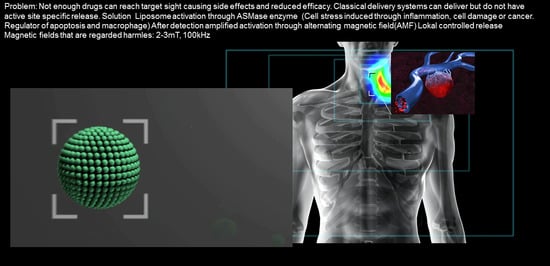
Lipid-Iron Nanoparticle with a Cell Stress Release Mechanism Combined with a Local Alternating Magnetic Field Enables Site-Activated Drug Release
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Biophysical In Vitro Characterization
2.2. Cell Studies
2.3. Animal Studies
2.4. Therapy Study
3. Discussion
4. Materials and Methods
4.1. Liposome Formulations
4.2. Transmission Electron Microscope (TEM) Imaging
4.3. Small Angle X-ray Scattering (SAXS)
4.4. AMF Treatment
4.5. Cell Experiments
Fluorescence Microscopy
4.6. Animal Experiments
4.6.1. Biodistribution and Blood Clearance
4.6.2. Immunohistological Staining
4.6.3. Liposomal Nanoparticle Injection, Imaging, and Biodistribution
4.6.4. In Vivo Tumor Imaging
4.6.5. Survival Studies
4.6.6. Photoacoustic Imaging
4.7. Sphingomyelinase Activity Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Seetharamu, N.; Kim, E.; Hochster, H.; Martin, F.; Muggia, F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer. Res. 2010, 30, 541–545. [Google Scholar] [PubMed]
- Gasselhuber, A.; Dreher, M.R.; Rattay, F.; Wood, B.J.; Haemmerich, D. Comparison of Conventional Chemotherapy, Stealth Liposomes and Temperature-Sensitive Liposomes in a Mathematical Model. PLoS ONE 2012, 7, e47453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Huang, P.; Chen, X. Stimuli-Responsive Programmed Specific Targeting in Nanomedicine. ACS Nano 2016, 10, 2991–2994. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Q.; Fumoto, S.; Suga, T.; Miyamoto, H.; Kuroda, N.; Kawakami, S.; Nishida, K. Targeted co-delivery of protein and drug to a tumor in vivo by sophisticated RGD-modified lipid-calcium carbonate nanoparticles. J. Control. Release 2019, 302, 42–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Un, K.; Kawakami, S.; Suzuki, R.; Maruyama, K.; Yamashita, F.; Hashida, M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials 2010, 31, 7813–7826. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]
- Schissel, S.L.; Keesler, G.A.; Schuchman, E.H.; Williams, K.J.; Tabas, I. The Cellular Trafficking and Zinc Dependence of Secretory and Lysosomal Sphingomyelinase, Two Products of the Acid Sphingomyelinase Gene. J. Biol. Chem. 1998, 273, 18250–18259. [Google Scholar] [CrossRef] [Green Version]
- Van Hell, A.J.; Haimovitz-Friedman, A.; Fuks, Z.; Tap, W.D.; Kolesnick, R.N. Gemcitabine kills proliferating endothelial cells exclusively via acid sphingomyelinase activation. Cell. Signal. 2017, 34, 86–91. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Luberto, C.; Argraves, K.M. Enzymes of Sphingolipid Metabolism: From Modular to Integrative Signaling. Biochemistry 2001, 40, 4893–4903. [Google Scholar] [CrossRef]
- Grassmé, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 Signaling via Ceramide-rich Membrane Rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremesti, A.; Paris, F.; Grassmé, H.; Holler, N.; Tschopp, J.; Fuks, Z.; Gulbins, E.; Kolesnick, R. Ceramide Enables Fas to Cap and Kill. J. Biol. Chem. 2001, 276, 23954–23961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanvicens, N.; Cotter, T.G. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. J. Neurochem. 2006, 98, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial Apoptosis as the Primary Lesion Initiating Intestinal Radiation Damage in Mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.; Peña, L.A.; Haimovitz-Friedman, A.; Martin, S.; Green, D.; McLoughlin, M.; Cordon-Cardo, C.; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Acid Sphingomyelinase–Deficient Human Lymphoblasts and Mice Are Defective in Radiation-Induced Apoptosis. Cell 1996, 86, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Lacour, S.; Hammann, A.; Grazide, S.; Lagadic-Gossmann, D.; Athias, A.; Sergent, O.; Laurent, G.; Gambert, P.; Solary, E.; Dimanche-Boitrel, M.-T. Cisplatin-Induced CD95 Redistribution into Membrane Lipid Rafts of HT29 Human Colon Cancer Cells. Cancer Res. 2004, 64, 3593–3598. [Google Scholar] [CrossRef] [Green Version]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine -1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef]
- Modrak, D.E.; Cardillo, T.M.; Newsome, G.A.; Goldenberg, D.M.; Gold, D.V. Synergistic Interaction between Sphingomyelin and Gemcitabine Potentiates Ceramide-Mediated Apoptosis in Pancreatic Cancer. Cancer Res. 2004, 64, 8405–8410. [Google Scholar] [CrossRef] [Green Version]
- Nurminen, T.A.; Holopainen, J.M.; Zhao, H.; Kinnunen, P.K.J. Observation of Topical Catalysis by Sphingomyelinase Coupled To Microspheres. J. Am. Chem. Soc. 2002, 124, 12129–12134. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rotolo, J.A.; Mesicek, J.; Penate-Medina, T.; Rimner, A.; Liao, W.-C.; Yin, X.; Ragupathi, G.; Ehleiter, D.; Gulbins, E.; et al. Mitochondrial Ceramide-Rich Macrodomains Functionalize Bax upon Irradiation. PLoS ONE 2011, 6, e19783. [Google Scholar] [CrossRef] [Green Version]
- Sot, J.; Bagatolli, L.A.; Goñi, F.M.; Alonso, A. Detergent-Resistant, Ceramide-Enriched Domains in Sphingomyelin/Ceramide Bilayers. Biophys. J. 2006, 90, 903–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Argüello, M.B.; Basáñez, G.; Goñi, F.M.; Alonso, A. Different Effects of Enzyme-generated Ceramides and Diacylglycerols in Phospholipid Membrane Fusion and Leakage. J. Biol. Chem. 1996, 271, 26616–26621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñate-Medina, T.; Kraas, E.; Luo, K.; Humbert, J.; Zhu, H.; Mertens, F.; Gerle, M.; Rohwedder, A.; Damoah, C.; Will, O.; et al. Utilizing ICG Spectroscopical Properties for Real-Time Nanoparticle Release Quantification In vitro and In vivo in Imaging Setups. Curr. Pharm. Des. 2020, 26, 3828–3833. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, R.; Estelrich, J.; Busquets, M.A. Liposomes Loaded with Hydrophobic Iron Oxide Nanoparticles: Suitable T2; Contrast Agents for MRI. Int. J. Mol. Sci. 2016, 17, e1209. [Google Scholar]
- Goya, G.F.; Lima, J.E.; Arelaro, A.D.; Torres, T.; Rechenberg, H.R.; Rossi, L.M.; Marquina, C.; Ibarra, M.R. Magnetic Hyperthermia With Fe3O4 Nanoparticles: The Influence of Particle Size on Energy Absorption. IEEE Trans. Magn. 2008, 44, 4444–4447. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.; Torres, T.; Andrés-Vergés, M.; Costo, R.; De La Presa, P.; Serna, C.; Morales, M.; Marquina, C.; Ibarra, M.; Goya, G.F. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. J. Solid State Chem. 2009, 182, 2779–2784. [Google Scholar] [CrossRef]
- De Biasi, E.; Lima, E., Jr.; Vargas, J.M.; Zysler, R.D.; Arbiol, J.; Ibarra, A.; Goya, G.F.; Ibarra, M.R. Exchange bias in ferrite hollow nanoparticles originated by complex internal magnetic structure. Mater. Res. Express 2015, 2, 105001. [Google Scholar] [CrossRef]
- Kong, G.; Anyarambhatla, G.; Petros, W.P.; Braun, R.D.; Colvin, O.M.; Needham, D.; Dewhirst, M.W. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res. 2000, 60, 6950–6957. [Google Scholar]
- Ruiz-Argüello, M.B.; Goñi, F.M.; Alonso, A. Vesicle Membrane Fusion Induced by the Concerted Activities of Sphingomyelinase and Phospholipase C. J. Biol. Chem. 1998, 273, 22977–22982. [Google Scholar] [CrossRef] [Green Version]
- Adelstein, D.J.; Li, Y.; Adams, G.L.; Wagner, H.; Kish, J.A.; Ensley, J.F.; Schuller, D.E.; Forastiere, A.A. An Intergroup Phase III Comparison of Standard Radiation Therapy and Two Schedules of Concurrent Chemoradiotherapy in Patients With Unresectable Squamous Cell Head and Neck Cancer. J. Clin. Oncol. 2003, 21, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachaud, J.-M.; Cohen-Jonathan, E.; Alzieu, C.; David, J.-M.; Serrano, E.; Daly-Schveitzer, N. Combined postoperative radiotherapy and Weekly Cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int. J. Radiat. Oncol. 1996, 36, 999–1004. [Google Scholar] [CrossRef]
- Gerle, M.; Medina, T.P.; Gülses, A.; Chu, H.; Naujokat, H.; Wiltfang, J.; Acil, Y. Acid sphingomyelinase activity as an indicator of the cell stress in HPV-positive and HPV-negative head and neck squamous cell carcinoma. Med. Oncol. 2018, 35, 58. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; McLean, J.W.; Rizen, M.; Baluk, P.; Haskell, A.; Murphy, T.J.; Hanahan, D.; McDonald, D.M. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J. Clin. Investig. 1998, 101, 1401–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuelsson, E.; Shen, H.; Blanco, E.; Ferrari, M.; Wolfram, J. Contribution of Kupffer cells to liposome accumulation in the liver. Colloids Surf. B Biointerfaces 2017, 158, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Knolle, P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 2010, 10, 753–766. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.D.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [Green Version]
- Benezra, M.; Peñate-Medina, O.; Zanzonico, P.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Srikant, I.; et al. Multimodal silica nanoparticles as cancer-targeted probes in a human melanoma model. J. Clin. Invest. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [Green Version]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor Response to Radiotherapy Regulated by Endothelial Cell Apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef] [Green Version]
- Grassmé, H.; Jendrossek, V.; Bock, J.; Riehle, A.; Gulbins, E. Ceramide-Rich Membrane Rafts Mediate CD40 Clustering. J. Immunol. 2002, 168, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Vinod, V.; Sharma, A.; Shailaja, S.; Sridevi, K. Chemotherapy—A helping hand in oral tumors. Univ. J. Dent. Sci. 2015, 1, 82–87. [Google Scholar]
- Lima, E.; De Biasi, E.; Mansilla, M.V.; Saleta, M.E.; Granada, M.; Troiani, H.E.; Effenberger, F.B.; Rossi, L.M.; Rechenberg, H.R.; Zysler, R.D. Heat generation in agglomerated ferrite nanoparticles in an alternating magnetic field. J. Phys. D Appl. Phys. 2012, 46, 045002. [Google Scholar] [CrossRef]
- Kneepkens, E.; Fernandes, A.; Nicolay, K.; Grüll, H. Iron(III)-Based Magnetic Resonance–Imageable Liposomal T1 Contrast Agent for Monitoring Temperature-Induced Image-Guided Drug Delivery. Investig. Radiol. 2016, 51, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Kang, T.; Li, F.; Baik, S.; Shao, W.; Ling, D.; Hyeon, T. Surface design of magnetic nanoparticles for stimuli-responsive cancer imaging and therapy. Biomaterials 2017, 136, 98–114. [Google Scholar] [CrossRef]
- Hossann, M.; Kneidl, B.; Peller, M.; Lindner, L.; Winter, G. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387. [Google Scholar] [CrossRef] [Green Version]
- Bizzozero, L.; Cazzato, D.; Cervia, D.; Assi, E.; Simbari, F.; Pagni, F.; De Palma, C.; Monno, A.; Verdelli, C.; Querini, P.R.; et al. Acid sphingomyelinase determines melanoma progression and metastatic behaviour via the microphtalmia-associated transcription factor signalling pathway. Cell Death Differ. 2014, 21, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.Y.; Liu, J.J.; Sun, X.F.; Wang, N. Acid sphingomyelinase, a novel negative biomarker of ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2076–2083. [Google Scholar]
- Hannun, Y.A.; Newcomb, B. A new twist to the emerging functions of ceramides in cancer: Novel role for platelet acid sphingomyelinase in cancer metastasis. EMBO Mol. Med. 2015, 7, 692–694. [Google Scholar] [CrossRef]
- Rebillard, A.; Rioux-Leclercq, N.; Müller, C.; Bellaud, P.; Jouan, F.; Meurette, O.; Jouan, E.; Vernhet, L.; Le Quément, C.; Carpinteiro, A.; et al. Acid sphingomyelinase deficiency protects from cisplatin-induced gastrointestinal damage. Oncogene 2008, 27, 6590–6595. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, I.; Takahashi, T.; Abe, T.; Watanabe, W.; Takada, G. Distribution of acid sphingomyelinase in human various body fluids. Tohoku J. Exp. Med. 2000, 192, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, L.-A.; Tsai, P.-J.; Wang, Y.-C.; Lo, L.-W.; Yang, C.-S.; Wang, Y.-J. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology 2009, 20, 135101. [Google Scholar] [CrossRef] [PubMed]
- Shaghasemi, B.S.; Virk, M.M.; Reimhult, E. Optimization of Magneto-thermally Controlled Release Kinetics by Tuning of Magnetoliposome Composition and Structure. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef]
- Maier-HauffFrank, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; Van Landeghem, F.K.; Waldoefner, N.; Teichgraeber, U.; Pinkernelle, J.; Bruhn, H.; Neumann, F.; Thiesen, B.; et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J. Neuro-Oncol. 2006, 78, 7–14. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldöfner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Peñate-Medina, T.; Damoah, C.; Benezra, M.; Will, O.; Kairemo, K.; Humbert, J.; Sebens, S. Alpha-MSH Targeted Liposomal Nanoparticle for Imaging in Inflammatory Bowel Disease (IBD). Curr. Pharm. Des. 2020, 26, 3840–3846. [Google Scholar] [CrossRef]
- Medina, T.P.; Tower, R.J.; Ashkenani, F.; Appold, L.; Bötcher, M.; Huber, L.; Will, O.; Ling, Q.; Hauser, C.; Rohwedder, A.; et al. Multimodal Targeted Nanoparticle-Based Delivery System for Pancreatic Tumor Imaging in Cellular and Animal Models. Curr. Pharm. Des. 2020, 26, 1–11. [Google Scholar] [CrossRef]
- Medina, O.P.; Medina, T.P.; Humbert, J.; Qi, B.; Baum, W.; Will, O.; Damm, T.; Glüer, C. Using Alendronic Acid Coupled Fluorescently Labelled SM Liposomes as a Vehicle for Bone Targeting. Curr. Pharm. Des. 2020, 26. [Google Scholar] [CrossRef]
- Humbert, J.; Will, O.; Peñate-Medina, T.; Jansen, O.; Both, M.; Glüer, C.-C. Comparison of photoacoustic and fluorescence tomography for the in vivo imaging of ICG-labelled liposomes in the medullary cavity in mice. Photoacoustics 2020, 20, 100210. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; Pavone, I.; Micarelli, A.; Caporale, C. Transoral robotic surgery for the base of tongue squamous cell carcinoma: A preliminary comparison between da Vinci Xi and Si. J. Robot. Surg. 2017, 12, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Wi, H.S.; Lee, K.; Pak, H.K. Interfacial energy consideration in the organization of a quantum dot–lipid mixed system. J. Phys. Condens. Matter 2008, 20, 494211. [Google Scholar] [CrossRef]
- Arnold, P.; Himmels, P.; Weiß, S.; Decker, T.-M.; Markl, J.; Gatterdam, V.; Tampé, R.; Bartholomäus, P.; Dietrich, U.; Duerr, R. Antigenic and 3D structural characterization of soluble X4 and hybrid X4-R5 HIV-1 Env trimers. Retrovirology 2014, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatter, O. A new method for the evaluation of small-angle scattering data. J. Appl. Crystallogr. 1977, 10, 415–421. [Google Scholar] [CrossRef]
- Pedersen, J.S. Analysis of small-angle scattering data from colloids and polymer solutions: Modeling and least-squares fitting. Adv. Colloid Interface Sci. 1997, 70, 171–210. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñate Medina, T.; Gerle, M.; Humbert, J.; Chu, H.; Köpnick, A.-L.; Barkmann, R.; Garamus, V.M.; Sanz, B.; Purcz, N.; Will, O.; et al. Lipid-Iron Nanoparticle with a Cell Stress Release Mechanism Combined with a Local Alternating Magnetic Field Enables Site-Activated Drug Release. Cancers 2020, 12, 3767. https://doi.org/10.3390/cancers12123767
Peñate Medina T, Gerle M, Humbert J, Chu H, Köpnick A-L, Barkmann R, Garamus VM, Sanz B, Purcz N, Will O, et al. Lipid-Iron Nanoparticle with a Cell Stress Release Mechanism Combined with a Local Alternating Magnetic Field Enables Site-Activated Drug Release. Cancers. 2020; 12(12):3767. https://doi.org/10.3390/cancers12123767
Chicago/Turabian StylePeñate Medina, Tuula, Mirko Gerle, Jana Humbert, Hanwen Chu, Anna-Lena Köpnick, Reinhard Barkmann, Vasil M. Garamus, Beatriz Sanz, Nicolai Purcz, Olga Will, and et al. 2020. "Lipid-Iron Nanoparticle with a Cell Stress Release Mechanism Combined with a Local Alternating Magnetic Field Enables Site-Activated Drug Release" Cancers 12, no. 12: 3767. https://doi.org/10.3390/cancers12123767