Fluctuations in Intracellular CheY-P Concentration Coordinate Reversals of Flagellar Motors in E. coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. E. coli Strains, Plasmids, and Cell Growth
2.2. Measurement of Rotation of Multiple Flagellar Motors
2.3. Measurement of the Response Time to the Instantaneously Applied Photoreleased Serine
2.4. Correlation Analysis
2.5. Estimation of Δτcorrelation, ∆τCCW-CW, and ∆τCW-CCW
2.6. Simulation of the Change in the Intracellular Concentration of CheY-P and the Estimation of Response Time for Photoreleased Serine
3. Results
3.1. Coordination of Flagellar Motors under Steady-State Conditions
3.2. Coordination between Flagellar Motors in the Absence of Polar Localization of CheZ
3.3. Distance-Dependent Time Lags in Steady-State Motor Switching
3.4. The Effect of Polar Localization of CheZ on Distance-Dependent Motor Response Times to an Instantaneously Applied Chemoattractant
3.5. Simulation of the Change in CheY-P Concentration and Response Time in the Presence and Absence of Polar Localization of CheZ
4. Discussion
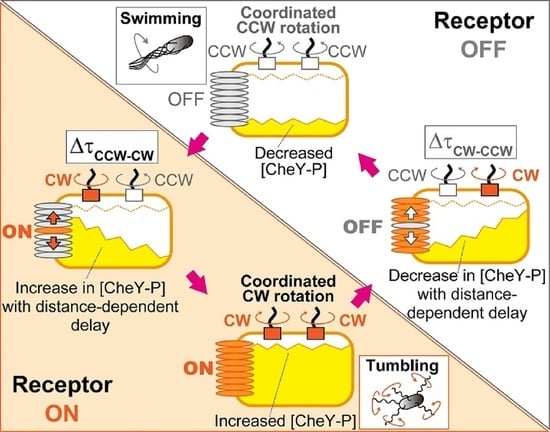
4.1. Mechanism for Coordination of Switching among Flagellar Motors on an E. coli Cell
4.2. Coordinated Motor Switching via Diffusive Signal Propagation from Receptor Arrays
4.3. Behavioral and Evolutionary Implications of the Blinking Array Model
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Segall, J.E.; Ishihara, A.; Berg, H.C. Chemotactic Signaling in Filamentous Cells of Escherichia coli. J. Bacteriol. 1985, 161, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipkow, K. Changing Cellular Location of CheZ Predicted by Molecular Simulations. PLoS Comput. Biol. 2006, 2, e39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kholodenko, B.N. Cell-Signalling Dynamics in Time and Space. Nat. Rev. 2006, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Wadhams, G.H.; Armitage, J.P. Making Sense of It All: Bacterial Chemotaxis. Nat. Rev. 2004, 5, 1024–1037. [Google Scholar] [CrossRef]
- Macnab, R. Flagela and Motility. In Eschericia coli and Salmonella; Neidhardt, F.C., Ed.; American Society for Microbiology: Washington, DC, USA, 1996; pp. 123–145. [Google Scholar]
- Fukuoka, H.; Sagawa, T.; Inoue, Y.; Takahashi, H.; Ishijima, A. Direct Imaging of Intracellular Signaling Components That Regulate Bacterial Chemotaxis. Sci. Signal. 2014, 7, ra32. [Google Scholar] [CrossRef]
- Stewart, R.C. Kinetic Characterization of Phosphotransfer between CheA and CheY in the Bacterial Chemotaxis Signal Transduction Pathway. Biochemistry 1997, 36, 2030–2040. [Google Scholar] [CrossRef]
- Sourjik, V.; Berg, H.C. Binding of the Escherichia coli Response Regulator Chey to Its Target Measured In Vivo by Fluorescence Resonance Energy Transfer. Proc. Natl. Acad. Sci. USA 2002, 99, 12669–12674. [Google Scholar] [CrossRef] [Green Version]
- Bren, A.; Eisenbach, M. The N Terminus of the Flagellar Switch Protein, Flim, Is the Binding Domain for the Chemotactic Response Regulator, CheY. J. Mol. Biol. 1998, 278, 507–514. [Google Scholar] [CrossRef]
- Welch, M.; Oosawa, K.; Aizawa, S.; Eisenbach, M. Phosphorylation-Dependent Binding of a Signal Molecule to the Flagellar Switch of Bacteria. Proc. Natl. Acad. Sci. USA 1993, 90, 8787–8791. [Google Scholar] [CrossRef] [Green Version]
- Cantwell, B.J.; Draheim, R.R.; Weart, R.B.; Nguyen, C.; Stewart, R.C.; Manson, M.D. CheZ Phosphatase Localizes to Chemoreceptor Patches via CheA-Short. J. Bacteriol. 2003, 185, 2354–2361. [Google Scholar] [CrossRef] [Green Version]
- Vaknin, A.; Berg, H.C. Single-Cell FRET Imaging of Phosphatase Activity in the Escherichia coli Chemotaxis System. Proc. Natl. Acad. Sci. USA 2004, 101, 17072–17077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasawa, S.; Fukuoka, H.; Inoue, Y.; Sagawa, T.; Takahashi, H.; Ishijima, A. Coordinated Reversal of Flagellar Motors on a Single Escherichia coli Cell. Biophys. J. 2011, 100, 2193–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkinson, J.S.; Houts, S.E. Isolation and Behavior of Escherichia coli Deletion Mutants Lacking Chemotaxis Functions. J. Bacteriol. 1982, 151, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skidmore, J.M.; Ellefson, D.D.; McNamara, B.P.; Couto, M.M.P.; Wolfe, A.J.; Maddock, J.R. Polar Clustering of the Chemoreceptor Complex in Escherichia coli Occurs in the Absence of Complete CheA Function. J. Bacteriol. 2000, 182, 967–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, W.S.; Berry, R.M.; Berg, H.C. Torque-Generating Units of the Flagellar Motor of Escherichia coli Have a High Duty Ratio. Nature 2000, 403, 444–447. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-Step Inactivation of Chromosomal Genes in Escherichia coli K-12 Using PCR Products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Maloy, S.R.; Nunn, W.D. Selection for Loss of Tetracycline Resistance by Escherichia coli. J. Bacteriol. 1981, 145, 1110–1111. [Google Scholar] [CrossRef] [Green Version]
- Sagawa, T.; Kikuchi, Y.; Inoue, Y.; Takahashi, H.; Muraoka, T.; Kinbara, K.; Ishijima, A.; Fukuoka, H. Single-Cell E. coli Response to an Instantaneously Applied Chemotactic Signal. Biophys. J. 2014, 107, 730–739. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Kennedy, R.A. Forward-Backward Non-Linear Filtering Technique for Extracting Small Biological Signals from Noise. J. Neurosci. Methods 1991, 40, 71–86. [Google Scholar] [CrossRef]
- Li, M.; Hazelbauer, G.L. Cellular Stoichiometry of the Components of the Chemotaxis Signaling Complex. J. Bacteriol. 2004, 186, 3687–3694. [Google Scholar] [CrossRef] [Green Version]
- Cluzel, P. An Ultrasensitive Bacterial Motor Revealed by Monitoring Signaling Proteins in Single Cells. Science 2000, 287, 1652–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.A.; Parkinson, J.S. Overlapping Genes at the CheA Locus of Escherichia coli. Proc. Natl. Acad. Sci. USA 1980, 77, 5370–5374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofoid, E.C.; Parkinson, J.S. Tandem Translation Starts in the CheA Locus of Escherichia coli. J. Bacteriol. 1991, 173, 2116–2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanatinia, H.; Kofoid, E.C.; Morrison, T.B.; Parkinson, J.S. The Smaller of Two Overlapping CheA Gene Products Is Not Essential for Chemotaxis in Escherichia coli. J. Bacteriol. 1995, 177, 2713–2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segall, J.E.; Manson, M.D.; Berg, H.C. Signal Processing Times in Bacterial Chemotaxis. Nature 1982, 296, 855–857. [Google Scholar] [CrossRef]
- Wang, H.; Matsumura, P. Characterization of the CheAS/CheZ Complex: A Specific Interaction Resulting in Enhanced Dephosphorylating Activity on CheY-Phosphate. Mol. Microbiol. 1996, 19, 695–703. [Google Scholar] [CrossRef]
- Bourret, R.B.; Hess, J.F.; Simon, M.I. Conserved Aspartate Residues and Phosphorylation in Signal Transduction by the Chemotaxis Protein CheY. Proc. Natl. Acad. Sci. USA 1990, 87, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Tu, Y. Coordinated Switching of Bacterial Flagellar Motors: Evidence for Direct Motor-Motor Coupling? Phys. Rev. Lett. 2013, 110, 158703. [Google Scholar] [CrossRef] [Green Version]
- Namba, T.; Shibata, T. Propagation of Regulatory Fluctuations Induces Coordinated Switching of Flagellar Motors in Chemotaxis Signaling Pathway of Single Bacteria. J. Theor. Biol. 2018, 454, 367–375. [Google Scholar] [CrossRef]
- Bray, D.; Levin, M.D.; Morton-Firth, C.J. Receptor Clustering as a Cellular Mechanism to Control Sensitivity. Nature 1998, 393, 85–88. [Google Scholar] [CrossRef]
- Shimizu, T.S.; Aksenov, S.; Bray, D. A Spatially Extended Stochastic Model of the Bacterial Chemotaxis Signalling Pathway. J. Mol. Biol. 2003, 329, 291–309. [Google Scholar] [CrossRef]
- Keegstra, J.M.; Kamino, K.; Anquez, F.; Lazova, M.D.; Emonet, T.; Shimizu, T.S. Phenotypic Diversity and Temporal Variability in a Bacterial Signaling Network Revealed by Single-Cell FRET. eLife 2017, 6, e27455. [Google Scholar] [CrossRef] [PubMed]
- Colin, R.; Rosazza, C.; Vaknin, A.; Sourjik, V. Multiple Sources of Slow Activity Fluctuations in a Bacterial Chemosensory Network. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mears, P.J.; Koirala, S.; Rao, C.V.; Golding, I.; Chemla, Y.R. Escherichia coli Swimming Is Robust Against Variations in Flagellar Number. eLife 2014, 3, e01916. [Google Scholar] [CrossRef]
- Turner, L.; Ryu, W.S.; Berg, H.C. Real-Time Imaging of Fluorescent Flagellar Filaments. J. Bacteriol. 2000, 182, 2793–2801. [Google Scholar] [CrossRef] [Green Version]
- Szurmant, H.; Ordal, G.W. Diversity in Chemotaxis Mechanisms among the Bacteria and Archaea. Microbiol. Mol. Biol. Rev. 2004, 68, 301–319. [Google Scholar] [CrossRef] [Green Version]
- Morales, V.M.; Bäckman, A.; Bagdasarian, M. A Series of Wide-Host-Range Low-Copy-Number Vectors That Allow Direct Screening for Recombinants. Gene 1991, 97, 39–47. [Google Scholar] [CrossRef]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight Regulation, Modulation, and High-Level Expression by Vectors Containing the Arabinose PBAD Promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, Y.-S.; Sagawa, T.; Inoue, Y.; Takahashi, H.; Hamamoto, T.; Ishijima, A.; Fukuoka, H. Fluctuations in Intracellular CheY-P Concentration Coordinate Reversals of Flagellar Motors in E. coli. Biomolecules 2020, 10, 1544. https://doi.org/10.3390/biom10111544
Che Y-S, Sagawa T, Inoue Y, Takahashi H, Hamamoto T, Ishijima A, Fukuoka H. Fluctuations in Intracellular CheY-P Concentration Coordinate Reversals of Flagellar Motors in E. coli. Biomolecules. 2020; 10(11):1544. https://doi.org/10.3390/biom10111544
Chicago/Turabian StyleChe, Yong-Suk, Takashi Sagawa, Yuichi Inoue, Hiroto Takahashi, Tatsuki Hamamoto, Akihiko Ishijima, and Hajime Fukuoka. 2020. "Fluctuations in Intracellular CheY-P Concentration Coordinate Reversals of Flagellar Motors in E. coli" Biomolecules 10, no. 11: 1544. https://doi.org/10.3390/biom10111544