia Induced by First- and Second-Generation PAN-PDE4 Inhibitors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Houslay, M.D.; Schafer, P.; Zhang, K.Y. Keynote review: Phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 2005, 10, 1503–1519. [Google Scholar] [CrossRef]
- Baillie, G.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Paes, D.; Schepers, M.; Rombaut, B.; Hove, D.V.D.; Vanmierlo, T.; Prickaerts, J. The Molecular Biology of Phosphodiesterase 4 Enzymes as Pharmacological Targets: An Interplay of Isoforms, Conformational States, and Inhibitors. Pharmacol. Rev. 2021, 73, 1016–1049. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Beavo, J. Biochemistry and Physiology of Cyclic Nucleotide Phosphodiesterases: Essential Components in Cyclic Nucleotide Signaling. Annu. Rev. Biochem. 2007, 76, 481–511. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kotera, J. Overview of PDEs and Their Regulation. Circ. Res. 2007, 100, 309–327. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef] [Green Version]
- Lugnier, C.; Meyer, A.; Talha, S.; Geny, B. Cyclic nucleotide phosphodiesterases: New targets in the metabolic syndrome? Pharmacol. Ther. 2020, 208, 107475. [Google Scholar] [CrossRef] [PubMed]
- Blokland, A.; Schreiber, R.; Prickaerts, J. Improving memory: A role for phosphodiesterases. Curr. Pharm. Des. 2006, 12, 2511–2523. [Google Scholar] [CrossRef]
- Blokland, A.; Menniti, F.S.; Prickaerts, J. PDE Inhibition and cognition enhancement. Expert Opin. Ther. Patents 2012, 22, 349–354. [Google Scholar] [CrossRef]
- Richter, W.; Menniti, F.S.; Zhang, H.-T.; Conti, M. PDE4 as a target for cognition enhancement. Expert Opin. Ther. Targets 2013, 17, 1011–1027. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-F.; Huang, Y.; Amsdell, S.L.; Xiao, L.; O’Donnell, J.M.; Zhang, H.-T. Antidepressant- and Anxiolytic-like Effects of the Phosphodiesterase-4 Inhibitor Rolipram on Behavior Depend on Cyclic AMP Response Element Binding Protein-Mediated Neurogenesis in the Hippocampus. Neuropsychopharmacology 2009, 34, 2404–2419. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Qi, B.; He, J.; Ke, H.; Shi, J. Advances in the Development of Phosphodiesterase-4 Inhibitors. J. Med. Chem. 2020, 63, 10594–10617. [Google Scholar] [CrossRef] [PubMed]
- Chervinsky, P.; Meltzer, E.; Busse, W.; Ohta, K.; Bardin, P.; Bredenbroker, D.; Bateman, E. Roflumilast for asthma: Safety findings from a pooled analysis of ten clinical studies. Pulm. Pharmacol. Ther. 2015, 35, S28–S34. [Google Scholar] [CrossRef] [PubMed]
- Cada, D.J.; Ingram, K.; Baker, D.E. Apremilast. Hosp. Pharm. 2014, 49, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.L.C.; Richter, W.; Conti, M. Insights into the physiological functions of PDE4 derived from knockout mice. In Cyclic Nucleotide Phosphodiesterases in Health and Disease; Beavo, J.A., Francis, S.H., Houslay, M.D., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 323–346. [Google Scholar]
- Huston, E.; Lynch, M.J.; Mohamed, A.; Collins, D.M.; Hill, E.V.; MacLeod, R.; Krause, E.; Baillie, G.; Houslay, M.D. EPAC and PKA allow cAMP dual control over DNA-PK nuclear translocation. Proc. Natl. Acad. Sci. USA 2008, 105, 12791–12796. [Google Scholar] [CrossRef] [Green Version]
- Boyd, A.; Aragon, I.V.; Saleh, L.A.; Southers, D.; Richter, W. The cAMP-phosphodiesterase 4 (PDE4) controls β-adrenoceptor- and CFTR-dependent saliva secretion in mice. Biochem. J. 2021, 478, 1891–1906. [Google Scholar] [CrossRef]
- Saleh, L.A.; Boyd, A.; Aragon, I.V.; Koloteva, A.; Spadafora, D.; Mneimneh, W.; Barrington, R.A.; Richter, W. Ablation of PDE4B protects from Pseudomonas aeruginosa -induced acute lung injury in mice by ameliorating the cytostorm and associated hypothermia. FASEB J. 2021, 35, e21797. [Google Scholar] [CrossRef]
- Ariga, M.; Neitzert, B.; Nakae, S.; Mottin, G.; Bertrand, C.; Pruniaux, M.P.; Jin, S.-L.C.; Conti, M. Nonredundant Function of Phosphodiesterases 4D and 4B in Neutrophil Recruitment to the Site of Inflammation. J. Immunol. 2004, 173, 7531–7538. [Google Scholar] [CrossRef] [Green Version]
- Hansen, R.T.; Conti, M.; Zhang, H.-T. Mice deficient in phosphodiesterase-4A display anxiogenic-like behavior. Psychopharmacol. 2014, 231, 2941–2954. [Google Scholar] [CrossRef]
- Jin, S.-L.C.; Lan, L.; Zoudilova, M.; Conti, M. Specific Role of Phosphodiesterase 4B in Lipopolysaccharide-Induced Signaling in Mouse Macrophages. J. Immunol. 2005, 175, 1523–1531. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.-L.C.; Goya, S.; Nakae, S.; Wang, D.; Bruss, M.; Hou, C.; Umetsu, D.; Conti, M. Phosphodiesterase 4B is essential for TH2-cell function and development of airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2010, 126, 1252–1259.e12. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.-L.; Lin, S.-C.; Ding, S.-L.; Slc, J.; Sl, D.; Sc, L. Phosphodiesterase 4 and its inhibitors in inflammatory diseases. Biomed. J. 2012, 35, 197–210. [Google Scholar] [CrossRef]
- Leroy, J.; Richter, W.; Mika, D.; Castro, L.R.; Abi-Gerges, A.; Xie, M.; Scheitrum, C.; Lefebvre, F.; Schittl, J.; Mateo, P.; et al. Phosphodiesterase 4B in the cardiac L-type Ca2+ channel complex regulates Ca2+ current and protects against ventricular arrhythmias in mice. J. Clin. Investig. 2011, 121, 2651–2661. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-F.; Cheng, Y.-F.; Huang, Y.; Conti, M.; Wilson, S.P.; O’Donnell, J.; Zhang, H.-T. Phosphodiesterase-4D Knock-Out and RNA Interference-Mediated Knock-Down Enhance Memory and Increase Hippocampal Neurogenesis via Increased cAMP Signaling. J. Neurosci. 2011, 31, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Vagena, E.; Ryu, J.K.; Baeza-Raja, B.; Walsh, N.M.; Syme, C.; Day, J.P.; Houslay, M.D.; Baillie, G.S. A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling. Transl. Psychiatry 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Srivani, P.; Usharani, D.; Jemmis, E.; Sastry, G. Subtype Selectivity in Phosphodiesterase 4 (PDE4): A Bottleneck in Rational Drug Design. Curr. Pharm. Des. 2008, 14, 3854–3872. [Google Scholar] [CrossRef]
- Bobin, P.; Belacel-Ouari, M.; Bedioune, I.; Zhang, L.; Leroy, J.; Leblais, V.; Fischmeister, R.; Vandecasteele, G. Cyclic nucleotide phosphodiesterases in heart and vessels: A therapeutic perspective. Arch. Cardiovasc. Dis. 2016, 109, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Houslay, M.D. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem. Sci. 2010, 35, 91–100. [Google Scholar] [CrossRef]
- Bolger, G.; Conti, M.; Houslay, M. Cellular Functions of PDE4 Enzymes. In Cyclic Nucleotide Phosphodiesterases in Health and Disease; CRC Press: Boca Raton, FL, USA, 2006; pp. 99–130. [Google Scholar]
- Azam, M.A. Selective Phosphodiesterase 4B Inhibitors: A Review. Sci. Pharm. 2014, 82, 453–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhang, H.-T.; O’Donnell, J.M. Phosphodiesterases in the Central Nervous System: Implications in Mood and Cognitive Disorders. Handb. Exp. Pharmacol. 2011, 447–485. [Google Scholar] [CrossRef]
- Reneerkens, O.A.H.; Rutten, K.; Steinbusch, H.W.M.; Blokland, A.; Prickaerts, J. Selective phosphodiesterase inhibitors: A promising target for cognition enhancement. Psychopharmacology 2008, 202, 419–443. [Google Scholar] [CrossRef] [Green Version]
- Kleppisch, T. Phosphodiesterases in the Central Nervous System. Handb. Exp. Pharmacol. 2009, 71–92. [Google Scholar] [CrossRef]
- Tibbo, A.J.; Baillie, G.S. Phosphodiesterase 4B: Master Regulator of Brain Signaling. Cells 2020, 9, 1254. [Google Scholar] [CrossRef]
- Navari, R.M. Managing Nausea and Vomiting in Patients with Cancer: What Works. Oncology (Williston Park) 2018, 32, 121–125, 131, 136. [Google Scholar] [PubMed]
- Horn, C.C. Why is the neurobiology of nausea and vomiting so important? Appetite 2008, 50, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Horn, C.C.; Kimball, B.A.; Wang, H.; Kaus, J.; Dienel, S.; Nagy, A.; Gathright, G.R.; Yates, B.; Andrews, P.L.R. Why Can’t Rodents Vomit? A Comparative Behavioral, Anatomical, and Physiological Study. PLoS ONE 2013, 8, e60537. [Google Scholar] [CrossRef]
- Horn, C.C. Measuring the nausea-to-emesis continuum in non-human animals: Refocusing on gastrointestinal vagal signaling. Exp. Brain Res. 2014, 232, 2471–2481. [Google Scholar] [CrossRef]
- Stern, R.M.; Koch, K.L.; Andrews, P.L.R. Nausea: Mechanisms and Management; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- McDonough, W.; Rich, J.; Aragon, I.V.; Saleh, L.A.; Boyd, A.; Richter, A.; Koloteva, A.; Richter, W. Inhibition of type 4 cAMP-phosphodiesterases (PDE4s) in mice induces hypothermia via effects on behavioral and central autonomous thermoregulation. Biochem. Pharmacol. 2020, 180, 114158. [Google Scholar] [CrossRef]
- A Rudd, J.; Nalivaiko, E.; Matsuki, N.; Wan, C.; Andrews, P.L. The involvement of TRPV1 in emesis and anti-emesis. Temperature 2015, 2, 258–276. [Google Scholar] [CrossRef]
- Nalivaiko, E.; A Rudd, J.; So, R.H. Motion sickness, nausea and thermoregulation: The “toxic” hypothesis. Temperature 2014, 1, 164–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngampramuan, S.; Cerri, M.; Del Vecchio, F.; Corrigan, J.J.; Kamphee, A.; Dragic, A.S.; Rudd, J.A.; Romanovsky, A.A.; Nalivaiko, E. Thermoregulatory correlates of nausea in rats and musk shrews. Oncotarget 2014, 5, 1565–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheshire, W.P., Jr. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nalivaiko, E. Thermoregulation and nausea. Cerebellum: From Embryol. Diagn. Investig. 2018, 156, 445–456. [Google Scholar] [CrossRef]
- Guimaraes, D.D.; Andrews, P.L.R.; A Rudd, J.; Braga, V.D.A.; Nalivaiko, E. Ondansetron and promethazine have differential effects on hypothermic responses to lithium chloride administration and to provocative motion in rats. Temperature 2015, 2, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, S.B. Effect of ondansetron and ICS 205-930 on radiation-induced hypothermia in rats. Radiat. Res. 1997, 147, 741. [Google Scholar] [CrossRef]
- Nobel, G.; Eiken, O.; Tribukait, A.; Kölegård, R.; Mekjavic, I.B. Motion sickness increases the risk of accidental hypothermia. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 98, 48–55. [Google Scholar] [CrossRef]
- Mekjavic, I.B.; Tipton, M.; Gennser, M.; Eiken, O. Motion sickness potentiates core cooling during immersion in humans. J. Physiol. 2001, 535, 619–623. [Google Scholar] [CrossRef]
- Robichaud, A.; Stamatiou, P.B.; Jin, S.-L.; Lachance, N.; Macdonald, D.; Laliberté, F.; Liu, S.; Huang, Z.; Conti, M.; Chan, C.-C. Deletion of phosphodiesterase 4D in mice shortens α2-adrenoceptor–mediated anesthesia, a behavioral correlate of emesis. J. Clin. Investig. 2002, 110, 1045–1052. [Google Scholar] [CrossRef]
- Saldou, N.; Obernolte, R.; Huber, A.; Baecker, P.; Wilhelm, R.; Alvarez, R.; Li, B.; Xia, L.; Callan, O.; Su, C.; et al. Comparison of Recombinant Human PDE4 Isoforms: Interaction with Substrate and Inhibitors. Cell. Signal. 1998, 10, 427–440. [Google Scholar] [CrossRef]
- Aoki, M.; Fukunaga, M.; Sugimoto, T.; Hirano, Y.; Kobayashi, M.; Honda, K.; Yamada, T. Studies on mechanisms of low emetogenicity of YM976, a novel phosphodiesterase type 4 inhibitor. J. Pharmacol. Exp. Ther. 2001, 298, 1142–1149. [Google Scholar] [PubMed]
- Thompson, W. Cyclic nucleotide phosphodiesterases: Pharmacology, biochemistry and function. Pharmacol. Ther. 1991, 51, 13–33. [Google Scholar] [CrossRef]
- Raeburn, D.; Underwood, S.L.; Lewis, S.A.; Woodman, V.R.; Battram, C.H.; Tomkinson, A.; Sharma, S.; Jordan, R.; Souness, J.E.; Webber, S.E.; et al. Anti-inflammatory and bronchodilator properties of RP 73401, a novel and selective phosphodiesterase type IV inhibitor. Br. J. Pharmacol. 1994, 113, 1423–1431. [Google Scholar] [CrossRef] [Green Version]
- Soilness, J.E.; Maslen, C.; Webber, S.; Foster, M.; Raeburn, D.; Palfreyman, M.N.; Ashton, M.J.; Karlsson, J.-A. Suppression of eosinophil function by RP 73401, a potent and selective inhibitor of cyclic AMP-specific phosphodiesterase: Comparison with rolipram. Br. J. Pharmacol. 1995, 115, 39–46. [Google Scholar] [CrossRef]
- Giembycz, M.A.; Field, S.K. Roflumilast: First phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug. Des. Devel. Ther. 2010, 4, 147–158. [Google Scholar]
- Hatzelmann, A.; Morcillo, E.; Lungarella, G.; Adnot, S.; Sanjar, S.; Beume, R.; Schudt, C.; Tenor, H. The preclinical pharmacology of roflumilast—A selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2010, 23, 235–256. [Google Scholar] [CrossRef]
- Bodkhe, S.; Nikam, M.; Sherje, A.P.; Khan, T.; Suvarna, V.; Patel, K. Current insights on clinical efficacy of roflumilast for treatment of COPD, asthma and ACOS. Int. Immunopharmacol. 2020, 88, 106906. [Google Scholar] [CrossRef]
- Jin, S.-L.C.; Conti, M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF- responses. Proc. Natl. Acad. Sci. USA 2002, 99, 7628–7633. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.L.; Richard, F.J.; Kuo, W.P.; D’Ercole, A.J.; Conti, M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc. Natl. Acad. Sci. USA 1999, 96, 11998–12003. [Google Scholar] [CrossRef] [Green Version]
- Robichaud, A.; Savoie, C.; Stamatiou, P.B.; Lachance, N.; Jolicoeur, P.; Rasori, R.; Chan, C.C. Assessing the emetic potential of PDE4 inhibitors in rats. Br. J. Pharmacol. 2002, 135, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Robichaud, A.; Tattersall, F.; Choudhury, I.; Rodger, I. Emesis induced by inhibitors of type IV cyclic nucleotide phosphodiesterase (PDE IV) in the ferret. Neuropharmacology 1999, 38, 289–297. [Google Scholar] [CrossRef]
- Robichaud, A.; Savoie, C.; Stamatiou, P.; Tattersall, F.; Chan, C. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology 2001, 40, 262–269. [Google Scholar] [CrossRef]
- A Giembycz, M. 4D or not 4D—The emetogenic basis of PDE4 inhibitors uncovered? Trends Pharmacol. Sci. 2002, 23, 548. [Google Scholar] [CrossRef]
- Nelissen, E.; van Goethem, N.P.; Bonassoli, V.T.; Heckman, P.R.; van Hagen, B.T.; Suay, D.; Wouters, C.; Prickaerts, J. Validation of the xylazine/ketamine anesthesia test as a predictor of the emetic potential of pharmacological compounds in rats. Neurosci. Lett. 2019, 699, 41–46. [Google Scholar] [CrossRef]
- Hagen, T.J.; Mo, X.; Burgin, A.B.; Fox, D.; Zhang, Z.; Gurney, M.E. Discovery of triazines as selective PDE4B versus PDE4D inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 4031–4034. [Google Scholar] [CrossRef] [Green Version]
- Kranz, M.; Wall, M.; Evans, B.; Miah, A.; Ballantine, S.; Delves, C.; Dombroski, B.; Gross, J.; Schneck, J.; Villa, J.P.; et al. Identification of PDE4B Over 4D subtype-selective inhibitors revealing an unprecedented binding mode. Bioorganic Med. Chem. 2009, 17, 5336–5341. [Google Scholar] [CrossRef]
- Naganuma, K.; Omura, A.; Maekawara, N.; Saitoh, M.; Ohkawa, N.; Kubota, T.; Nagumo, H.; Kodama, T.; Takemura, M.; Ohtsuka, Y.; et al. Discovery of selective PDE4B inhibitors. Bioorganic Med. Chem. Lett. 2009, 19, 3174–3176. [Google Scholar] [CrossRef]
- Suzuki, O.; Mizukami, K.; Etori, M.; Sogawa, Y.; Takagi, N.; Tsuchida, H.; Morimoto, K.; Goto, T.; Yoshino, T.; Mikkaichi, T.; et al. Evaluation of the Therapeutic Index of a Novel Phosphodiesterase 4B–Selective Inhibitor Over Phosphodiesterase 4D in Mice. J. Pharmacol. Sci. 2013, 123, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Contreras, S.; Milara, J.; Morcillo, E.; Cortijo, J. Selective Inhibition of Phosphodiesterases 4A, B, C and D Isoforms in Chronic Respiratory Diseases: Current and Future Evidences. Curr. Pharm. Des. 2017, 23, 2073–2083. [Google Scholar] [CrossRef]
- Clapcote, S.J. Phosphodiesterase-4B as a Therapeutic Target for Cognitive Impairment and Obesity-Related Metabolic Diseases. Adv. Neurobiol. 2017, 17, 103–131. [Google Scholar] [CrossRef]
- Przegaliński, E.; Bigajska, K.; Lewandowska, A. The Influence of Rolipram on the Central Serotoninergic System. Pharmacopsychiatry 1981, 14, 162–166. [Google Scholar] [CrossRef]
- Wachtel, H. Species differences in behavioural effects of rolipram and other adenosine cyclic 3h, 5h-monophosphate phosphodiesterase inhibitors. J. Neural Transm. 1983, 56, 139–152. [Google Scholar] [CrossRef]
- E Souness, J.; Rao, S. Proposal for Pharmacologically Distinct Conformers of PDE4 Cyclic AMP Phosphodiesterases. Cell. Signal. 1997, 9, 227–236. [Google Scholar] [CrossRef]
- Aragon, I.V.; Boyd, A.; Saleh, L.A.; Rich, J.; McDonough, W.; Koloteva, A.; Richter, W. Inhibition of cAMP-phosphodiesterase 4 (PDE4) potentiates the anesthetic effects of Isoflurane in mice. Biochem. Pharmacol. 2021, 186, 114477. [Google Scholar] [CrossRef] [PubMed]
- Sette, C.; Conti, M. Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J. Biol. Chem. 1996, 271, 16526–16534. [Google Scholar] [CrossRef] [Green Version]
- Richter, W.; Conti, M. The Oligomerization State Determines Regulatory Properties and Inhibitor Sensitivity of Type 4 cAMP-specific Phosphodiesterases. J. Biol. Chem. 2004, 279, 30338–30348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duplantier, A.J.; Biggers, M.S.; Chambers, R.J.; Cheng, J.B.; Cooper, K.; Damon, D.B.; Eggler, J.F.; Kraus, K.G.; Marfat, A.; Masamune, H.; et al. Biarylcarboxylic Acids and -amides: Inhibition of Phosphodiesterase Type IV versus [3H]Rolipram Binding Activity and Their Relationship to Emetic Behavior in the Ferret. J. Med. Chem. 1996, 39, 120–125. [Google Scholar] [CrossRef]
- Huang, Z.; Dias, R.; Jones, T.; Liu, S.; Styhler, A.; Claveau, D.; Otu, F.; Ng, K.; Laliberté, F.; Zhang, L.; et al. L-454,560, a potent and selective PDE4 inhibitor with in vivo efficacy in animal models of asthma and cognition. Biochem. Pharmacol. 2007, 73, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- McDonough, W.; Aragon, I.V.; Rich, J.; Murphy, J.M.; Saleh, L.A.; Boyd, A.; Koloteva, A.; Richter, W. PAN-selective inhibition of cAMP-phosphodiesterase 4 (PDE4) induces gastroparesis in mice. FASEB J. 2020, 34, 12533–12548. [Google Scholar] [CrossRef] [PubMed]
- Boomkamp, S.D.; McGrath, M.A.; Houslay, M.; Barnett, S.C. Epac and the high affinity rolipram binding conformer of PDE4 modulate neurite outgrowth and myelination using anin vitrospinal cord injury model. Br. J. Pharmacol. 2014, 171, 2385–2398. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Zhao, Y.; Huang, Y.; Deng, C.; Hopper, A.T.; De Vivo, M.; Rose, G.M.; O’Donnell, J.M. Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology 2006, 186, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.-T.; O’Donnell, J.M. Antidepressant-Induced Increase in High-Affinity Rolipram Binding Sites in Rat Brain: Dependence on Noradrenergic and Serotonergic Function. J. Pharmacol. Exp. Ther. 2003, 307, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; O’Donnell, J.M.; Zhang, H.-T. Inhibitor Binding to Type 4 Phosphodiesterase (PDE4) Assessed Using [3H]Piclamilast and [3H]Rolipram. J. Pharmacol. Exp. Ther. 2003, 305, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Navari, R.M.; Qin, R.; Ruddy, K.J.; Liu, H.; Powell, S.F.; Bajaj, M.; Dietrich, L.; Biggs, D.; Lafky, J.M.; Loprinzi, C.L. Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting. N. Engl. J. Med. 2016, 375, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Houslay, M.D.; Conti, M. Phosphodiesterase Inhibitors: Factors That Influence Potency, Selectivity, and Action. Phosphodiesterases Drug Targets 2011, 47–84. [Google Scholar] [CrossRef]
- Houslay, M.D.; Baillie, G.; Maurice, D.H. cAMP-Specific Phosphodiesterase-4 Enzymes in the Cardiovascular System. Circ. Res. 2007, 100, 950–966. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Huang, Y.; Masood, A.; Stolinski, L.R.; Li, Y.; Zhang, L.; Dlaboga, D.; Jin, S.-L.C.; Conti, M.; O’Donnell, J. Anxiogenic-Like Behavioral Phenotype of Mice Deficient in Phosphodiesterase 4B (PDE4B). Neuropsychopharmacology 2007, 33, 1611–1623. [Google Scholar] [CrossRef] [Green Version]
- Bruss, M.D.; Richter, W.; Horner, K.; Jin, S.-L.C.; Conti, M. Critical Role of PDE4D in β2-Adrenoceptor-dependent cAMP Signaling in Mouse Embryonic Fibroblasts. J. Biol. Chem. 2008, 283, 22430–22442. [Google Scholar] [CrossRef] [Green Version]
- Peter, D.; Jin, S.L.C.; Conti, M.; Hatzelmann, A.; Zitt, C. Differential Expression and Function of Phosphodiesterase 4 (PDE4) Subtypes in Human Primary CD4+ T Cells: Predominant Role of PDE4D. J. Immunol. 2007, 178, 4820–4831. [Google Scholar] [CrossRef] [Green Version]
- Surawski, R.J.; Quinn, D.K. Metoclopramide and Homicidal Ideation: A Case Report and Literature Review. Psychosomatics 2011, 52, 403–409. [Google Scholar] [CrossRef]
- Matsui, A.; Matsuo, H.; Takanaga, H.; Sasaki, S.; Maeda, M.; Sawada, Y. Prediction of catalepsies induced by amiodarone, aprindine and procaine: Similarity in conformation of diethylaminoethyl side chain. J. Pharmacol. Exp. Ther. 1998, 287, 725–732. [Google Scholar]
- Tonini, M.; Candura, S.M.; Messori, E.; Rizzi, C.A. Therapeutic potential of drugs with mixed 5-HT4 agonist/5-HT3 antagonist action in the control of emesis. Pharmacol. Res. 1995, 31, 257–260. [Google Scholar] [CrossRef]
- Burgin, A.B.; Magnusson, O.T.; Singh, J.; Witte, P.; Staker, B.; Bjornsson, J.M.; Thorsteinsdottir, M.; Hrafnsdottir, S.; Hagen, T.; Kiselyov, A.S.; et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010, 28, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, Y.; Chowdhary, A.; Fox, D.; Gurney, M.E.; Zhang, H.-T.; Auerbach, B.D.; Salvi, R.J.; Yang, M.; Li, G.; et al. Memory enhancing effects of BPN14770, an allosteric inhibitor of phosphodiesterase-4D, in wild-type and humanized mice. Neuropsychopharmacology 2018, 43, 2299–2309. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyd, A.; Aragon, I.V.; Rich, J.; McDonough, W.; Oditt, M.; Irelan, D.; Fiedler, E.; Abou Saleh, L.; Richter, W. Assessment of PDE4 Inhibitor-Induced Hypothermia as a Correlate of Nausea in Mice. Biology 2021, 10, 1355. https://doi.org/10.3390/biology10121355
Boyd A, Aragon IV, Rich J, McDonough W, Oditt M, Irelan D, Fiedler E, Abou Saleh L, Richter W. Assessment of PDE4 Inhibitor-Induced Hypothermia as a Correlate of Nausea in Mice. Biology. 2021; 10(12):1355. https://doi.org/10.3390/biology10121355
Chicago/Turabian StyleBoyd, Abigail, Ileana V. Aragon, Justin Rich, Will McDonough, Marianna Oditt, Daniel Irelan, Edward Fiedler, Lina Abou Saleh, and Wito Richter. 2021. "Assessment of PDE4 Inhibitor-Induced Hypothermia as a Correlate of Nausea in Mice" Biology 10, no. 12: 1355. https://doi.org/10.3390/biology10121355