Examining the Effects of Anabolic–Androgenic Steroids on Repetitive Mild Traumatic Brain Injury (RmTBI) Outcomes in Adolescent Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
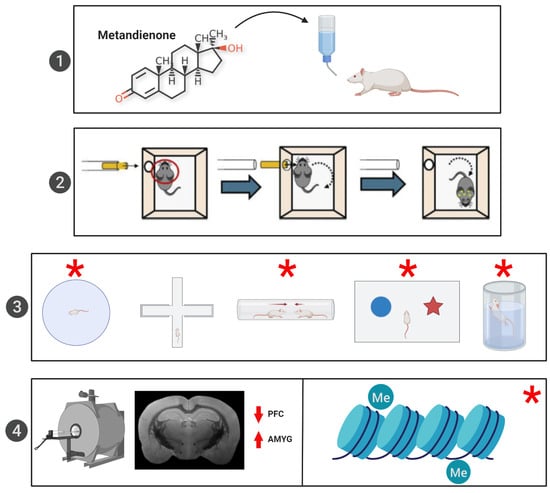
2.2. AAS Administration Protocol
2.3. RmTBI Procedure
2.4. Behavioural Testing
2.5. mRNA Analysis
2.6. MRI Analysis
2.7. Telomere Length Analysis
2.8. Serum Hormone Analysis
2.9. Statistical Analysis
3. Results
3.1. Animal Characteristics
3.2. MRI Volumetric Analysis
3.3. DTI Analysis
3.4. Behavioural Testing
3.5. mRNA Expression
3.6. Telomere Length
3.7. Serum Testosterone Levels
3.8. Serum Growth Hormone Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, M.P. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurology 1995, 45, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talavage, T.M.; Nauman, E.A.; Breedlove, E.L.; Yoruk, U.; Dye, A.E.; Morigaki, K.E.; Feuer, H.; Leverenz, L.J. Functionally-detected cognitive impairm ent in high school football players without clinically-diagnosed concussion. J. Neurotrauma 2014, 31, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, C.; Geburek, A.J.; Rist, F.; Blumenroth, H.; Fischer, B.; Husstedt, I.; Arolt, V.; Schiffbauer, H.; Lohmann, H. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol. Med. 2011, 41, 1197–1211. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Mihalik, J.P. Biomechanics of sport concussion: Quest for the elusive injury threshold. Exerc. Sport Sci. Rev. 2011, 39, 4–11. [Google Scholar] [CrossRef]
- Prins, M.; Giza, C.C. Repeat traumatic brain injury in the developing brain. Int. J. Dev. Neurosci. 2012, 30, 185–190. [Google Scholar] [CrossRef]
- Giza, C.C.; Kolb, B.; Harris, N.G.; Asarnow, R.F.; Prins, M.L. Hitting a moving target: Basic mechanisms of recovery from acquired developmental brain injury. Dev. Neurorehabil. 2009, 12, 255–268. [Google Scholar] [CrossRef]
- Bahrke, M.S.; Yesalis, C.E.; Kopstein, A.N.; Stephens, J.A. Risk factors associated with anabolic-androgenic steroid use among adolescents. Sports Med. 2000, 29, 397–405. [Google Scholar] [CrossRef]
- Sagoe, D.; Molde, H.; Andreassen, C.S.; Torsheim, T.; Pallesen, S. The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Ann. Epidemiol. 2014, 24, 383–398. [Google Scholar] [CrossRef] [Green Version]
- Steidle, C.; Schwartz, S.; Jacoby, K.; Sebree, T.; Smith, T.; Bachand, R.; North American AA2500 T Gel Study Group. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J. Clin. Endocrinol. Metab. 2003, 88, 2673–2681. [Google Scholar] [CrossRef] [Green Version]
- Basaria, S.; Wahlstrom, J.T.; Dobs, A.S. Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J. Clin. Endocrinol. Metab. 2001, 86, 5108–5117. [Google Scholar] [CrossRef] [PubMed]
- Pope, H.G., Jr.; Kanayama, G.; Athey, A.; Ryan, E.; Hudson, J.I.; Baggish, A. The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am. J. Addict. 2014, 23, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, M.J.; Janes, A.C.; Hudson, J.I.; Brennan, B.P.; Kanayama, G.; Kerrigan, A.R.; Jensen, J.E.; Pope, H.G., Jr. Brain and cognition abnormalities in long-term anabolic-androgenic steroid users. Drug Alcohol. Depend. 2015, 152, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, R.C.; Hall, R.C.; Chapman, M.J. Psychiatric complications of anabolic steroid abuse. Psychosomatics 2005, 46, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.S.; Henderson, L.P. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci. Biobehav. Rev. 2003, 27, 413–436. [Google Scholar] [CrossRef]
- Lincoln, A.E.; Caswell, S.V.; Almquist, J.L.; Dunn, R.E.; Norris, J.B.; Hinton, R.Y. Trends in concussion incidence in high school sports: a prospective 11-year study. Am. J. Sports Med. 2011, 39, 958–963. [Google Scholar] [CrossRef]
- Zemper, E.D. Two-year prospective study of relative risk of a second cerebral concussion. Am. J. Phys. Med. Rehabil. 2003, 82, 653–659. [Google Scholar] [CrossRef]
- Germanakis, I.; Tsarouhas, K.; Fragkiadaki, P.; Tsitsimpikou, C.; Goutzourelas, N.; Champsas, M.C.; Stagos, D.; Rentoukas, E.; Tsatsakis, A.M. Oxidative stress and myocardial dysfunction in young rabbits after short term anabolic steroids administration. Food Chem. Toxicol. 2013, 61, 101–105. [Google Scholar] [CrossRef]
- Mychasiuk, R.; Hehar, H.; Ma, I.; Candy, S.; Esser, M.J. Reducing the time interval between concussion and voluntary exercise restores motor impairment, short-term memory, and alterations to gene expression. Eur. J. Neurosci. 2016, 44, 2407–2417. [Google Scholar] [CrossRef] [Green Version]
- Davis, M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992, 15, 353–375. [Google Scholar] [CrossRef]
- Wright, D.; O’Brien, T.; Shultz, S.R.; Mychasiuk, R. Sex matters: Repetitive mild traumatic brain injury in adolescent rats. Ann. Clin. Transl. Neurol. 2017, 4, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Bjørnebekk, A.; Walhovd, K.B.; Jørstad, M.L.; Due-Tønnessen, P.; Hullstein, I.R.; Fjell, A.M. Structural brain imaging of long-term anabolic-androgenic steroid users and nonusing weightlifters. Biol. Psychiatry 2017, 82, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Mychasiuk, R.; Hehar, H.; Candy, S.; Ma, I.; Esser, M.J. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J. Neurosci. Methods 2016, 257, 168–178. [Google Scholar] [CrossRef]
- Hehar, H.; Mychasiuk, R. The use of telomere length as a predictive biomarker for injury prognosis in juvenile rats folowing a concussion/mild traumatic brain injury. Neurobiol. Dis. 2016, 87, 11–18. [Google Scholar] [CrossRef]
- Whishaw, I.Q.; Kolb, B. The Behavior of the Laboratory Rat: A Handbook with Tests; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Lindzey, G.; Winston, H.; Manosevitz, M. Social dominance in inbred mouse strains. Nature 1961, 191, 474–476. [Google Scholar] [CrossRef]
- Spanswick, S.C.; Sutherland, R.J. Object/context-specific memory deficits associated with loss of hippocampal granule cells after adrenalectomy in rats. Learn. Mem. 2010, 17, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Yadid, G.; Overstreet, D.H.; Zangen, A. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res. 2001, 896, 43–47. [Google Scholar] [CrossRef]
- Zilles, K. The Cortex of the Rat: A Stereotaxic Atlas Springer-Verlag. Berl. N. Y. 1985, VI, 1–123. [Google Scholar]
- Wohleb, E.S.; Patterson, J.M.; Sharma, V.; Quan, N.; Godbout, J.P.; Sheridan, J.F. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J. Neurosci. 2014, 34, 2583–2591. [Google Scholar] [CrossRef]
- Aid, T.; Kazantseva, A.; Piirsoo, M.; Palm, K.; Timmusk, T. Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 2007, 85, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Kim, Y.-K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, C.M.; Falo, M.C.; Alonso, O.F.; Bramlett, H.M.; Dietrich, W.D. Deficits in ERK and CREB activation in the hippocampus after traumatic brain injury. Neurosci. Lett. 2009, 459, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metting, Z.; Wilczak, N.; Rodiger, L.; Schaaf, J.; Van Der Naalt, J. GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 2012, 78, 1428–1433. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Avants, B.; Tustison, N.; Song, G.; Cook, P.; Klein, A.; Gee, J. A reproducible evaluation of ANTs similarity metric perfrormance in brain image registration. NeuroImage 2011, 54, 2033–2044. [Google Scholar] [CrossRef] [Green Version]
- Jarow, J.P.; Lipshultz, L.I. Anabolic steroid-induced hypogonadotropic hypogonadism. Am. J. Sports Med. 1990, 18, 429–431. [Google Scholar] [CrossRef]
- Lithgow, K.; Chin, A.; Debert, C.T.; Kline, G.A. Utility of serum IGF-1 for diagnosis of growth hormone deficiency following traumatic brain injury and sport-related concussion. BMC Endocr. Disord. 2018, 18, 20. [Google Scholar] [CrossRef] [Green Version]
- Meinhardt, U.J.; Ho, K.K. Modulation of growth hormone action by sex steroids. Clin. Endocrinol. 2006, 65, 413–422. [Google Scholar] [CrossRef]
- Rose, S.C.; Weber, K.D.; Collen, J.B.; Heyer, G.L. The diagnosis and management of concussion in children and adolescents. Pediatric Neurol. 2015, 53, 108–118. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Epstein, D.J.; Legarreta, M.; Bueler, E.; King, J.; McGlade, E.; Yurgelun-Todd, D. Orbitofrontal cortical thinning and aggression in mild traumatic brain injury patients. Brain Behav. 2016, 6, e00581. [Google Scholar] [CrossRef]
- Kikinis, Z.; Muehlmann, M.; Pasternak, O.; Peled, S.; Kulkarni, P.; Ferris, C.; Bouix, S.; Rathi, Y.; Koerte, I.K.; Pieper, S.; et al. Diffusion imaging of mild traumatic brain injury in the impact accelerated rodent model: A pilot study. Brain Inj. 2017, 31, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Derntl, B.; Windischberger, C.; Robinson, S.; Kryspin-Exner, I.; Gur, R.C.; Moser, E.; Habel, U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology 2009, 34, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.M.; Tang, L.; Thomason, M.E.; Diamond, M.P.; Hariri, A.R.; Carré, J.M. Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol. Psychiatry 2014, 76, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, D.; Fillard, P.; Paradot, G.; Tadie, M.; Lasjaunias, P.; Ducreux, D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. Am. J. Neuroradiol. 2008, 29, 1730–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seitz, J.; Lyall, A.E.; Kanayama, G.; Makris, N.; Hudson, J.I.; Kubicki, M.; Pope, H.G., Jr.; Kaufman, M.J. White matter abnormalities in long-term anabolic-androgenic steroid users: A pilot study. Psychiatry Res. Neuroimaging 2017, 260, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lowe, M.J.; Horenstein, C.; Hirsch, J.G.; Marrie, R.A.; Stone, L.; Bhattacharyya, P.K.; Gass, A.; Phillips, M.D. Functional pathway-defined MRI diffusion measures reveal increased transverse diffusivity of water in multiple sclerosis. Neuroimage 2006, 32, 1127–1133. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [Green Version]
- RF Silva, F.; Cabral, A.; PN Lima, A.; L Dierschnabel, A.; B do Nascimento, E.; T Macedo, P.; S Izidio, G.; M Ribeiro, A.; H Silva, R. The Anabolic Androgenic Steroid Testosterone Propionate Decreases Recognition Memory in Adult Male Rats. Curr. Psychopharmacol. 2013, 2, 247–253. [Google Scholar] [CrossRef]
- Frodl, T.; Meisenzahl, E.; Zetzsche, T.; Bottlender, R.; Born, C.; Groll, C.; Jäger, M.; Leinsinger, G.; Hahn, K.; Möller, H.-J. Enlargement of the amygdala in patients with a first episode of major depression. Biol. Psychiatry 2002, 51, 708–714. [Google Scholar] [CrossRef]
- Aikey, J.L.; Nyby, J.G.; Anmuth, D.M.; James, P.J. Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav. 2002, 42, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Minkin, D.M.; Meyer, M.E.; Van Haaren, F. Behavioral effects of long-term administration of an anabolic steroid in intact and castrated male Wistar rats. Pharmacol. Biochem. Behav. 1993, 44, 959–963. [Google Scholar] [CrossRef]
- Melloni, R.H., Jr.; Connor, D.F.; Hang, P.T.X.; Harrison, R.J.; Ferris, C.F. Anabolic-androgenic steroid exposure during adolescence and aggressive behavior in golden hamsters. Physiol. Behav. 1997, 61, 359–364. [Google Scholar] [CrossRef]
- Morrison, T.R.; Ricci, L.A.; Melloni, R.H., Jr. Anabolic/androgenic steroid administration during adolescence and adulthood differentially modulates aggression and anxiety. Horm. Behav. 2015, 69, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, S.F.; McGinnis, M.Y. Long-term effects of pubertal anabolic–androgenic steroid exposure on reproductive and aggressive behaviors in male rats. Horm. Behav. 2004, 46, 193–203. [Google Scholar] [CrossRef]
- Wallace, T.L.; Stellitano, K.E.; Neve, R.L.; Duman, R.S. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol. Psychiatry 2004, 56, 151–160. [Google Scholar] [CrossRef]
- Odagaki, Y.; Garcı́a-Sevilla, J.A.; Huguelet, P.; La Harpe, R.; Koyama, T.; Guimón, J. Cyclic AMP-mediated signaling components are upregulated in the prefrontal cortex of depressed suicide victims. Brain Res. 2001, 898, 224–231. [Google Scholar] [CrossRef]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 1993, 14, 421–429. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets CNS Neurol. Disord. 2007, 6, 219–233. [Google Scholar] [CrossRef] [Green Version]
- Petraglia, A.L.; Plog, B.A.; Dayawansa, S.; Dashnaw, M.L.; Czerniecka, K.; Walker, C.T.; Chen, M.; Hyrien, O.; Iliff, J.J.; Deane, R.; et al. The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg. Neurol. Int. 2014, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.T.; Schneider, A.; DonCarlos, L.L.; Breedlove, S.M.; Jordan, C.L. Astrocytes in the rat medial amygdala are responsive to adult androgens. J. Comp. Neurol. 2012, 520, 2531–2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.P.; Achua, J.K.; Summers, T.R.; Ronan, P.J.; Summers, C.H. Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Front. Behav. Neurosci. 2014, 8, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldt, S.A.; Zimmermann, K.; Parker, K.; Gaval, M.; Ressler, K.J. BDNF deletion or TrkB impairment in amygdala inhibits both appetitive and aversive learning. J. Neurosci. 2014, 34, 2444–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Pham, L.; Shultz, S.R.; Kim, H.A.; Brady, R.D.; Wortman, R.C.; Genders, S.G.; Hale, M.W.; O’Shea, R.D.; Djouma, E.; van den Buuse, M. Mild closed-head injury in conscious rats causes transient neurobehavioral and glial disturbances: a novel experimental model of concussion. J. Neurotrauma 2019, 36, 2260–2271. [Google Scholar] [CrossRef]
- Jasnow, A.M.; Shi, C.; Israel, J.E.; Davis, M.; Huhman, K.L. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav. Neurosci. 2005, 119, 1125. [Google Scholar] [CrossRef]
- Kota, L.N.; Bharath, S.; Purushottam, M.; Moily, N.S.; Sivakumar, P.T.; Varghese, M.; Pal, P.K.; Jain, S. Reduced telomere length in neurodegenerative disorders may suggest shared biology. J. Neuropsychiatry Clin. Neurosci. 2015, 27, e92–e96. [Google Scholar] [CrossRef]
- Greco, T.; Hovda, D.; Prins, M. The effects of repeat traumatic brain injury on the pituitary in adolescent rats. J. Neurotrauma 2013, 30, 1983–1990. [Google Scholar] [CrossRef] [Green Version]
- Van den Berghe, G. Novel insights into the neuroendocrinology of critical illness. Eur. J. Endocrinol. 2000, 143, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Christophidis, L.J.; Gorba, T.; Gustavsson, M.; Williams, C.E.; Werther, G.A.; Russo, V.C.; Scheepens, A. Growth hormone receptor immunoreactivity is increased in the subventricular zone of juvenile rat brain after focal ischemia: a potential role for growth hormone in injury-induced neurogenesis. Growth Horm. IGF Res. 2009, 19, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Salberg, S.; Christensen, J.; Yamakawa, G.R.; Lengkeek, C.; Malik, H.; Tabor, J.; Hazari, A.; Mychasiuk, R. A bump on the head or late to bed: Behavioral and pathophysiological effects of sleep deprivation after repetitive mild traumatic brain injury in adolescent rats. J. Neurotrauma 2018, 35, 1895–1905. [Google Scholar] [CrossRef] [PubMed]




| MRI Measure | Brain Region | Effect of RmTBI F (p) | Effect of Steroid Treatment F (p) | Steroid Treatment x RmTBI F (p) |
|---|---|---|---|---|
| Volumetric Analysis | IL PFC | 0.12 (0.73) | 7.37 (0.01) | 0.01 (0.91) |
| CL PFC | 4.43 (0.05) | 4.30 (0.05) | 0.02 (0.89) | |
| IL AMYG | 0.22 (0.64) | 5.45 (0.03) | 0.22 (0.64) | |
| CL AMYG | 2.31 (0.14) | 4.62 (0.04) | 0.03 (0.86) | |
| CC | 0.35 (0.56) | 0.21 (0.65) | 0.12 (0.73) | |
| DTI Measure in the Corpus Callosum | FA | 1.59 (0.22) | 4.64 (0.04) | 0.38 (0.55) |
| RD | 0.01 (0.94) | 3.43 (0.08) | 0.06 (0.81) | |
| AD | 0.36 (0.55) | 10.73 (<0.01) | 0.49 (0.49) | |
| ADC | 0.10 (0.75) | 6.07 (0.02) | 0.19 (0.67) |
| Behavioural Test | Effect of RmTBI F (p) | Effect of Steroid Treatment F (p) | Steroid Treatment x RmTBI F (p) |
|---|---|---|---|
| Time-to-right | 18.74 (<0.01) | 0.04 (.95) | 0.79 (.38) |
| Open field: distance | 4.23 (0.04) | 0.01 (.92) | 0.23 (.64) |
| Open field: time in center | 2.20 (0.14) | 0.07 (.79) | 0.00 (.96) |
| EPM | 1.27 (0.26) | 0.10 (.75) | 0.16 (.69) |
| NCM | 1.32 (0.25) | 5.00 (.03) | 6.74 (0.01) |
| Dominance Tube | <0.01 (0.96) | 0.77 (.38) | 4.25 (0.04) |
| Forced Swim | 0.19 (0.67) | 12.31 (<0.01) | 0.07 (0.79) |
| Brain Region | Gene | Effect of RmTBI F (p) | Effect of Steroid Treatment F (p) | Steroid Treatment x RmTBI F (p) |
|---|---|---|---|---|
| PFC | Bdnf | 0.06 (0.82) | 0.46 (0.50) | 0.03 (0.86) |
| Iba1 | 0.27 (0.61) | 2.51 (0.12) | 0.11 (0.74) | |
| Creb | <0.01 (0.99) | 15.28 (<0.01) | 2.11 (0.16) | |
| Gfap | 0.44 (0.52) | 7.73 (0.02) | <0.01 (0.98) | |
| AMYG | Bdnf | 1.24 (0.27) | 3.94 (0.05) | 9.64 (<0.01) |
| Iba1 | <0.01 (0.98) | 0.01 (0.91) | 0.13 (0.72) | |
| Creb | 0.84 (0.37) | 1.14 (0.29) | 7.44 (0.01) | |
| Gfap | 53.11 (<0.01) | 0.61 (0.46) | 6.45 (0.04) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabor, J.; Wright, D.K.; Christensen, J.; Zamani, A.; Collins, R.; Shultz, S.R.; Mychasiuk, R. Examining the Effects of Anabolic–Androgenic Steroids on Repetitive Mild Traumatic Brain Injury (RmTBI) Outcomes in Adolescent Rats. Brain Sci. 2020, 10, 258. https://doi.org/10.3390/brainsci10050258
Tabor J, Wright DK, Christensen J, Zamani A, Collins R, Shultz SR, Mychasiuk R. Examining the Effects of Anabolic–Androgenic Steroids on Repetitive Mild Traumatic Brain Injury (RmTBI) Outcomes in Adolescent Rats. Brain Sciences. 2020; 10(5):258. https://doi.org/10.3390/brainsci10050258
Chicago/Turabian StyleTabor, Jason, David. K. Wright, Jennaya Christensen, Akram Zamani, Reid Collins, Sandy R. Shultz, and Richelle Mychasiuk. 2020. "Examining the Effects of Anabolic–Androgenic Steroids on Repetitive Mild Traumatic Brain Injury (RmTBI) Outcomes in Adolescent Rats" Brain Sciences 10, no. 5: 258. https://doi.org/10.3390/brainsci10050258