Linking Eco-Energetics and Eco-Hydrology to Select Sites for the Assisted Colonization of Australia’s Rarest Reptile
Abstract
:1. Introduction

2. Results and Discussion
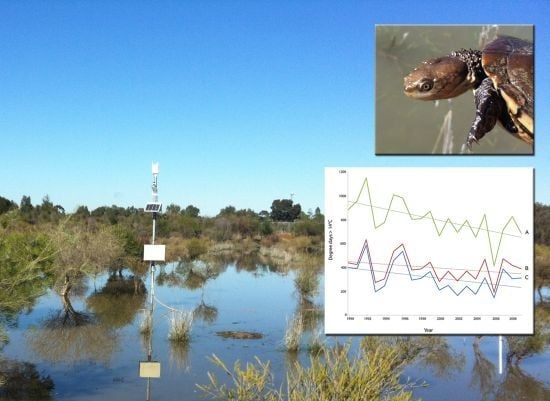
2.1. Model Performance and Assessment of Current Habitat

| Year | Hydroperiod (mo.) | Average water temperature °C |
|---|---|---|
| 1990 | 6.45 | 15.43 |
| 1991 | 6.08 | 15.17 |
| 1992 | 7.77 | 15.78 |
| 1993 | 5.36 | 14.48 |
| 1994 | 5.51 | 14.48 |
| 1995 | 6.74 | 15.17 |
| 1996 | 5.82 | 15.85 |
| 1997 | 5.38 | 14.65 |
| 1998 | 5.61 | 15.14 |
| 1999 | 6.03 | 15.08 |
| 2000 | 5.06 | 14.99 |
| 2001 | 6.51 | 14.93 |
| 2002 | 6.57 | 14.63 |
| 2003 | 6.17 | 14.80 |
| 2004 | 5.67 | 14.53 |
| 2005 | 6.75 | 14.80 |
| 2006 | 3.02 | 15.36 |
| 2007 | 5.09 | 15.10 |
| 2008 | 7.26 | 14.72 |
| 2009 | 4.49 | 14.75 |
| Mean | 5.87 | 14.99 |


2.2. Screening of Wetland Hydroperiods under Current and Future Climates

2.3. Screening of Tortoise Activity Potential under Current and Future Climates

2.4. Implications of Screening on the Selection of Sites for the Assisted Colonization of P. umbrina


2.5. Caveats of the Existing Framework and Future Directions
3. Experimental Section
3.1. Wetland Water Balance and Thermodynamic Model: WET-R






 is the incident solar radiation above the vegetation (W/m2). The last term of Equation 6 accounts for the effect of wetland vegetation shading on the incident solar intensity, with ks defined as a constant to increase the shading effect, and AMAX is the maximum area of inundation extent (m2).
is the incident solar radiation above the vegetation (W/m2). The last term of Equation 6 accounts for the effect of wetland vegetation shading on the incident solar intensity, with ks defined as a constant to increase the shading effect, and AMAX is the maximum area of inundation extent (m2).3.2. WET-R Setup and Calibration
3.3. Modeling the Heat Budget and Activity Constraints of P. umbrina
| Parameter | Value | Source |
|---|---|---|
| Morphological Traits | ||
| Insulative fat layer thickness | 0 | Default value |
| Thermal conductivity of flesh | 0.5 W/mC | Default value |
| Specific heat of flesh | 4,185 J/(kg-K) | Default value |
| Density of flesh | 1,000 kg/m3 | Default value |
| Maximum solar absorptivity | 0.85% | Default value |
| Minimum solar absorptivity | 0.85% | Default value |
| Emissivity of animal | 1 | Default value |
| Reflectance of animal | 0.9 | Assumed |
| Proportion of body surface area in water while basking | 30% | Assumed |
| Configuration factor to sky | 0.4 | Default value |
| Configuration factor to substrate | 0.4 | Default value |
| Physiological Traits | ||
| Voluntary thermal maximum (upper body temperature for foraging, leaving pond, seeking deeper burrows) | 28 °C | [31,50,51,52] |
| Voluntary thermal minimum (lower body temperature for foraging) | 14 °C | [50,52] |
| Temperature difference between expired and inspired air | 0.1 | Default |
| Proportion of surface area acting like a free water surface | 1% | Default |
| Behavioral Traits | ||
| Daily activity | Diurnal * | [51], this study |
| Retreat underground in 80% shade | Yes—max depth of 1 m | GK unpublished |
| Shade seeking | Yes—0 to 80% | [53] |
| Allometric Respiration | ||
| Typical mass of an animal | 6 g (hatchling) | [11] |
| Metabolic Rate | Q = 0.013 M0.810(0.038*Tb) | [54] |
3.4. Regional Screening for Suitable Translocation Sites
4. Conclusions
Acknowledgments
References and Notes
- Skelly, D.K.; Joseph, L.N.; Possingham, H.P.; Kealoha-Freidenburg, L.; Farrugia, T.S.; Kinnison, M.T.; Hendry, A.P. Evolutionary responses to climate change. Conserv. Biol. 2007, 21, 1353–1355. [Google Scholar] [CrossRef]
- Seddon, P.J. From reintroduction to assisted colonization: Moving along the conservation translocation spectrum. Restor. Ecol. 2010, 18, 796–802. [Google Scholar] [CrossRef]
- Ricciardi, A.; Simberloff, D. Assisted colonization: Good intentions and dubious risk assessment. Trends Ecol. Evol. 2009, 24, 476–477. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Soorae, P.; Launay, F.; Walker, S.; Ruiz-Miranda, C.R.; Molur, S.; Koldewey, H.; Kleiman, D.G. The risks of assisted colonization. Conserv. Biol. 2009, 23, 788–789. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Hughes, H.; McIntyre, S.; Lindenmayer, D.B.; Parmesan, C.; Possingham, H.P.; Thomas, C.D. Assisted colonization and rapid climate change. Science 2008, 321, 345–346. [Google Scholar] [CrossRef]
- Thomas, C.D. Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol. Amp Evol. 2011, 26, 216–221. [Google Scholar] [CrossRef]
- Weller, R. Boomtown 2050: Scenarios for a Rapidly Growing City; UWA Publishing: Perth, Australia, 2009; p. 9781921401213. [Google Scholar]
- Charles, S.; Silberstein, R.; Teng, J.; Fu, G.; Hodgson, G.; Gabrovsek, C.; Crute, J.; Chiew, F.; Smith, I.; Kirono, D.; et al. Climate Analyses for South-West Western Australia. Technical Report for the Australian Government from the CSIRO South-West Western Australia Sustainable Yields Project, CSIRO: Perth, Australia, 2010; p. 83. [Google Scholar]
- Petrone, K.C.; Hughes, J.D.; van Niel, T.G.; Silberstein, R.P. Streamflow decline in southwestern Australia. Geophys. Res. Lett. 2010, 37, 1950–2008. [Google Scholar]
- Mitchell, N.J.; Jones, T.; Kuchling, G. Simulated climate change increases juvenile growth in a Critically Endangered tortoise. Endanger. Species Res. 2012, 17, 73–82. [Google Scholar] [CrossRef]
- Burbidge, A.A.; Kuchling, G.; Olejnik, C.; Mutter, L. Western Swamp Tortoise (Pseudemydura umbrina) Recovery Plan, 4th ed; Department of Environment and Conservation: Perth, WA, Australia, 2010. [Google Scholar]
- McFarlane, D.; Strawbridge, M.; Stone, R.; Paton, A. Managing groundwater levels in the face of uncertainty and change: A case study from Gnangara. Water Sci. Technol. 2012, 12, 321–328. [Google Scholar] [CrossRef]
- Kuchling, G.; DeJose, J.P. A captive breeding operation to rescue the critically endangered western swamp turtle Pseudemydura umbrina from extinction. Int. Zoo Yearb. 1989, 28, 103–109. [Google Scholar]
- Bates, B.C.; Hope, P.; Rya, B.; Smith, I.; Charles, S. Key findings from the Indian Ocean climate initiative and their impact on policy development in Australia. Clim. Change 2008, 89, 339–354. [Google Scholar] [CrossRef]
- Stephen, G.W.; Jane, K.H.; Chris, D.T.; David, B.R.; Richard, F.; David, S.B.; Brian, H. Assisted colonization in a changing climate: A test-study using two U.K. Butterflies. Conserv. Lett. 2009, 2, 46–52. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Dormann, C.F.; Schymanski, S.J.; Cabral, J.; Chuine, I.; Graham, C.; Hartig, F.; Kearney, M.; Morin, X.; Römermann, C.; Schröder, B.; et al. Correlation and process in species distribution models: bridging a dichotomy. J. Biogeogr. 2012, 39, 2119–2131. [Google Scholar] [CrossRef]
- Davis, A.J.; Jenkinson, L.S.; Lawton, J.H.; Shorrocks, B.; Wood, S. Making mistakes when predicting shifts in species range in response to global warming. Nature 1998, 391, 783–786. [Google Scholar] [CrossRef]
- Dormann, C.F. Promising the future? Global change projections of species distributions. Basic Appl. Ecol. 2007, 8, 387–397. [Google Scholar] [CrossRef]
- Kearney, M.R.; Wintle, B.A.; Porter, W.P. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 2010, 3, 203–213. [Google Scholar] [CrossRef]
- Chauvenet, A.L.M.; Ewen, J.G.; Armstrong, D.P.; Blackburn, T.M.; Pettorelli, N. Maximizing the success of assisted colonizations. Anim. Conserv. 2012, in press. [Google Scholar]
- Kearney, M.; Porter, W.P. Mapping the fundamental niche: Physiology, climate, and the distribution of a nocturnal lizard. Ecology 2004, 85, 3119–3131. [Google Scholar] [CrossRef]
- Kearney, M.R.; Simpson, S.J.; Raubenheimer, D.; Kooijman, S.A.L.M. Balancing heat, water and nutrients under environmental change: A thermodynamic niche framework. Funct. Ecol. 2012, in press. [Google Scholar]
- Mitchell, N.J.; Kearney, M.R.; Nelson, N.J.; Porter, W.P. Predicting the fate of a living fossil: How will global warming affect sex determination and hatching phenology in tuatara? Proc. Roy. Soc. B Biol. Sci. 2008, 275, 2185–2193. [Google Scholar] [CrossRef]
- Kearney, M.; Phillips, B.L.; Tracy, C.R.; Betts, G.; Porter, W.P. Modelling species distributions without using species distributions: The cane toad in Australia under current and future climates. Ecography 2008, 31, 423–434. [Google Scholar] [CrossRef]
- Wilson, J.N.; Bekessy, S.; Parris, K.M.; Gordon, A.; Heard, G.W.; Wintle, B.A. Impacts of climate change and urban development on the spotted marsh frog (Limnodynastes tasmaniensis). Aust. Ecol. 2012, in press. [Google Scholar]
- Kuchling, G.; Bradshaw, S.D. Ovarian cycle and egg-production of the Western Swamp Tortoise Pseudemydura umbrina (Testudines, Chelidae) in the wild and in captivity. J. Zool. 1993, 229, 405–419. [Google Scholar] [CrossRef]
- Coletti, J.Z.; Hinz, C.; Vogwill, R.; Hipsey, M.R. Wetland ecohydrological response to rainfall variability in a semi-arid climate. 2012. submitted. [Google Scholar]
- Raupach, M.R.; Briggs, P.R.; Haverd, V.; King, E.A.; Paget, M.; Trudinger, C.M. CSIRO AWAP Run 26c Historical Monthly and Annual Model Results for 1900-2009; Bureau of Meteorology and CSIRO: Canberra, Australia, 2011. [Google Scholar]
- Murphy, B.M. Seasonal climate summary southern hemisphere (winter 2006): El niño develops late but australian drought already severe. Aust. Meteorol. Mag. 2007, 56, 111–121. [Google Scholar]
- Burbidge, A.A. The biology of south western Australian tortoises. Ph.D. thesis, The University of Western Australia, Perth, Australia, 1967. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Hajkowicz, S.A. Supporting multi-stakeholder environmental decisions. J. Environ. Manag. 2008, 88, 607–614. [Google Scholar] [CrossRef]
- Dade, M. Identifying translocation sites for the western swamp tortoise (Pseudemydura umbrina) through a multiple criteria analysis. Honours thesis, University of Western Australia, Perth, Australia, 2012. [Google Scholar]
- Hipsey, M.R.; Sivapalan, M.; Menabde, M. A risk-based approach to the design of rural water supply catchments across Western Australia. Hydrol. Sci. J. 2003, 48, 709–727. [Google Scholar] [CrossRef]
- Charles, S.P.; Bates, B.C.; Whetton, P.H.; Hughes, J.P. Validation of downscaling models for changed climate conditions: Case study of southwestern Australia. Clim. Res. 1999, 12, 1–14. [Google Scholar] [CrossRef]
- Coletti, J.Z.; Hinz, C.; Vogwill, R.; Hipsey, M.R. Hydrological controls on carbon metabolism in wetlands. Ecol. Model. 2012. [Google Scholar] [CrossRef]
- Koojman, S.A. Dynamic Energy Budget Theory for Metabolic Organisation; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Kearney, M. Metabolic theory, life history and the distribution of a terrestrial ectotherm. Funct. Ecol. 2012, 26, 167–179. [Google Scholar] [CrossRef]
- Kuchling, G. Nesting of Pseudemydura umbrina (Testudines, Chelidae)-The other way round. Herpetologica 1993, 49, 479–487. [Google Scholar]
- Assessments of the Hydrology of the Western Swamp Tortoise Area; Consultant’s report for Geo and Hydro Environmental Management Pty Ltd.: Perth, Australia, 2011.
- Byrd, R.H.; Lu, P.; Nocedal, J.; Zhu, C. A limited memory algorithm for bound constrained optimization. SIAM J. Sci. Comput. 1995, 16, 1190–1208. [Google Scholar] [CrossRef]
- Belisle, C.J.P. Convergence theorems for a class of simulated annealing algorithms on rd. J. Appl. Prob. 1992, 19, 885–895. [Google Scholar] [CrossRef]
- Porter, W.P.; Mitchell, J.W. System and method for calculating the spatial-temporal and other effects of climate and other environmental conditions on animals. US Patent 2006. [Google Scholar]
- Porter, W.P.; Mitchell, J.W.; Beckman, W.A.; DeWitt, C.B. Behavioral implications of mechanistic ecology. Thermal and behavioral modeling of desert ectotherms and their microenvironment. Oecologia 1973, 13, 1–54. [Google Scholar] [CrossRef]
- Beckman, W.A.; Mitchell, J.W.; Porter, W.P. Thermal model for prediction of a desert iguana’s daily and seasonal behavior. J. Heat Trans. 1973, 95, 257–262. [Google Scholar] [CrossRef]
- Houlder, D.J.; Hutchinson, M.F.; Nix, H.A.; McMahon, J.P. ANUCLIM User Guide, Version 5.0; Centre for Resource and Environmental Studies, Australian National University: Canberra, Australia, 1999. [Google Scholar]
- Raupach, M.R.; Briggs, P.R.; Haverd, V.; King, E.A.; Paget, M.; Trudinger, C.M. Australian Water Availability Project (AWAP), CSIRO Marine and Atmospheric Research Component: Final Report for Phase 3 Cawcr Technical Report No. 013; Centre for Australian Weather and Climate Research (Bureau of Meteorology and CSIRO): Melbourne, Australia, 2009; pp. 1–67. [Google Scholar]
- Gobron, N.; Pinty, B.; Mélin, F.; Taberner, M.; Verstraete, M.M. Sea Wide Field-of-View Sensor (SeaWiFS)-An optimized FAPAR algorithm-Theoretical Basis Document; Institute for Environment and Sustainability: Ispra, Italy, 2002. [Google Scholar]
- Lucas, J.; Burbidge, A.; Graham, M.; Leung, S.; Lindgren, E.; Philips, B. Ecology and conservation of Pseudemydura umbrina Siebenrock 1901. Honours thesis, The University of Western Australia, Perth, Australia, 1963. [Google Scholar]
- Burbidge, A.A. The ecology of the western swamp tortoise Pseudemydura umbrina (testudines, chelidae). Austr. Wildl. Res. 1981, 8, 203–223. [Google Scholar] [CrossRef]
- Leung, S. Temperature responses of two western australian tortoises Pseudemydura umbrina and Chelodina oblonga. Honours thesis, The University of Western Australia, Perth, Australia, 1963. [Google Scholar]
- King, J.M.; Kuchling, G.; Bradshaw, S.D. Thermal environment, behavior, and body condition of wild Pseudemydura umbrina (Testudines: Chelidae) during late winter and early spring. Herpetologica 1998, 54, 103–112. [Google Scholar]
- Andrews, R.M.; Pough, F.H. Metabolism of squamate reptiles: Allometric and ecological relationships. Physiol. Zool. 1985, 58, 214–231. [Google Scholar]
- Arnall, S. The University of Western Australia: Perth, WA, Australia, 2011; Unpublished work.
- Kuchling, G. Department of Environment and Conservation: Wanneroo, WA, Australia, 2012; Unpublished work.
- Burbidge, A.A.; Byrne, M.; Coates, D.; Garnett, S.T.; Harris, S.; Hayward, M.W.; Martin, T.G.; McDonald-Madden, E.; Mitchell, N.J.; Nally, S.; et al. Is Australia ready for assisted colonisation? Policy changes required to facilitate translocation under climate change. Pac. Conserv. Biol. 2011, 17, 259–269. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mitchell, N.; Hipsey, M.R.; Arnall, S.; McGrath, G.; Tareque, H.B.; Kuchling, G.; Vogwill, R.; Sivapalan, M.; Porter, W.P.; Kearney, M.R. Linking Eco-Energetics and Eco-Hydrology to Select Sites for the Assisted Colonization of Australia’s Rarest Reptile. Biology 2013, 2, 1-25. https://doi.org/10.3390/biology2010001
Mitchell N, Hipsey MR, Arnall S, McGrath G, Tareque HB, Kuchling G, Vogwill R, Sivapalan M, Porter WP, Kearney MR. Linking Eco-Energetics and Eco-Hydrology to Select Sites for the Assisted Colonization of Australia’s Rarest Reptile. Biology. 2013; 2(1):1-25. https://doi.org/10.3390/biology2010001
Chicago/Turabian StyleMitchell, Nicola, Matthew R. Hipsey, Sophie Arnall, Gavan McGrath, Hasnein Bin Tareque, Gerald Kuchling, Ryan Vogwill, Murugesu Sivapalan, Warren P. Porter, and Michael R. Kearney. 2013. "Linking Eco-Energetics and Eco-Hydrology to Select Sites for the Assisted Colonization of Australia’s Rarest Reptile" Biology 2, no. 1: 1-25. https://doi.org/10.3390/biology2010001