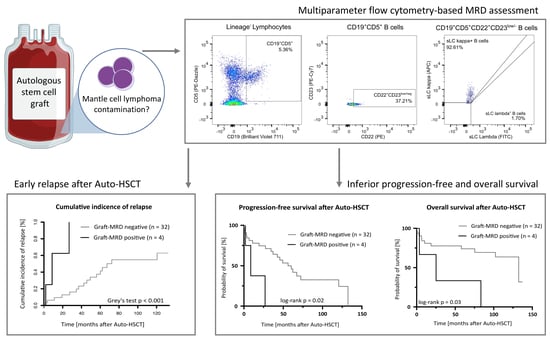
Impact of Mantle Cell Lymphoma Contamination of Autologous Stem Cell Grafts on Outcome after High-Dose Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Multiparameter Flow Cytometry Based MRD Assessment
2.3. Software and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Outcome after HDCT/Auto-HSCT
3.3. Sensitivity and Specificity of MFC Panel for MRD Assessment
3.4. Frequency and Prognostic Significance of MRD Detection in Autologous Stem Cell Grafts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dreyling, M.; Campo, E.; Hermine, O.; Jerkeman, M.; Le Gouill, S.; Rule, S.; Shpilberg, O.; Walewski, J.; Ladetto, M. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv62–iv71. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Lenz, G.; Hoster, E.; van Hoof, A.; Gisselbrecht, C.; Schmits, R.; Metzner, B.; Truemper, L.; Reiser, M.; Steinhauer, H. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood 2005, 105, 2677–2684. [Google Scholar] [PubMed] [Green Version]
- Dietrich, S.; Boumendil, A.; Finel, H.; Avivi, I.; Volin, L.; Cornelissen, J.; Jarosinska, R.; Schmid, C.; Finke, J.; Stevens, W. Outcome and prognostic factors in patients with mantle-cell lymphoma relapsing after autologous stem-cell transplantation: A retrospective study of the European Group for Blood and Marrow Transplantation (EBMT). Ann. Oncol. 2014, 25, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.; Kopp, H.-G.; Kanz, L.; Einsele, H. Myeloma cell contamination of peripheral blood stem-cell grafts can predict the outcome in multiple myeloma patients after high-dose chemotherapy and autologous stem-cell transplantation. J. Cancer Res. Clin. Oncol. 2005, 131, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kopp, H.; Yildirim, S.; Weisel, K.; Kanz, L.; Vogel, W. Contamination of autologous peripheral blood progenitor cell grafts predicts overall survival after high-dose chemotherapy in multiple myeloma. J. Cancer Res. Clin. Oncol. 2009, 135, 637–642. [Google Scholar] [CrossRef]
- Sharp, J.G.; Kessinger, A.; Mann, S.; Crouse, D.; Armitage, J.O.; Bierman, P.; Weisenburger, D. Outcome of high-dose therapy and autologous transplantation in non-Hodgkin’s lymphoma based on the presence of tumor in the marrow or infused hematopoietic harvest. J. Clin. Oncol. 1996, 14, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.G.; Joshi, S.S.; Armitage, J.O.; Bierman, P.; Coccia, P.; Harrington, D.; Kessinger, A.; Crouse, D.; Mann, S.; Weisenburger, D. Significance of detection of occult non-Hodgkin’s lymphoma in histologically uninvolved bone marrow by a culture technique. Blood 1992, 79, 1074–1080. [Google Scholar] [CrossRef]
- Andersen, N.S.; Pedersen, L.; Elonen, E.; Johnson, A.; Kolstad, A.; Franssila, K.; Langholm, R.; Ralfkiær, E.; Åkerman, M.; Eriksson, M. Primary treatment with autologous stem cell transplantation in mantle cell lymphoma: Outcome related to remission pretransplant. Eur. J. Haematol. 2003, 71, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Demirkazik, A.; Kessinger, A.; Armitage, J.O.; Bierman, P.J.; Lynch, J.; Vose, J.; Chan, W.; Sharp, J.G. Progenitor and lymphoma cells in blood stem cell harvests: Impact on survival following transplantation. Bone Marrow Transplant. 2001, 28, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Vose, J.M.; Sharp, G.; Chan, W.C.; Nichols, C.; Loh, K.; Inwards, D.; Rifkin, R.; Bierman, P.J.; Lynch, J.C.; Weisenburger, D.D. Autologous transplantation for aggressive non-Hodgkin’s lymphoma: Results of a randomized trial evaluating graft source and minimal residual disease. J. Clin. Oncol. 2002, 20, 2344–2352. [Google Scholar] [CrossRef]
- Lopez-Perez, R.; Garcia-Sanz, R.; Gonzalez, D.; Balanzategui, A.; Chillon, M.; Alaejos, I.; Mateos, M.; Caballero, M.; Mateo, G.; Nieto, M. The detection of contaminating clonal cells in apheresis products is related to response and outcome in multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Leukemia 2000, 14, 1493–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.; Yang, L.; Banihashemi, B.; Martin, L.; Halpenny, M.; Atkins, H.; Sabloff, M.; McDiarmid, S.A.; Huebsch, L.B.; Bence-Bruckler, I.; et al. Contaminating tumour cells in autologous PBSC grafts do not influence survival or relapse following transplant for multiple myeloma or B-cell non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2009, 43, 223–228. [Google Scholar] [CrossRef]
- Blystad, A.; Delabie, J.; Kvaløy, S.; Holte, H.; Vålerhaugen, H.; Ikonomou, I.; Kvalheim, G. Infused CD34+ cell dose, but not tumour cell content of peripheral blood progenitor cell grafts, predicts clinical outcome in patients with diffuse large B-cell lymphoma and follicular lymphoma grade 3 treated with high-dose therapy. Br. J. Haematol. 2004, 125, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Hoster, E.; Delfau-Larue, M.-H.; Beldjord, K.; Böttcher, S.; Asnafi, V.; Plonquet, A.; Siebert, R.; Callet-Bauchu, E.; Andersen, N. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: A European MCL intergroup study. Blood 2010, 115, 3215–3223. [Google Scholar] [CrossRef]
- Pott, C.; Schrader, C.; Gesk, S.; Harder, L.; Tiemann, M.; Raff, T.; Brüggemann, M.; Ritgen, M.; Gahn, B.; Unterhalt, M. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood 2006, 107, 2271–2278. [Google Scholar] [CrossRef]
- Liu, H.; Johnson, J.L.; Koval, G.; Malnassy, G.; Sher, D.; Damon, L.E.; Hsi, E.D.; Bucci, D.M.; Linker, C.A.; Cheson, B.D. Detection of minimal residual disease following induction immunochemotherapy predicts progression free survival in mantle cell lymphoma: Final results of CALGB 59909. Haematologica 2012, 97, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Cowan, A.J.; Stevenson, P.A.; Cassaday, R.D.; Graf, S.A.; Fromm, J.R.; Wu, D.; Holmberg, L.A.; Till, B.G.; Chauncey, T.R.; Smith, S.D. Pretransplantation minimal residual disease predicts survival in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in complete remission. Biol. Blood Marrow Transplant. 2016, 22, 380–385. [Google Scholar] [CrossRef]
- Pott, C. Minimal residual disease detection in mantle cell lymphoma: Technical aspects and clinical relevance. Semin. Hematol. 2011, 48, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Herrera, A.F.; Armand, P. Minimal residual disease assessment in lymphoma: Methods and applications. J. Clin. Oncol. 2017, 35, 3877–3887. [Google Scholar] [CrossRef]
- Budde, L.E.; Guthrie, K.A.; Till, B.G.; Press, O.W.; Chauncey, T.R.; Pagel, J.M.; Petersdorf, S.H.; Bensinger, W.I.; Holmberg, L.A.; Shustov, A.R. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J. Clin. Oncol. 2011, 29, 3023–3029. [Google Scholar] [CrossRef] [Green Version]
- Cassaday, R.D.; Guthrie, K.A.; Budde, E.L.; Thompson, L.; Till, B.G.; Press, O.W.; Chauncey, T.R.; Pagel, J.M.; Petersdorf, S.H.; Palanca-Wessels, M.C. Specific features identify patients with relapsed or refractory mantle cell lymphoma benefitting from autologous hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2013, 19, 1403–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisler, C.H.; Kolstad, A.; Laurell, A.; Räty, R.; Jerkeman, M.; Eriksson, M.; Nordström, M.; Kimby, E.; Boesen, A.M.; Nilsson-Ehle, H. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT). Blood 2010, 115, 1530–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoster, E.; Dreyling, M.; Klapper, W.; Gisselbrecht, C.; Van Hoof, A.; Kluin-Nelemans, H.C.; Pfreundschuh, M.; Reiser, M.; Metzner, B.; Einsele, H. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008, 111, 558–565. [Google Scholar] [CrossRef] [PubMed]
- McKay, P.; Leach, M.; Jackson, B.; Robinson, S.; Rule, S. Guideline for the management of mantle cell lymphoma. Br. J. Haematol. 2018, 182, 46–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Comprehensive Cancer Network. B-Cell Lymphomas (Version 3.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf (accessed on 5 May 2021).
- Forstpointner, R.; Unterhalt, M.; Dreyling, M.; Böck, H.-P.; Repp, R.; Wandt, H.; Pott, C.; Seymour, J.F.; Metzner, B.; Hänel, A. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006, 108, 4003–4008. [Google Scholar]
- Vidal, L.; Gafter-Gvili, A.; Dreyling, M.; Ghielmini, M.; Witzens-Harig, M.; Shpilberg, O.; Unterhalt, M.; Rummel, M.; Gurion, R. Maintenance treatment for patients with mantle cell lymphoma: A systematic review and meta-analysis of randomized trials. HemaSphere 2018, 2, e136. [Google Scholar] [CrossRef]
- Graf, S.; Stevenson, P.; Holmberg, L.; Till, B.; Press, O.; Chauncey, T.; Smith, S.; Philip, M.; Orozco, J.; Shustov, A. Maintenance rituximab after autologous stem cell transplantation in patients with mantle cell lymphoma. Ann. Oncol. 2015, 26, 2323–2328. [Google Scholar] [CrossRef]
- Hilal, T.; Wang, Z.; Almader-Douglas, D.; Rosenthal, A.; Reeder, C.B.; Jain, T. Rituximab maintenance therapy for mantle cell lymphoma: A systematic review and meta-analysis. Am. J. Hematol. 2018, 93, 1220–1226. [Google Scholar] [CrossRef]
- Vogel, W.; Scheding, S.; Kanz, L.; Brugger, W. Clinical applications of CD34+ peripheral blood progenitor cells (PBPC). Stem Cells 2000, 18, 87–92. [Google Scholar] [CrossRef]
- Le Gouill, S.; Thieblemont, C.; Oberic, L.; Moreau, A.; Bouabdallah, K.; Dartigeas, C.; Damaj, G.; Gastinne, T.; Ribrag, V.; Feugier, P. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N. Engl. J. Med. 2017, 377, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Anderson, M.A.; Pott, C.; Agarwal, R.; Handunnetti, S.; Hicks, R.J.; Burbury, K.; Turner, G.; Di Iulio, J.; Bressel, M. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N. Engl. J. Med. 2018, 378, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Jurczak, W.; Jerkeman, M.; Silva, R.S.; Rusconi, C.; Trneny, M.; Offner, F.; Caballero, D.; Joao, C.; Witzens-Harig, M. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet 2016, 387, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, R.; Lewis, D.; Crosbie, N.; Eyre, T.A.; Bolam, S.; Arasaretnam, A.; Creasey, T.; Goradia, H.; McMillan, A.; Dawi, S.; et al. Ibrutinib for mantle cell lymphoma at first relapse: A United Kingdom real-world analysis of outcomes in 211 patients. Br. J. Haematol. 2021, 193, 290–298. [Google Scholar] [CrossRef]
- Fisher, R.I.; Bernstein, S.H.; Kahl, B.S.; Djulbegovic, B.; Robertson, M.J.; De Vos, S.; Epner, E.; Krishnan, A.; Leonard, J.P.; Lonial, S. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2006, 24, 4867–4874. [Google Scholar] [CrossRef] [Green Version]
- Kane, R.C.; Dagher, R.; Farrell, A.; Ko, C.-W.; Sridhara, R.; Justice, R.; Pazdur, R. Bortezomib for the treatment of mantle cell lymphoma. Clin. Cancer Res. 2007, 13, 5291–5294. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.; Martin, P.; Shah, B.; Schuster, S.J.; Smith, S.M.; Furman, R.R.; Christos, P.; Rodriguez, A.; Svoboda, J.; Lewis, J. Lenalidomide plus rituximab as initial treatment for mantle-cell lymphoma. N. Engl. J. Med. 2015, 373, 1835–1844. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Fayad, L.; Wagner-Bartak, N.; Zhang, L.; Hagemeister, F.; Neelapu, S.S.; Samaniego, F.; McLaughlin, P.; Fanale, M.; Younes, A. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: A phase 1/2 clinical trial. Lancet Oncol. 2012, 13, 716–723. [Google Scholar] [CrossRef]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Cohen, J.B.; Hall, N.C.; Ruppert, A.S.; Jones, J.A.; Porcu, P.; Baiocchi, R.; Christian, B.A.; Penza, S.; Benson, D.M., Jr.; Flynn, J.; et al. Association of pre-transplantation positron emission tomography/computed tomography and outcome in mantle cell lymphoma. Bone Marrow Transpl. 2013, 48, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Bodet-Milin, C.; Touzeau, C.; Leux, C.; Sahin, M.; Moreau, A.; Maisonneuve, H.; Morineau, N.; Jardel, H.; Moreau, P.; Gallazini-Crépin, C. Prognostic impact of 18 F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: A retrospective study from the GOELAMS group. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1633–1642. [Google Scholar] [CrossRef]
- Damon, L.E.; Johnson, J.L.; Niedzwiecki, D.; Cheson, B.D.; Hurd, D.D.; Bartlett, N.L.; LaCasce, A.S.; Blum, K.A.; Byrd, J.C.; Kelly, M. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J. Clin. Oncol. 2009, 27, 6101–6108. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, S.; Tielesch, B.; Rieger, M.; Nickelsen, M.; Pott, C.; Witzens-Harig, M.; Kneba, M.; Schmitz, N.; Ho, A.D.; Dreger, P. Patterns and outcome of relapse after autologous stem cell transplantation for mantle cell lymphoma. Cancer 2011, 117, 1901–1910. [Google Scholar] [CrossRef]
- Cheminant, M.; Derrieux, C.; Touzart, A.; Schmit, S.; Grenier, A.; Trinquand, A.; Delfau-Larue, M.-H.; Lhermitte, L.; Thieblemont, C.; Ribrag, V. Minimal residual disease monitoring by 8-color flow cytometry in mantle cell lymphoma: An EU-MCL and LYSA study. Haematologica 2016, 101, 336–345. [Google Scholar] [CrossRef]
- Cheson, B.D.; Ansell, S.; Schwartz, L.; Gordon, L.I.; Advani, R.; Jacene, H.A.; Hoos, A.; Barrington, S.F.; Armand, P. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 2016, 128, 2489–2496. [Google Scholar] [CrossRef] [Green Version]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Pandey, P.; Choudhry, R.; Kashyap, R.; Mehrotra, M.; Naseem, S.; Nityanand, S. A prospective comparison of four techniques for diagnosis of paroxysmal nocturnal hemoglobinuria. Int. J. Lab. Hematol. 2007, 29, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Rawstron, A.; Böttcher, S.; Letestu, R.; Villamor, N.; Fazi, C.; Kartsios, H.; De Tute, R.; Shingles, J.; Ritgen, M.; Moreno, C. Improving efficiency and sensitivity: European Research Initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia 2013, 27, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Rawstron, A.; Fazi, C.; Agathangelidis, A.; Villamor, N.; Letestu, R.; Nomdedeu, J.; Palacio, C.; Stehlíková, O.; Kreuzer, K.; Liptrot, S. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: An European Research Initiative on CLL study. Leukemia 2016, 30, 929–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef] [Green Version]
- Wood, B.L. Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry. Cytom. Part B Clin. Cytom. 2016, 90, 47–53. [Google Scholar] [CrossRef]
- Borowitz, M.; Pullen, D.; Shuster, J.; Viswanatha, D.; Montgomery, K.; Willman, C.; Camitta, B. Minimal residual disease detection in childhood precursor–B-cell acute lymphoblastic leukemia: Relation to other risk factors. A Children’s Oncology Group study. Leukemia 2003, 17, 1566–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böttcher, S.; Ritgen, M.; Buske, S.; Gesk, S.; Klapper, W.; Hoster, E.; Hiddemann, W.; Unterhalt, M.; Dreyling, M.; Siebert, R. Minimal residual disease detection in mantle cell lymphoma: Methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica 2008, 93, 551–559. [Google Scholar] [CrossRef] [Green Version]
- Scrucca, L.; Santucci, A.; Aversa, F. Competing risk analysis using R: An easy guide for clinicians. Bone Marrow Transplant. 2007, 40, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.T. Cumulative incidence in competing risks data and competing risks regression analysis. Clin. Cancer Res. 2007, 13, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Gray, R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988, 16, 1141–1154. [Google Scholar] [CrossRef]
- Geisler, C.H.; Kolstad, A.; Laurell, A.; Andersen, N.S.; Pedersen, L.B.; Jerkeman, M.; Eriksson, M.; Nordström, M.; Kimby, E.; Boesen, A.M. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo–purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood J. Am. Soc. Hematol. 2008, 112, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Lefrere, F.; Delmer, A.; Suzan, F.; Levy, V.; Belanger, C.; Djabarri, M.; Arnulf, B.; Damaj, G.; Maillard, N.; Ribrag, V. Sequential chemotherapy by CHOP and DHAP regimens followed by high-dose therapy with stem cell transplantation induces a high rate of complete response and improves event-free survival in mantle cell lymphoma: A prospective study. Leukemia 2002, 16, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.; Dreger, P.; Caballero, D.; Corradini, P.; Geisler, C.; Ghielmini, M.; Le Gouill, S.; Kimby, E.; Rule, S.; Vitolo, U. The EBMT/EMCL consensus project on the role of autologous and allogeneic stem cell transplantation in mantle cell lymphoma. Leukemia 2015, 29, 464–473. [Google Scholar] [CrossRef]
- Van Dongen, J.; Lhermitte, L.; Böttcher, S.; Almeida, J.; van der Velden, V.; Flores-Montero, J.; Rawstron, A.; Asnafi, V.; Lecrevisse, Q.; Lucio, P. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012, 26, 1908–1975. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Lin, P.; Saksena, A.; Xu, J.; Wang, M.; Romaguera, J.; Yin, C.C.; Medeiros, L.J.; Li, S. CD5-negative mantle cell lymphoma. Am. J. Surg. Pathol. 2019, 43, 1052–1060. [Google Scholar] [CrossRef]
- DiRaimondo, F.; Albitar, M.; Huh, Y.; O’Brien, S.; Montillo, M.; Tedeschi, A.; Kantarjian, H.; Lerner, S.; Giustolisi, R.; Keating, M. The clinical and diagnostic relevance of CD23 expression in the chronic lymphoproliferative disease. Cancer 2002, 94, 1721–1730. [Google Scholar] [CrossRef] [Green Version]
- Dorfman, D.M.; Pinkus, G.S. Distinction between small lymphocytic and mantle cell lymphoma by immunoreactivity for CD23. Mod. Pathol. 1994, 7, 326–331. [Google Scholar] [PubMed]
- Bartee, E.; Chan, W.M.; Moreb, J.S.; Cogle, C.R.; McFadden, G. Selective purging of human multiple myeloma cells from autologous stem cell transplantation grafts using oncolytic myxoma virus. Biol. Blood Marrow Transplant. 2012, 18, 1540–1551. [Google Scholar] [CrossRef] [Green Version]
- Yahng, S.A.; Yoon, J.H.; Shin, S.H.; Lee, S.E.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Lee, S.; Kim, H.J.; Min, C.K. Influence of ex vivo purging with C lini MACS CD 34+ selection on outcome after autologous stem cell transplantation in non-H odgkin lymphoma. Br. J. Haematol. 2014, 164, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Robinson, S.N.; Nieto, Y.; Jones, R.J.; Gocke, C.D.; Lu, J.; Giralt, S.A.; Jones, R.B.; Decker, W.K.; Xing, D. Ex vivo graft purging and expansion of autologous blood progenitor cell products from patients with multiple myeloma. Cancer Res. 2011, 71, 5040–5049. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Characteristic | All Patients | Graft-MRD Negative Patients | Graft-MRD Positive Patients | p |
|---|---|---|---|---|
| Demographics | ||||
| Number of patients | 36 | 32 | 4 | |
| Follow-up time (months) # | 104 | 104 | Undefined | |
| Male (no.[%)] | 29 (81) | 26 (81) | 4 (100) | 1.0 † |
| Median age (range) | 58 (43–69) | 58 (43–69) | 57 (53–62) | 1.0 $ |
| Disease characteristics (no.[%)] | ||||
| Stage I–II | 2 (6) | 2 (6) | 0 (0) | 0.70 § |
| Stage III–IV | 34 (94) | 30 (94) | 4 (100) | |
| BM involvement at diagnosis | 28 (78) | 24 (75) | 4 (100) | 0.56 † |
| Extranodal disease at diagnosis | 14 (39) | 12 (38) | 2 (50) | 0.63 † |
| MIPI risk group (no.[%)] | 0.39 § | |||
| Low risk | 13 (36) | 12 (38) | 1 (33) | |
| Intermediate risk | 11 (31) | 9 (28) | 1 (33) | |
| High risk | 8 (22) | 8 (25) | 1 (33) | |
| n.a. | 4 (11) | 3 (9) | 1 (33) | |
| First-line therapy (no.[%)] | 0.08 § | |||
| R-CHOP / R-DHAP | 27 (75) | 25 (78) | 2 (50) | |
| R-CHOP | 4 (11) | 4 (13) | 0 (0) | |
| Other (Rituximab-containing) | 5 (14) | 3 (9) | 2 (50) | |
| Auto-HSCT setting | 0.39 † | |||
| First-line consolidation | 32 (89) | 29 (91) | 3 (75) | |
| Second-line therapy | 4 (11) | 3 (9) | 1 (25) | |
| Response prior to Auto-HSCT | 0.28 † | |||
| CR | 22 (61) | 21 (66) | 1 (25) | |
| PR | 14 (39) | 11 (34) | 3 (75) | |
| Mobilization regimen (no.[%)] | 1.0 † | |||
| R-DHAP | 29 (81) | 26 (81) | 3 (75) | |
| Other | 7 (19) | 6 (19) | 1 (25) | |
| HDCT regimen (no.[%)] | 0.39 † | |||
| BEAM | 27 (75) | 23 (72) | 4 (100) | |
| Other | 9 (25) | 9 (28) | 0 (0) | |
| Maintenance therapy (no.[%)] | 0.76 § | |||
| Rituximab | 5 (14) | 4 (13) | 1 (25) | |
| Ibrutinib | 1 (3) | 1 (3) | 0 (0) | |
| None | 30 (83) | 27 (84) | 3 (75) | |
| Median CD34+ cell count / kg body weight (range) | ||||
| Total harvested | 8.0 (4–29) | 7.9 (4–29) | 11.3 (9–14) | 0.10 $ |
| Re-transfused at Auto-HSCT | 5.2 (2–11) | 5.0 (2–11) | 5.8 (4–7) | 0.53 $ |
| Unit | Total Events | CD45 + Cells | CD19 + CD5 + | % CD19 + CD5 + | CD19 + CD5 + CD22 + CD23 low/− | % CD19 + CD5 + CD22 + CD23 low/− |
|---|---|---|---|---|---|---|
| Events Median (SD) | 1,027,505 (238,823) | 701,150 (215,308) | 5382 (4443) | 0.64 (0.53) | 1650 (750) | 0.27% (0.12) |
| LC ratio Kappa/Lambda Median (SD) | 1.67 (0.65) | |||||
| Level of blank | 3144 | 0.48% | ||||
| Level of detection | 4160 | 0.64% | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roerden, M.; Wirths, S.; Sökler, M.; Bethge, W.A.; Vogel, W.; Walz, J.S. Impact of Mantle Cell Lymphoma Contamination of Autologous Stem Cell Grafts on Outcome after High-Dose Chemotherapy. Cancers 2021, 13, 2558. https://doi.org/10.3390/cancers13112558
Roerden M, Wirths S, Sökler M, Bethge WA, Vogel W, Walz JS. Impact of Mantle Cell Lymphoma Contamination of Autologous Stem Cell Grafts on Outcome after High-Dose Chemotherapy. Cancers. 2021; 13(11):2558. https://doi.org/10.3390/cancers13112558
Chicago/Turabian StyleRoerden, Malte, Stefan Wirths, Martin Sökler, Wolfgang A. Bethge, Wichard Vogel, and Juliane S. Walz. 2021. "Impact of Mantle Cell Lymphoma Contamination of Autologous Stem Cell Grafts on Outcome after High-Dose Chemotherapy" Cancers 13, no. 11: 2558. https://doi.org/10.3390/cancers13112558