Tailored Exercise during Hematopoietic Stem Cell Transplantation Hospitalization in Children with Cancer: A Prospective Cohort Study
Abstract
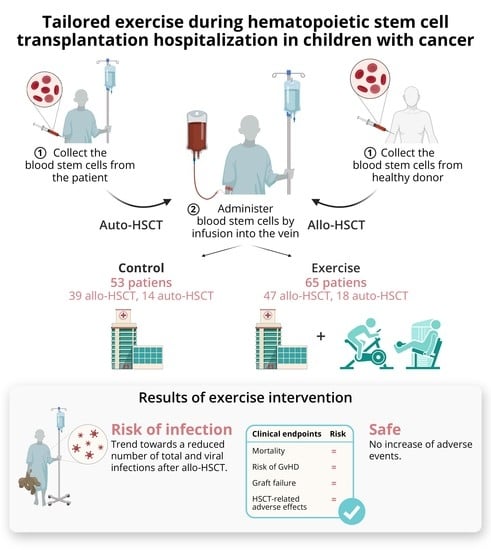
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Primary Outcomes
2.3. Secondary Outcomes
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Supervised Exercise Intervention
4.3. Outcomes
4.3.1. Primary Outcomes
4.3.2. Secondary Outcomes
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miano, M.; Labopin, M.; Hartmann, O.; Angelucci, E.; Cornish, J.; Gluckman, E.; Locatelli, F.; Fischer, A.; Egeler, R.M.; Or, R.; et al. Haematopoietic stem cell transplantation trends in children over the last three decades: A survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transpl. 2007, 39, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.J.; Cushing-Haugen, K.L.; Cheng, G.S.; Boeckh, M.; Khera, N.; Lee, S.J.; Leisenring, W.M.; Martin, P.J.; Mueller, B.A.; Schwartz, S.M.; et al. Morbidity and Mortality Differences Between Hematopoietic Cell Transplantation Survivors and Other Cancer Survivors. J. Clin. Oncol. 2017, 35, 306–313. [Google Scholar] [CrossRef]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.S.; Valenzuela, P.L.; Rincón-Castanedo, C.; Takken, T.; Fiuza-Luces, C.; Santos-Lozano, A.; Lucia, A. Exerc.ise training in childhood cancer: A systematic review and meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2018, 70, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Martha, B.A.; Vacchi, C.O.; Fattori, R.A.; Macagnan, F.E. Effect of physical Exerc.ise on the functional capacity of children and adolescents submitted to transplantation of hematopoietic stem cells-A systematic review with meta-analysis. J. Child. Health Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Padilla, J.R.; Valentín, J.; Santana-Sosa, E.; Santos-Lozano, A.; Sanchis-Gomar, F.; Pareja-Galeano, H.; Morales, J.S.; Fleck, S.J.; Pérez, M.; et al. Effects of Exerc.ise on the Immune Function of Pediatric Patients With Solid Tumors: Insights From the PAPEC Randomized Trial. Am. J. Phys. Med. Rehabil. 2017, 96, 831–837. [Google Scholar] [CrossRef]
- Chamorro-Viña, C.; Valentín, J.; Fernández, L.; González-Vicent, M.; Pérez-Ruiz, M.; Lucía, A.; Culos-Reed, S.N.; Díaz, M.; Pérez-Martínez, A. Influence of a Moderate-Intensity Exercise Program on Early NK Cell Immune Recovery in Pediatric Patients After Reduced-Int.ensity Hematopoietic Stem Cell Transplantation. Integr. Cancer 2017, 16, 464–472. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.F.; Chamorro-Viña, C.; Moral, S.; Fernández del Valle, M.; Madero, L.; Ramírez, M.; Pérez, M.; Lucia, A. Benefits of Int.rahospital Exerc.ise training after Pediatr.ic bone marrow transplantation. Int. J. Sports Med. 2008, 29, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Viña, C.; Ruiz, J.R.; Santana-Sosa, E.; González Vicent, M.; Madero, L.; Pérez, M.; Fleck, S.J.; Pérez, A.; Ramírez, M.; Lucía, A. Exerc.ise during hematopoietic stem cell transplant hospitalization in children. Med. Sci. Sports Exerc. 2010, 42, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Sorror, M.L. How I assess comorbidities before hematopoietic cell transplantation. Blood 2013, 121, 2854–2863. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.S.; Santana-Sosa, E.; Santos-Lozano, A.; Baño-Rodrigo, A.; Valenzuela, P.L.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Vicent, M.G.; Pérez-Somarriba, M.; Madero, L.; et al. Inhospital Exerc.ise benefits in childhood cancer: A prospective cohort study. Scand. J. Med. Sci. Sports 2019. [Google Scholar] [CrossRef]
- Yildiz Kabak, V.; Duger, T.; Uckan Cetinkaya, D. Investigation of the Effects of an Exerc.ise Program on Physical Functions and Activities of Daily Life in Pediatric Hematopoietic Stem Cell Transplantation. Pediatr. Blood Cancer 2016, 63, 1643–1648. [Google Scholar] [CrossRef]
- Senn-Malashonak, A.; Wallek, S.; Schmidt, K.; Rosenhagen, A.; Vogt, L.; Bader, P.; Banzer, W. Psychophysical effects of an Exerc.ise therapy during Pediatr.ic stem cell transplantation: A randomized controlled trial. Bone Marrow Transpl. 2019, 54, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.H.; Kurimo, M.; Kanerva, J.; Hovi, L. Physical performance of nontransplanted childhood ALL survivors is comparable to healthy controls. J. Pediatr. Hematol. Oncol. 2013, 35, 276–280. [Google Scholar] [CrossRef]
- Diaz, M.A.; Pérez-Martínez, A.; Herrero, B.; Deltoro, N.; Martinez, I.; Ramirez, M.; Abad, L.; Sevilla, J.; Merino, E.; Ruiz, J.; et al. Prognostic factors and outcomes for Pediatr.ic patients receiving an haploidentical relative allogeneic transplant using CD3/CD19-depleted grafts. Bone Marrow Transpl. 2016, 51, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; Counts, G.W.; Appelbaum, F.R.; Lee, S.J.; Sanders, J.E.; Deeg, H.J.; Flowers, M.E.; Syrjala, K.L.; Hansen, J.A.; Storb, R.F.; et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J. Clin. Oncol. 2010, 28, 1011–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiskemann, J.; Kleindienst, N.; Kuehl, R.; Dreger, P.; Schwerdtfeger, R.; Bohus, M. Effects of physical Exerc.ise on survival after allogeneic stem cell transplantation. Int. J. Cancer 2015, 137, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; Rogers, C.J.; et al. Position statement. Part one: Immune function and Exerc.ise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Kruijsen-Jaarsma, M.; Révész, D.; Bierings, M.B.; Buffart, L.M.; Takken, T. Effects of Exerc.ise on immune function in patients with cancer: A systematic review. Exerc. Immunol. Rev. 2013, 19, 120–143. [Google Scholar]
- Kesting, S.; Weeber, P.; Schönfelder, M.; Renz, B.W.; Wackerhage, H.; von Luettichau, I. Exerc.ise as a Potential Int.ervention to Modulate Cancer Outcomes in Children and Adults? Front. Oncol. 2020, 10, 196. [Google Scholar] [CrossRef]
- Liu, D.; Wang, R.; Grant, A.R.; Zhang, J.; Gordon, P.M.; Wei, Y.; Chen, P. Immune adaptation to chronic Int.ense Exerc.ise training: New microarray evidence. BMC Genom. 2017, 18, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, A.; Zimmer, P.; Schmidt, M.E.; Hummel, M.; Ulrich, C.M.; Wiskemann, J.; Steindorf, K. No Evidence for Effect of Exerc.ise on Transcriptome of NK Cells in Breast Cancer Patients Undergoing Adjuvant Therapy: Results From a Pilot Study. Front. Physiol. 2019, 10, 959. [Google Scholar] [CrossRef]
- Morales, J.S.; Padilla, J.R.; Valenzuela, P.L.; Santana-Sosa, E.; Rincón-Castanedo, C.; Santos-Lozano, A.; Herrera-Olivares, A.M.; Madero, L.; San Juan, A.F.; Fiuza-Luces, C.; et al. Inhospital Exerc.ise Training in Children With Cancer: Does It Work for All? Front. Pediatr. 2018, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Is infection risk linked to Exerc.ise workload? Med. Sci. Sports Exerc. 2000, 32, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Pedersen, B.K. Exerc.ise and immune function. Recent developments. Sports Med. 1999, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A.; Davis, J.M.; Smith, J.A.; Nieman, D.C. Exerc.ise and cellular innate immune function. Med. Sci. Sports Exerc. 1999, 31, 57–66. [Google Scholar] [CrossRef]
- Santana Sosa, E.; Groeneveld, I.F.; Gonzalez-Saiz, L.; López-Mojares, L.M.; Villa-Asensi, J.R.; Barrio Gonzalez, M.I.; Fleck, S.J.; Pérez, M.; Lucia, A. Int.rahospital weight and aerobic training in children with cystic fibrosis: A randomized controlled trial. Med. Sci. Sports Exerc. 2012, 44, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.J.; Altman, D.G. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Variable | Control Group (n = 53) | Exercise Group (n = 65) | p-Value |
|---|---|---|---|
| Age (mean ± SD [range], years) | |||
| At diagnosis | 9 ± 4 (4, 17) | 10 ± 4 (4, 17) | 0.070 |
| At HSCT | 10 ± 4 (4, 18) | 11 ± 4 (5, 18) | 0.081 |
| Sex (% male) | |||
| Recipient | 64% | 63% | 0.904 |
| Donor | 50% | 46% | 0.751 |
| Diagnosis (%) | |||
| ALL/other leukemias | 70/30% | 68/32% | 0.805 |
| Disease status (%) | |||
| 1st CR | 28% | 32% | 0.638 |
| 2nd CR | 36% | 34% | 0.820 |
| >2nd CR | 17% | 22% | 0.534 |
| Not in remission | 19% | 12% | 0.324 |
| Type of HSCT (%) | |||
| Allogeneic/autologous | 74/26% | 72/28% | 0.877 |
| Source of donor cells (%) | |||
| Peripheral blood/umbilical cord | 98/2% | 94/6% | 0.377 |
| Origin of cells in allo-HSCT (%) | |||
| Parent | 62% | 60% | 0.853 |
| Sibling | 15% | 23% | 0.353 |
| Unrelated donor | 23% | 17% | 0.483 |
| Conditioning regimen (%) | |||
| Myeloablative/Nonmyeloablative | 91/9% | 88/12% | 0.599 |
| GvHD prophylaxis in allo-HSCT (%) | |||
| Cyclosporine | 28% | 23% | 0.650 |
| Cyclosporine + Methylprednisolone | 72% | 77% | 0.650 |
| HLA-match status in allo-HSCT (%) | |||
| HLA-matched and related | 8% | 15% | 0.300 |
| HLA-matched and unrelated | 18% | 11% | 0.330 |
| HLA-mismatched (related or unrelated) | 74% | 74% | 0.991 |
| Graft manipulation method (%) | |||
| Manipulated/Unmanipulated | 73/27% | 74/26% | 0.778 |
| HCT-CI * (median [range]) | 0 (0, 1) | 0 (0, 2) | 0.197 |
| Anthropometrical variables (mean ± SD) | |||
| Body weight (kg) | 37.4 ± 22.8 | 39.6 ± 15 | 0.541 |
| BMI (kg/m2) | 19.1 ± 5.3 | 18.5 ± 3.5 | 0.459 |
| Karnofsky/Lansky’s performance scale ** (mean ± SD) | 93 ± 6 | 93 ± 8 | 0.726 |
| Allogeneic-HSCT | ||||||
| Control Group (n = 39) | Exercise Group (n = 47) | |||||
| Outcome | Unadjusted | Adjusted * | Unadjusted | Adjusted * | Unadjusted between-group Difference p-value | Adjusted * between-group Difference p-value |
| Mortality | ||||||
| OR (95% CI) for death | 1.00 (Reference) | 1.00 (Reference) | 0.78 (0.46, 2.77) | 2.55 (0.65, 9.98) | 0.784 | 0.179 |
| Time since HSCT to death (days) | 276 ± 111 (50, 503) | 396 ± 373 (364, 1256) | 393 ± 97 (195, 591) | 351 ± 258 (243, 947) | 0.436 | 0.915 |
| HSCT | ||||||
| Number of HSCT | 1 ± 0 (1, 1) | 1 ± 0 (1, 2) | 1 ± 0 (1, 1) | 1 ± 0 (1, 2) | 0.293 | 0.556 |
| Graft failure (OR (95% CI)) | 1.00 (Reference) | 1.00 (Reference) | 0.60 (0.21, 1.69) | 0.57 (0.13, 2.49) | 0.331 | 0.459 |
| Time from first to second HSCT (days) | 256 ± 67 (114, 398) | 358 ± 85 (226, 1441) | 298 ± 75 (139, 456) | 455 ± 51 (189, 1099) | 0.684 | 0.587 |
| GvHD | ||||||
| Risk of aGvHD, (OR (95% CI)) | 1.00 (Reference) | 1.00 (Reference) | 1.38 (0.59, 3.25) | 1.59 (0.49, 5.20) | 0.464 | 0.441 |
| Grade of aGvHD | 2 ± 0 (2, 3) | 1 ± 1 (−1, 3) | 2 ± 0 (2, 3) | 1 ± 1 (−1, 3) | 0.464 | 0.521 |
| Time from HSCT to aGvHD, (days) | 40 ± 6 (29, 52) | 58 ± 19 (18, 98) | 39 ± 5 (30, 49) | 59 ± 22 (11, 106) | 0.876 | 0.953 |
| Risk of cGvHD (OR (95% CI)) | 1.00 (Reference) | 1.00 (Reference) | 2.37 (0.68, 8.24) | 2.16 (0.51, 9.22) | 0.177 | 0.298 |
| Grade of cGvHD | 2 ± 0 (1, 2) | 1 ± 0 (1, 2) | 1 ± 0 (1, 2) | 1 ± 0 (0, 2) | 0.271 | 0.542 |
| Time from HSCT to cGvHD (days) | 116 ± 39 (31, 201) | 72 ± 93 (63, 307) | 137 ± 25 (83, 191) | 103 ± 61 (91, 297) | 0.665 | 0.220 |
| Autologous-HSCT | ||||||
| Control Group (n = 14) | Exercise Group (n = 18) | |||||
| Unadjusted | Adjusted ** | Unadjusted | Adjusted ** | Unadjusted between-group Difference p-value | Adjusted ** between-group Difference p-value | |
| Mortality | ||||||
| OR (95% CI) for death | 1.00 (Reference) | 1.00 (Reference) | 0.75 (0.09, 6.11) | 0.60 (0.61, 5,88) | 0.788 | 0.658 |
| Time since HSCT to death (days) | 1232 ± 514 (981, 3445) | N/A | 528 ± 514 (486, 2741) | N/A | 0.435 | N/A |
| HSCT | ||||||
| Number of HSCT | 1 ± 0 (1, 1) | 1 ± 0 (1, 1) | 1 ± 0 (1, 1) | 1 ± 0 (1, 1) | 0.860 | 0.952 |
| Graft failure (OR (95% CI)) | 1.00 (Reference) | 1.00 (Reference) | 0.77 (0.04, 13.41) | 1.4 (0.04, 49.88) | 0.854 | 0.854 |
| Time from first to second HSCT (days) | N/A | N/A | N/A | N/A | N/A | N/A |
| Allogeneic-HSCT | ||||||
| Control Group (n = 39) | Exercise Group (n = 47) | |||||
| Outcome | Unadjusted | Adjusted * | Unadjusted | Adjusted * | Unadjusted between-group Difference p-Value | Adjusted * between-group Difference p-Value |
| Time to neutrophil engraftment (days) | 16 ± 1 (14, 17) | 15 ± 2 (11, 19) | 14 ± 1 (13, 16) | 13 ± 2 (9, 17) | 0.126 | 0.170 |
| Time to platelet count ≥ 20 × 109/L (days) | 13 ± 1 (11, 15) | 13 ± 3 (7, 19) | 12 ± 1 (10, 14) | 11 ± 3 (6, 17) | 0.368 | 0.454 |
| Time to platelet count ≥ 50 × 109/L (days) | 15 ± 1 (12, 18) | 21 ± 4 (13, 29) | 14 ± 1 (12, 17) | 19 ± 4 (11, 27) | 0.857 | 0.489 |
| Time to platelet count ≥ 100 × 109/L (days) | 19 ± 3 (14, 25) | 73 ± 8 (56, 89) | 22 ± 2 (17, 27) | 73 ± 7 (58, 88) | 0.382 | 0.960 |
| Myelosuppression (days) | 12 ± 1 (10, 14) | 11 ± 3 (4, 17) | 10 ± 1 (8, 12) | 9 ± 3 (3, 16) | 0.329 | 0.563 |
| Autologous-HSCT | ||||||
| Control Group (n = 14) | Exercise Group (n = 18) | |||||
| Unadjusted | Adjusted ** | Unadjusted | Adjusted ** | Unadjusted between-Group Difference p-Value | Adjusted ** between-Group Difference p-Value | |
| Time to neutrophil engraftment (days) | 13 ± 1 (12, 14) | 13 ± 1 (12, 14) | 12 ± 0 (11, 13) | 13 ± 1 (12, 14) | 0.403 | 0.920 |
| Time to platelet count ≥ 20 × 109/L (days) | 10 ± 1 (8, 13) | 10 ± 2 (7, 14) | 12 ± 1 (11, 13) | 13 ± 1 (10, 16) | 0.251 | 0.282 |
| Time to platelet count ≥ 50 × 109/L (days) | 13 ± 1 (10, 17) | 12 ± 1 (7, 18) | 15 ± 1 (12, 17) | 16 ± 1 (11, 20) | 0.460 | 0.195 |
| Time to platelet count ≥ 100 × 109/L (days) | 16 ± 4 (15, 19) | N/A | 19 ± 3 (16, 21) | N/A | 0.667 | N/A |
| Myelosuppression (days) | 7 ± 1 (6, 8) | 7 ± 1 (5, 8) | 8 ± 1 (7, 9) | 9 ± 1 (7, 10) | 0.135 | 0.071 |
| Allogeneic-HSCT | ||||||
| Control Group (n = 39) | Exercise Group (n = 47) | |||||
| Outcome | Unadjusted | Adjusted * | Unadjusted | Adjusted * | Unadjusted between-group Difference p-value | Adjusted * between-group Difference p-value |
| Platelet transfusions (number) | 3 ± 1 (2, 5) | 3 ± 2 (1, 6) | 3 ± 1 (2, 4) | 3 ± 2 (0, 6) | 0.758 | 0.474 |
| Red blood cell transfusions (number) | 3 ± 0 (2, 4) | 2 ± 1 (1, 4) | 2 ± 0 (1, 3) | 2 ± 1 (1, 4) | 0.147 | 0.546 |
| Fever (days) | 3 ± 0 (2, 4) | 1 ± 1 (0, 3) | 2 ± 0 (1, 3) | 0 ± 1 (0, 2) | 0.108 | 0.040 |
| Antibiotic treatment (days) | 18 ± 2 (15, 22) | 18 ± 4 (9, 26) | 16 ± 2 (13, 19) | 14 ± 4 (6, 22) | 0.256 | 0.206 |
| Parenteral nutrition (days) | 6 ± 2 (2, 10) | 7 ± 4 (1, 15) | 6 ± 2 (3, 10) | 7 ± 4 (1, 15) | 0.862 | 0.787 |
| Time between HSCT and hospital discharge (days) | 21 ± 1 (18, 24) | 20 ± 4 (12, 28) | 17 ± 1 (14, 20) | 15 ± 4 (8, 23) | 0.052 | 0.082 |
| Autologous-HSCT | ||||||
| Control Group (n = 14) | Exercise Group (n = 18) | |||||
| Unadjusted | Adjusted ** | Unadjusted | Adjusted ** | Unadjusted between-group Difference p-value | Adjusted ** between-group Difference p-value | |
| Platelet transfusions (number) | 4 ± 1 (3, 5) | 4 ± 1 (3, 5) | 3 ± 0 (2, 4) | 3 ± 1 (2, 5) | 0.099 | 0.566 |
| Red blood cell transfusions (number) | 2 ± 0 (2, 2) | 2 ± 0 (1, 3) | 2 ± 0 (1, 2) | 2 ± 0 (1, 2) | 0.141 | 0.371 |
| Fever (days) | 1 ± 0 (1, 2) | 1 ± 0 (1, 2) | 1 ± 0 (1, 2) | 1 ± 0 (0, 2) | 0.690 | 0.311 |
| Antibiotic treatment (days) | 12 ± 1 (10, 14) | 12 ± 1 (10, 14) | 13 ± 1 (11, 15) | 14 ± 1 (12, 16) | 0.703 | 0.242 |
| Parenteral nutrition (days) | 10 ± 2 (6, 13) | 9 ± 1 (6, 11) | 9 ± 2 (5, 12) | 8 ± 1 (6, 11) | 0.595 | 0.793 |
| Time between HSCT and hospital discharge (days) | 15 ± 1 (14, 17) | 16 ± 1 (14, 17) | 16 ± 1 (15, 18) | 17 ± 1 (16, 18) | 0.243 | 0.158 |
| Allogeneic-HSCT | ||||||
| Control Group (n = 39) | Exercise Group (n = 47) | |||||
| Risk of Outcomes | Unadjusted | Adjusted * | Unadjusted | Adjusted * | Unadjusted between-group Difference p-Value | Adjusted * between-group Difference p-Value |
| Mucositis | 1.00 (Reference) | 1.00 (Reference) | 0.89 (0.35, 2.23) | 1.57 (0.45, 5.50) | 0.802 | 0.478 |
| Vomiting | 1.00 (Reference) | 1.00 (Reference) | 0.84 (0.34, 2.07) | 0.80 (0.23, 2.78) | 0.705 | 0.729 |
| Diarrhea | 1.00 (Reference) | 1.00 (Reference) | 1.45 (0.58, 3.66) | 1.21 (0.34, 4.26) | 0.430 | 0.764 |
| Engraftment syndrome | 1.00 (Reference) | 1.00 (Reference) | 0.79 (0.33, 1.92) | 2.53 (0.66, 9.70) | 0.607 | 0.176 |
| Hemorrhagic cystitis | 1.00 (Reference) | 1.00 (Reference) | 0.50 (0.13, 1.94) | 0.74 (0.12, 4.46) | 0.312 | 0.741 |
| Neurologic toxicity | 1.00 (Reference) | 1.00 (Reference) | 0.89 (0.38, 2.13) | 1.02 (0.24, 4.34) | 0.797 | 0.982 |
| Liver toxicity | 1.00 (Reference) | 1.00 (Reference) | 0.39 (0.13, 1.14) | 0.29 (0.06, 1.39) | 0.086 | 0.121 |
| Renal toxicity | 1.00 (Reference) | 1.00 (Reference) | 0.97 (0.34, 2.74) | 0.83 (0.21, 3.33) | 0.953 | 0.789 |
| Autologous-HSCT | ||||||
| Control Group (n = 14) | Exercise Group (n = 18) | |||||
| Unadjusted | Adjusted ** | Unadjusted | Adjusted ** | Unadjusted between-Group Difference p-Value | Adjusted ** between-group Difference p-Value | |
| Mucositis | 1.00 (Reference) | 1.00 (Reference) | 1.36 (0.23, 8.08) | 2.23 (0.14, 34.98) | 0.733 | 0.569 |
| Vomiting | 1.00 (Reference) | 1.00 (Reference) | 4.50 (0.72, 28.15) | 2.83 (0.35, 22.56) | 0.108 | 0.327 |
| Diarrhea | 1.00 (Reference) | 1.00 (Reference) | 2.24 (0.45, 11.11) | 1.96 (0.28, 13.64) | 0.324 | 0.498 |
| Engraftment syndrome | 1.00 (Reference) | 1.00 (Reference) | 2.19 (0.47, 10.21) | 2.76 (0.39, 19.34) | 0.319 | 0.307 |
| Hemorrhagic cystitis | 1.00 (Reference) | 1.00 (Reference) | 2.38 (0.42, 13.39) | 7.16 (0.59, 87.28) | 0.325 | 0.123 |
| Neurologic toxicity | 1.00 (Reference) | 1.00 (Reference) | 1.50 (0.17, 12.94) | 2.65 (0.15, 48.29) | 0.712 | 0.511 |
| Liver toxicity | 1.00 (Reference) | 1.00 (Reference) | 4.40 (0.77, 25.15) | 10.84 (0.70, 167.13) | 0.096 | 0.088 |
| Renal toxicity | 1.00 (Reference) | 1.00 (Reference) | 2.22 (0.37, 13.54) | 3.84 (0.43, 34.13) | 0.386 | 0.228 |
| Allogeneic-HSCT | ||||||
| Control Group (n = 39) | Exercise Group (n = 47) | |||||
| Outcome | Unadjusted | Adjusted * | Unadjusted | Adjusted * | Unadjusted between-group Difference p-Value | Adjusted * between-group Difference p-Value |
| Number of infections after HSCT | 2 ± 0 (1, 2) | 2 ± 0 (1, 3) | 1 ± 0 (1, 1) | 2 ± 0 (1, 2) | 0.005 | 0.023 |
| Number of viral infections after HSCT | 1 ± 0 (1, 1) | 1 ± 0 (0, 2) | 0 ± 0 (0, 1) | 1 ± 0 (0, 1) | 0.005 | 0.083 |
| Number of bacterial infections after HSCT | 0 ± 0 (0, 1) | 1 ± 0 (0, 1) | 0 ± 0 (0, 1) | 1 ± 0 (0, 1) | 0.947 | 0.942 |
| Number of fungal infections after HSCT | 1 ± 0 (0, 1) | 1 ± 0 (0,1) | 0 ± 0 (0, 0) | 0 ± 0 (0, 1) | 0.022 | 0.026 |
| Autologous-HSCT | ||||||
| Control Group (n = 14) | Exercise Group (n = 18) | |||||
| Unadjusted | Adjusted ** | Unadjusted | Adjusted ** | Unadjusted between-group Difference p-value | Adjusted ** between-group Difference p-value | |
| Number of infections after HSCT | 1 ± 0 (1, 2) | 1 ± 0 (1, 2) | 1 ± 0 (0, 1) | 1 ± 0 (0, 1) | 0.273 | 0.213 |
| Number of viral infections after HSCT | 1 ± 0 (0, 1) | 0 ± 0 (0, 1) | 0 ± 0 (0, 1) | 0 ± 0 (0, 1) | 0.678 | 0.959 |
| Number of bacterial infections after HSCT | 0 ± 0 (0, 1) | 1 ± 0 (0, 1) | 0 ± 0 (0, 1) | 0 ± 0 (0, 1) | 0.525 | 0.230 |
| Number of fungal infections after HSCT | 0 ± 0 (0, 0) | 0 ± 0 (0, 0) | 0 ± 0 (0, 0) | 0 ± 0 (0, 0) | 0.518 | 0.728 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, J.S.; González Vicent, M.; Valenzuela, P.L.; Castillo-García, A.; Santana-Sosa, E.; Lassaletta, A.; Santos-Lozano, A.; Fiuza-Luces, C.; Lucia, A. Tailored Exercise during Hematopoietic Stem Cell Transplantation Hospitalization in Children with Cancer: A Prospective Cohort Study. Cancers 2020, 12, 3020. https://doi.org/10.3390/cancers12103020
Morales JS, González Vicent M, Valenzuela PL, Castillo-García A, Santana-Sosa E, Lassaletta A, Santos-Lozano A, Fiuza-Luces C, Lucia A. Tailored Exercise during Hematopoietic Stem Cell Transplantation Hospitalization in Children with Cancer: A Prospective Cohort Study. Cancers. 2020; 12(10):3020. https://doi.org/10.3390/cancers12103020
Chicago/Turabian StyleMorales, Javier S., Marta González Vicent, Pedro L. Valenzuela, Adrián Castillo-García, Elena Santana-Sosa, Alvaro Lassaletta, Alejandro Santos-Lozano, Carmen Fiuza-Luces, and Alejandro Lucia. 2020. "Tailored Exercise during Hematopoietic Stem Cell Transplantation Hospitalization in Children with Cancer: A Prospective Cohort Study" Cancers 12, no. 10: 3020. https://doi.org/10.3390/cancers12103020