Characterization of the DNAM-1, TIGIT and TACTILE Axis on Circulating NK, NKT-Like and T Cell Subsets in Patients with Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Results
2.1. Peripheral Blood Distribution of NK and T Cells in AML Patients at Diagnostic
2.2. Expression of DNAM-1, TIGIT and TACTILE Receptors in NK and T Cells
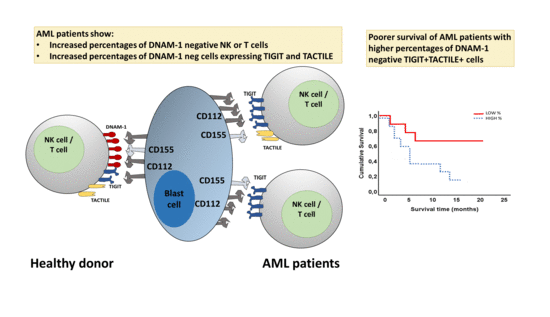
2.3. Boolean Analysis of the Co-Expression of DNAM-1, TIGIT and TACTILE in NK and T Cells
2.4. Expression of DNAM-1, TIGIT and TACTILE on CD4 and CD8 T Cell Subsets
2.5. Boolean Analysis of the Co-Expression of DNAM-1, TIGIT and TACTILE on CD4 and CD8 T Cell Subsets
2.6. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Samples
4.2. Antibodies
4.3. Flow Cytometry
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khwaja, A.; Bjorkholm, M.; Gale, R.E.; Levine, R.L.; Jordan, C.T.; Ehninger, G.; Bloomfield, C.D.; Estey, E.; Burnett, A.; Cornelissen, J.J.; et al. Acute myeloid leukaemia. Nat. Rev. Dis. Primers. 2016, 2, e16010. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J. Acute myeloid leukaemia and the immune system: Implications for immunotherapy. Br. J. Haematol. 2020, 188, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, D.O.; Adokoh, C.K.; Asante, D.B.; Asiamah, E.A.; Barnie, P.A.; Bonsu, D.O.M.; Kyei, F. Immunotherapy for acute myeloid leukemia (AML): A potent alternative therapy. Biomed. Pharmacother. 2018, 97, 225–232. [Google Scholar] [CrossRef]
- Winer, E.S.; Stone, R.M. Novel therapy in Acute myeloid leukemia (AML): Moving toward targeted approaches. Ther. Adv. Hematol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Correa, B.; Morgado, S.; Gayoso, I.; Bergua, J.M.; Casado, J.G.; Arcos, M.J.; Bengochea, M.L.; Duran, E.; Solana, R.; Tarazona, R. Human NK cells in acute myeloid leukaemia patients: Analysis of NK cell-activating receptors and their ligands. Cancer Immunol. Immunother. 2011, 60, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Correa, B.; Gayoso, I.; Bergua, J.M.; Casado, J.G.; Morgado, S.; Solana, R.; Tarazona, R. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol. Cell Biol. 2012, 90, 109–115. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, Q.; Xin, N.; Wang, W.; Zhao, C. CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 2017, 108, 1934–1938. [Google Scholar] [CrossRef] [Green Version]
- Martinet, L.; Smyth, M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015, 15, 243–254. [Google Scholar] [CrossRef]
- Chan, C.J.; Andrews, D.M.; Smyth, M.J. Receptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancer. Curr. Opin. Immunol. 2012, 24, 246–251. [Google Scholar] [CrossRef]
- Pende, D.; Bottino, C.; Castriconi, R.; Cantoni, C.; Marcenaro, S.; Rivera, P.; Spaggiari, G.M.; Dondero, A.; Carnemolla, B.; Reymond, N.; et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: Involvement in tumor cell lysis. Mol. Immunol. 2005, 42, 463–469. [Google Scholar] [CrossRef]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N.; et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, G.F.; Triglia, T.; Werkmeister, J.A.; Begley, C.G.; Boyd, A.W. TLiSA1, a human T lineage-specific activation antigen involved in the differentiation of cytotoxic T lymphocytes and anomalous killer cells from their precursors. J. Exp. Med. 1985, 161, 1063–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; Franz-Bacon, K.; McClanahan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef] [Green Version]
- Kwon, H.J.; Choi, G.E.; Ryu, S.; Kwon, S.J.; Kim, S.C.; Booth, C.; Nichols, K.E.; Kim, H.S. Stepwise phosphorylation of p65 promotes NF-kappaB activation and NK cell responses during target cell recognition. Nat. Commun. 2016, 7, e11686. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bu, J.; Zhou, M.; Sido, J.; Lin, Y.; Liu, G.; Lin, Q.; Xu, X.; Leavenworth, J.W.; Shen, E. CD8(+)T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clin. Immunol. 2018, 190, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhu, L.; Schell, T.D.; Zhang, J.; Claxton, D.F.; Ehmann, W.C.; Rybka, W.B.; George, M.R.; Zeng, H.; Zheng, H. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clin. Cancer Res. 2016, 22, 3057–3066. [Google Scholar] [CrossRef] [Green Version]
- Boles, K.S.; Vermi, W.; Facchetti, F.; Fuchs, A.; Wilson, T.J.; Diacovo, T.G.; Cella, M.; Colonna, M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur. J. Immunol. 2009, 39, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA. 2009, 106, 17858–17863. [Google Scholar] [CrossRef] [Green Version]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Sanchez-Correa, B.; Valhondo, I.; Hassouneh, F.; Lopez-Sejas, N.; Pera, A.; Bergua, J.M.; Arcos, M.J.; Banas, H.; Casas-Aviles, I.; Duran, E.; et al. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers 2019, 11, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, H.; Ravens, I.; Papadogianni, G.; Bernhardt, G. Coming of Age: CD96 Emerges as Modulator of Immune Responses. Front. Immunol. 2018, 9, e1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, N.; Tsukerman, P.; Mandelboim, O. The paired receptors TIGIT and DNAM-1 as targets for therapeutic antibodies. Hum. Antibodies. 2017, 25, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Stamm, H.; Wellbrock, J.; Fiedler, W. Interaction of PVR/PVRL2 with TIGIT/DNAM-1 as a novel immune checkpoint axis and therapeutic target in cancer. Mamm. Genome. 2018, 29, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, H.S. Targeting Checkpoint Receptors and Molecules for Therapeutic Modulation of Natural Killer Cells. Front. Immunol. 2018, 9, e2041. [Google Scholar] [CrossRef]
- Solomon, B.L.; Garrido-Laguna, I. TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018, 67, 1659–1667. [Google Scholar] [CrossRef]
- Blake, S.J.; Dougall, W.C.; Miles, J.J.; Teng, M.W.; Smyth, M.J. Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 5183–5188. [Google Scholar] [CrossRef] [Green Version]
- Lepletier, A.; Lutzky, V.P.; Mittal, D.; Stannard, K.; Watkins, T.S.; Ratnatunga, C.N.; Smith, C.; McGuire, H.M.; Kemp, R.A.; Mukhopadhyay, P.; et al. The immune checkpoint CD96 defines a distinct lymphocyte phenotype and is highly expressed on tumor-infiltrating T cells. Immunol. Cell Biol. 2019, 97, 152–164. [Google Scholar] [CrossRef] [Green Version]
- Stannard, K.A.; Lemoine, S.; Waterhouse, N.J.; Vari, F.; Chatenoud, L.; Gandhi, M.K.; Martinet, L.; Smyth, M.J.; Guillerey, C. Human peripheral blood DNAM-1(neg) NK cells are a terminally differentiated subset with limited effector functions. Blood Adv. 2019, 3, 1681–1694. [Google Scholar] [CrossRef]
- Chauvin, J.M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.H.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef]
- Carlsten, M.; Norell, H.; Bryceson, Y.T.; Poschke, I.; Schedvins, K.; Ljunggren, H.G.; Kiessling, R.; Malmberg, K.J. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J. Immunol. 2009, 183, 4921–4930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauvin, J.M.; Ka, M.; Pagliano, O.; Menna, C.; Ding, Q.; DeBlasio, R.; Sander, C.; Hou, J.; Li, X.Y.; Ferrone, S.; et al. IL-15 stimulation with TIGIT blockade reverses CD155-mediated NK cell dysfunction in melanoma. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Tarazona, R.; (University of Extremadura, Caceres, Spain). Personal observation. 2019. [Google Scholar]
- Fuchs, A.; Cella, M.; Giurisato, E.; Shaw, A.S.; Colonna, M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155). J. Immunol. 2004, 172, 3994–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, G.; Iguchi-Manaka, A.; Murata, R.; Yamashita-Kanemaru, Y.; Shibuya, A.; Shibuya, K. Tumor-derived soluble CD155 inhibits DNAM-1-mediated antitumor activity of natural killer cells. J. Exp. Med. 2020, 217, e1. [Google Scholar] [CrossRef] [Green Version]
- Hattori, N.; Kawaguchi, Y.; Sasaki, Y.; Shimada, S.; Murai, S.; Abe, M.; Baba, Y.; Watanuki, M.; Fujiwara, S.; Arai, N.; et al. Monitoring TIGIT/DNAM-1 and PVR/PVRL2 Immune Checkpoint Expression Levels in Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2019, 25, 861–867. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014, 26, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Correa, B.; Bergua, J.M.; Pera, A.; Campos, C.; Arcos, M.J.; Banas, H.; Duran, E.; Solana, R.; Tarazona, R. In Vitro Culture with Interleukin-15 Leads to Expression of Activating Receptors and Recovery of Natural Killer Cell Function in Acute Myeloid Leukemia Patients. Front Immunol. 2017, 8, e931. [Google Scholar] [CrossRef] [Green Version]
- Mittal, D.; Lepletier, A.; Madore, J.; Aguilera, A.R.; Stannard, K.; Blake, S.J.; Whitehall, V.L.J.; Liu, C.; Bettington, M.L.; Takeda, K.; et al. CD96 Is an Immune Checkpoint That Regulates CD8(+) T-cell Antitumor Function. Cancer Immunol. Res. 2019, 7, 559–571. [Google Scholar] [CrossRef]
- Chiang, E.Y.; de Almeida, P.E.; de Almeida Nagata, D.E.; Bowles, K.H.; Du, X.; Chitre, A.S.; Banta, K.L.; Kwon, Y.; McKenzie, B.; Mittman, S.; et al. CD96 functions as a co-stimulatory receptor to enhance CD8(+) TÂ cell activation and effector responses. Eur. J. Immunol. 2020. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Q.; Huang, M.; Wen, H.; Lin, R.; Zheng, M.; Qu, K.; Li, K.; Wei, H.; Xiao, W.; et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology 2019. [Google Scholar] [CrossRef]
- Peng, L.S.; Mao, F.Y.; Zhao, Y.L.; Wang, T.T.; Chen, N.; Zhang, J.Y.; Cheng, P.; Li, W.H.; Lv, Y.P.; Teng, Y.S.; et al. Altered phenotypic and functional characteristics of CD3+CD56+ NKT-like cells in human gastric cancer. Oncotarget 2016, 7, 55222–55230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Minden, M.D.; Chen, W.C.; Streck, E.; Chen, B.; Kang, H.; Arruda, A.; Ly, D.; Der, S.D.; Kang, S.; et al. Allogeneic Human Double Negative T Cells as a Novel Immunotherapy for Acute Myeloid Leukemia and Its Underlying Mechanisms. Clin. Cancer Res. 2018, 24, 370–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Ly, D.; Dervovic, D.; Fang, L.; Lee, J.B.; Kang, H.; Wang, Y.H.; Pham, N.A.; Pan, H.; Tsao, M.S.; et al. Human double negative T cells target lung cancer via ligand-dependent mechanisms that can be enhanced by IL-15. J. Immunother. Cancer 2019, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Whelan, S.; Ophir, E.; Kotturi, M.F.; Levy, O.; Ganguly, S.; Leung, L.; Vaknin, I.; Kumar, S.; Dassa, L.; Hansen, K.; et al. PVRIG and PVRL2 Are Induced in Cancer and Inhibit CD8(+) T-cell Function. Cancer Immunol. Res. 2019, 7, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roederer, M.; Nozzi, J.L.; Nason, M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytom. Part A 2011, 79, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Cell Subset | HD | AML | p |
|---|---|---|---|
| % NK cells (CD3− CD56+) * | 26.3 ± 14.3 | 14.7 ± 13.9 | 0.003 |
| % Conventional T cells (CD3+CD56−) * | 44.3 ± 17.2 | 50.3 ± 17.2 | 0.53 |
| CD4+ T cells ** | 53.4 ± 16.9 | 54.1 ± 15.4 | 0.89 |
| CD8+ T cells ** | 35.9 ± 13.5 | 35.4 ± 13.3 | 0.90 |
| DN T cells ** | 7.1 ± 4.4 | 7.7 ± 4.4 | 0.63 |
| DP T cells ** | 0.6 ± 0.2 | 0.6 ± 0.4 | 0.62 |
| % NKT-like cells (CD3+CD56+) * | 2.7 ± 3.1 | 4.7 ± 5.1 | 0.13 |
| CD4+ T cells ** | 15.6 ± 14.5 | 8.3 ± 12.4 | 0.08 |
| CD8+ T cells ** | 59.9 ± 17.8 | 62.1 ± 20.6 | 0.71 |
| DN T cells ** | 17.0 ± 11.0 | 22.3 ± 15.6 | 0.21 |
| DP T cells ** | 1.0 ± 1.1 | 0.8 ± 1.3 | 0.58 |
| Characteristic | Value |
|---|---|
| No. Patients | 36 |
| Age | |
| Median (Range) | 74.5 (25–90) |
| Mean (SD) | 69.5 ± 16.5 |
| Sex no. (%) | |
| Male | 26 (72.2%) |
| Female | 10 (27.8%) |
| FAB classification no. (%) | |
| M0 | 0% |
| M1 | 6 (16.7%) |
| M2 | 3 (8.3%) |
| M3 | 1 (2.8%) |
| M4 | 5 (13.9%) |
| M5 | 9 (25%) |
| M6 | 1 (2.8%) |
| M7 | 1 (2.8%) |
| Secondary AML no. (%) | 6 (16.7%) |
| AML classification not available | 4 (11.1%) |
| Cytogenetic risk no. (%) | |
| Favorable | 4 |
| Intermediate | 17 |
| Adverse | 8 |
| Not available | 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valhondo, I.; Hassouneh, F.; Lopez-Sejas, N.; Pera, A.; Sanchez-Correa, B.; Guerrero, B.; Bergua, J.M.; Arcos, M.J.; Bañas, H.; Casas-Avilés, I.; et al. Characterization of the DNAM-1, TIGIT and TACTILE Axis on Circulating NK, NKT-Like and T Cell Subsets in Patients with Acute Myeloid Leukemia. Cancers 2020, 12, 2171. https://doi.org/10.3390/cancers12082171
Valhondo I, Hassouneh F, Lopez-Sejas N, Pera A, Sanchez-Correa B, Guerrero B, Bergua JM, Arcos MJ, Bañas H, Casas-Avilés I, et al. Characterization of the DNAM-1, TIGIT and TACTILE Axis on Circulating NK, NKT-Like and T Cell Subsets in Patients with Acute Myeloid Leukemia. Cancers. 2020; 12(8):2171. https://doi.org/10.3390/cancers12082171
Chicago/Turabian StyleValhondo, Isabel, Fakhri Hassouneh, Nelson Lopez-Sejas, Alejandra Pera, Beatriz Sanchez-Correa, Beatriz Guerrero, Juan M. Bergua, Maria Jose Arcos, Helena Bañas, Ignacio Casas-Avilés, and et al. 2020. "Characterization of the DNAM-1, TIGIT and TACTILE Axis on Circulating NK, NKT-Like and T Cell Subsets in Patients with Acute Myeloid Leukemia" Cancers 12, no. 8: 2171. https://doi.org/10.3390/cancers12082171