Deep Phenotypic Characterisation of CTCs by Combination of Microfluidic Isolation (IsoFlux) and Imaging Flow Cytometry (ImageStream)
Abstract
:Simple Summary
Abstract
1. Introduction
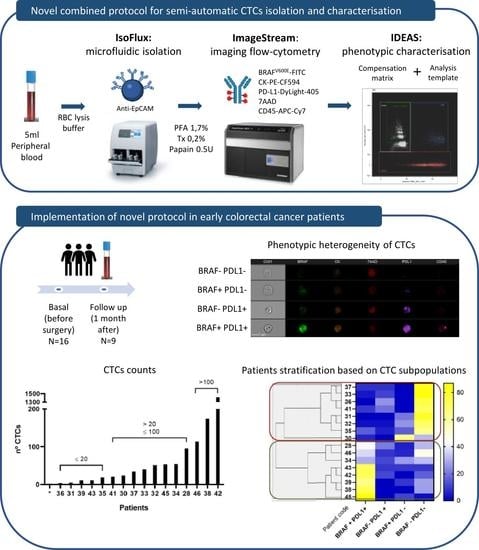
2. Materials and Methods
2.1. Protocol Optimisation Experiments
2.2. Validation Experiments with Early Colorectal Cancer Samples
2.3. Statistical Analyses
3. Results
3.1. Protocol Implementation for CTC Enrichment and Phenotypic Characterisation Combining the IsoFlux and ImageStream Platforms
3.2. Analysis Protocol on the IDEAS Software
3.3. Isolation and Phenotypic Characterisation of CTCs from Early Colorectal Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabel, L.; Proudhon, C.; Gortais, H.; Loirat, D.; Coussy, F.; Pierga, J.Y.; Bidard, F.C. Circulating tumor cells: Clinical validity and utility. Int. J. Clin. Oncol. 2017, 22, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ureña, M.; Ortega, F.G.; de Miguel-Pérez, D.; Rodriguez-Martínez, A.; García-Puche, J.L.; Ilyine, H.; Lorente, J.A.; Exposito-Hernandez, J.; Garrido-Navas, M.C.; Delgado-Ramirez, M.; et al. Circulating tumor cells criteria (CyCAR) versus standard RECIST criteria for treatment response assessment in metastatic colorectal cancer patients. J. Transl. Med. 2018, 16, 251. [Google Scholar] [CrossRef]
- De Miguel-Pérez, D.; Bayarri-Lara, C.I.; Ortega, F.G.; Russo, A.; Rodriguez, M.J.M.; Alvarez-Cubero, M.J.; Serrano, E.M.; Lorente, J.A.; Rolfo, C.; Serrano, M.J. Post-surgery circulating tumor cells and AXL overexpression as new poor prognostic biomarkers in resected lung adenocarcinoma. Cancers 2019, 11, 1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadal, R.; Ortega, F.G.; Salido, M.; Lorente, J.A.; Rodríguez-Rivera, M.; Delgado-Rodríguez, M.; Macià, M.; Fernández, A.; Corominas, J.M.; García-Puche, J.L.; et al. CD133 expression in circulating tumor cells from breast cancer patients: Potential role in resistance to chemotherapy. Int. J. Cancer 2013, 133, 2398–2407. [Google Scholar] [CrossRef] [Green Version]
- Cabezas-Camarero, S.; De la Orden-García, V.; Veganzones-de-Castro, S.; Mediero-Valeros, B.; Fuentes-Ferrer, M.E.; Sánchez Ruiz, A.C.; Provencio, M.; Aranda, E.; Sastre Valera, J.; Diaz-Rubio, E. Performance of two immunoafinity-based methods for CTC detection and molecular characterization in advanced colorectal cancer. J. Clin. Oncol. 2018, 36, e15648. [Google Scholar] [CrossRef]
- Harb, W.; Fan, A.; Tran, T.; Danila, D.C.; Keys, D.; Schwartz, M.; Ionescu-Zanetti, C. Mutational Analysis of Circulating Tumor Cells Using a Novel Microfluidic Collection Device and qPCR Assay. Transl. Oncol. 2013, 6, 528. [Google Scholar] [CrossRef] [Green Version]
- Alva, A.; Friedlander, T.; Clark, M.; Huebner, T.; Daignault, S.; Hussain, M.; Lee, C.; Hafez, K.; Hollenbeck, B.; Weizer, A.; et al. Circulating tumor cells as potential biomarkers in bladder cancer. J. Urol. 2015, 194, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Luk, A.; Young, F.P.; Lynch, D.; Chua, W.; Balakrishnar, B.; De Souza, P.; Becker, T.M. Droplet Digital PCR Based Androgen Receptor Variant 7 (AR-V7) Detection from Prostate Cancer Patient Blood Biopsies. Int. J. Mol. Sci. 2016, 17, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, Q.; Zhang, Q.; Zheng, C.; Dai, W.; Zhang, B.; Ionescu-Zanetti, C.; Lin, Z.; Zhang, L. Detection of circulating tumour cells in patients with epithelial ovarian cancer by a microfluidic system. Int J. Clin. Exp. Pathol. 2017, 10, 9599–9606. [Google Scholar]
- Sánchez-Lorencio, M.I.; Ramirez, P.; Saenz, L.; Martínez Sánchez, M.V.; De La Orden, V.; Mediero-Valeros, B.; Veganzones-De-Castro, S.; Baroja-Mazo, A.; Revilla Nuin, B.; Gonzalez, M.R.; et al. Comparison of Two Types of Liquid Biopsies in Patients with Hepatocellular Carcinoma Awaiting Orthotopic Liver Transplantation. Transplant. Proc. 2015, 47, 2639–2642. [Google Scholar] [CrossRef]
- Ramirez, P.; Sáenz, L.; Cascales-Campos, P.A.; González Sánchez, M.R.; Llàcer-Millán, E.; Sánchez-Lorencio, M.I.; Díaz-Rubio, E.; De La Orden, V.; Mediero-Valeros, B.; Navarro, J.L.; et al. Oncological Evaluation by Positron-emission Tomography, Circulating Tumor Cells and Alpha Fetoprotein in Patients With Hepatocellular Carcinoma on the Waiting List for Liver Transplantation. Transplant. Proc. 2016, 48, 2962–2965. [Google Scholar] [CrossRef] [PubMed]
- Vilhav, C.; Engström, C.; Naredi, P.; Novotny, A.; Bourghardt-Fagman, J.; Iresjö, B.-M.; Asting, A.G.; Lundholm, K. Fractional uptake of circulating tumor cells into liver-lung compartments during curative resection of periampullary cancer. Oncol. Lett. 2018, 16, 6331. [Google Scholar] [CrossRef] [Green Version]
- Amado, V.; González-Rubio, S.; Zamora, J.; Alejandre, R.; Espejo-Cruz, M.L.; Linares, C.; Sánchez-Frías, M.; García-Jurado, G.; Montero, J.L.; Ciria, R.; et al. Clearance of Circulating Tumor Cells in Patients with Hepatocellular Carcinoma Undergoing Surgical Resection or Liver Transplantation. Cancers 2021, 13, 2476. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Raba, K.; Guglielmi, R.; Behrens, B.; Van Dalum, G.; Flügen, G.; Koch, A.; Patel, S.; Knoefel, W.T.; Stoecklein, N.H.; et al. Magnetic-Based Enrichment of Rare Cells from High Concentrated Blood Samples. Cancers 2020, 12, 933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toh, J.W.T.; Lim, S.H.; MacKenzie, S.; de Souza, P.; Bokey, L.; Chapuis, P.; Spring, K.J. Association between Microsatellite Instability Status and Peri-Operative Release of Circulating Tumour Cells in Colorectal Cancer. Cells 2020, 9, 425. [Google Scholar] [CrossRef] [Green Version]
- Ortyn, W.E.; Hall, B.E.; George, T.C.; Frost, K.; Basiji, D.A.; Perry, D.J.; Zimmerman, C.A.; Coder, D.; Morrissey, P.J. Sensitivity measurement and compensation in spectral imaging. Cytom. Part A 2006, 69, 852–862. [Google Scholar] [CrossRef] [PubMed]
- López-Riquelme, N.; Minguela, A.; Villar-Permuy, F.; Ciprian, D.; Castillejo, A.; Álvarez-López, M.R.; Soto, J.L. Imaging cytometry for counting circulating tumor cells: Comparative analysis of the CellSearch vs ImageStream systems. Apmis 2013, 121, 1139–1143. [Google Scholar] [CrossRef]
- Ogle, L.F.; Orr, J.G.; Willoughby, C.E.; Hutton, C.; McPherson, S.; Plummer, R.; Boddy, A.V.; Curtin, N.J.; Jamieson, D.; Reeves, H.L. Imagestream detection and characterisation of circulating tumour cells–A liquid biopsy for hepatocellular carcinoma? J. Hepatol. 2016, 65, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, B.M.; Ogle, L.F.; O’donnell, R.L.; Hayes, N.; Malik, U.; Curtin, N.J.; Boddy, A.V.; Plummer, E.R.; Edmondson, R.J.; Reeves, H.L.; et al. High-resolution imaging for the detection and characterisation of circulating tumour cells from patients with oesophageal, hepatocellular, thyroid and ovarian cancers. Int. J. Cancer 2016, 138, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Shirai, K.; Ijiri, Y.; Morita, E.; Yoshida, T.; Iwanaga, S.; Yanagida, M. Integrated system for detection and molecular characterization of circulating tumor cells. PLoS ONE 2020, 15, e0237506. [Google Scholar] [CrossRef]
- Merugu, S.; Chen, L.; Gavens, E.; Gabra, H.; Brougham, M.; Makin, G.; Ng, A.; Murphy, D.; Gabriel, A.S.; Robinson, M.L.; et al. Detection of Circulating and Disseminated Neuroblastoma Cells Using the ImageStream Flow Cytometer for Use as Predictive and Pharmacodynamic Biomarkers. Clin. Cancer Res. 2020, 26, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhuang, W.; Hu, Y.; Zhu, L. Clinical significance of peripheral circulating tumor cell counts in colorectal polyps and non-metastatic colorectal cancer. World J. Surg. Oncol. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, W.-S.; Chen, J.-S.; Shao, H.-J.; Wu, J.-C.; Lai, J.-M.; Lu, S.-H.; Hung, T.-F.; Chiu, Y.-C.; You, J.-F.; Hsieh, P.-S.; et al. Circulating Tumor Cell Count Correlates with Colorectal Neoplasm Progression and Is a Prognostic Marker for Distant Metastasis in Non-Metastatic Patients. Sci. Rep. 2016, 6, 24517. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, A.; Dall, K.; Brandt, B.; Geisen, R.; Röder, C.; Schafmayer, C.; Becker, T.; Hinz, S.; Sebens, S. Longitudinal Analysis of Circulating Tumor Cells in Colorectal Cancer Patients by a Cytological and Molecular Approach: Feasibility and Clinical Application. Front. Oncol. 2021, 11, 646885. [Google Scholar] [CrossRef] [PubMed]
- Bork, U.; Rahbari, N.N.; Schölch, S.; Reissfelder, C.; Kahlert, C.; Büchler, M.W.; Weitz, J.; Koch, M. Circulating tumour cells and outcome in non-metastatic colorectal cancer: A prospective study. Br. J. Cancer 2015, 112, 1306–1313. [Google Scholar] [CrossRef]
- Sotelo, M.J.; Sastre, J.; Maestro, M.L.; Veganzones, S.; Viéitez, J.M.; Alonso, V.; Grávalos, C.; Escudero, P.; Vera, R.; Aranda, E.; et al. Role of circulating tumor cells as prognostic marker in resected stage III colorectal cancer. Ann. Oncol. 2015, 26, 535–541. [Google Scholar] [CrossRef]
- Chu, H.-Y.; Yang, C.-Y.; Yeh, P.-H.; Hsu, C.-J.; Chang, L.-W.; Chan, W.-J.; Lin, C.-P.; Lyu, Y.-Y.; Wu, W.-C.; Lee, C.-W.; et al. Highly Correlated Recurrence Prognosis in Patients with Metastatic Colorectal Cancer by Synergistic Consideration of Circulating Tumor Cells/Microemboli and Tumor Markers CEA/CA19-9. Cells 2021, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.-Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF Mutation and Microsatellite Instability on the Pattern of Metastatic Spread and Prognosis in Metastatic Colorectal Cancer. Cancer 2011, 117, 4623. [Google Scholar] [CrossRef] [Green Version]
- Takeda, H.; Sunakawa, Y. Management of BRAF Gene Alterations in Metastatic Colorectal Cancer: From Current Therapeutic Strategies to Future Perspectives. Front. Oncol. 2021, 11, 942. [Google Scholar] [CrossRef]
- Hummel, M.; Hegewisch-Becker, S.; Neumann, J.H.L.; Vogel, A. BRAF testing in metastatic colorectal carcinoma and novel, chemotherapy-free therapeutic options. Der. Pathol. 2021, 42, 98–109. [Google Scholar] [CrossRef]
- Rowland, A.; Dias, M.M.; Wiese, M.D.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer 2015, 112, 1888–1894. [Google Scholar] [CrossRef] [Green Version]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair deficient/microsatellite instability–high colorectal cancer (CheckMate 142): Results of an open-label, multicentre, phase 2 study. Lancet. Oncol. 2017, 18, 1182. [Google Scholar] [CrossRef]
- Feng, D.; Qin, B.; Pal, K.; Sun, L.; Dutta, S.; Dong, H.; Liu, X.; Mukhopadhyay, D.; Huang, S.; Sinicrope, F.A. BRAFV600E-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene 2019, 38, 6752–6766. [Google Scholar] [CrossRef]
- Cohen, R.; Svrcek, M.; Dreyer, C.; Cervera, P.; Duval, A.; Pocard, M.; Fléjou, J.-F.; de Gramont, A.; André, T. New Therapeutic Opportunities Based on DNA Mismatch Repair and BRAF Status in Metastatic Colorectal Cancer. Curr. Oncol. Reports 2016, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- ELfishawy, M.; Abd-ELaziz, S.A.; Hegazy, A.; EL-yasergy, D.F. Immunohistochemical Expression of Programmed Death Ligand-1 (PDL-1) in Colorectal carcinoma and Its Correlation with Stromal Tumor Infiltrating Lymphocytes. Asian Pac. J. Cancer Prev. 2020, 21, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, G.; Peng, C.; Chen, Y.; Li, J.; Yang, R.; Wu, M.; Lu, P. Expression of STING and PD-L1 in colorectal cancer and their correlation with clinical prognosis. Int. J. Clin. Exp. Pathol. 2018, 11, 1256. [Google Scholar]
- Satelli, A.; Batth, I.S.; Brownlee, Z.; Rojas, C.; Meng, Q.H.; Kopetz, S.; Li, S. Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci. Rep. 2016, 6, 28910. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, T.S.A.; Meiners, J.; Riethdorf, S.; König, A.; Melling, N.; Gorges, T.; Karstens, K.-F.; Izbicki, J.R.; Pantel, K.; Reeh, M. Prognostic value of preoperative circulating tumor cells counts in patients with UICC stage I-IV colorectal cancer. PLoS ONE 2021, 16, e0252897. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-J.; Hong, H.-J.; Sun, J.; Yu, C.-R.; Liu, H.-S.; Li, P.-Y.; Zheng, M.-H. Detection and Clinical Value of Circulating Tumor Cells as an Assisted Prognostic Marker in Colorectal Cancer Patients. Cancer Manag. Res. 2021, 13, 4567. [Google Scholar] [CrossRef]
- Ishizawa, K.; Yamanaka, M.; Saiki, Y.; Miyauchi, E.; Fukushige, S.; Akaishi, T.; Asao, A.; Mimori, T.; Saito, R.; Tojo, Y.; et al. CD45+CD326+ Cells are Predictive of Poor Prognosis in Non–Small Cell Lung Cancer Patients. Clin. Cancer Res. 2019, 25, 6756–6763. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Lin, J.; Zhang, T.; Luo, J.; Nie, D.; Li, M.; Hu, X.; Zheng, Y.; Huang, X.; Xiao, Z. Metastatic Colorectal Cancer Patient With Microsatellite Stability and BRAFV600E Mutation Showed a Complete Metabolic Response to PD-1 Blockade and Bevacizumab: A Case Report. Front. Oncol. 2021, 11, 652394. [Google Scholar] [CrossRef]
- Mostert, B.; Jiang, Y.; Sieuwerts, A.M.; Wang, H.; Bolt-de Vries, J.; Biermann, K.; Kraan, J.; Lalmahomed, Z.; van Galen, A.; de Weerd, V.; et al. KRAS and BRAF mutation status in circulating colorectal tumor cells and their correlation with primary and metastatic tumor tissue. Int. J. Cancer 2013, 133, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Azcue, P.; Encío, I.; Setas, D.G.; Alecha, J.S.; Galbete, A.; Mercado, M.; Vera, R.; Gomez-Dorronsoro, M.L. PD-L1 as a Prognostic Factor in Early-Stage Colon Carcinoma within the Immunohistochemical Molecular Subtype Classification. Cancers 2021, 13, 1943. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; del Bene, G.; Prete, A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Jiang, Y.; Li, P.; Wang, Y.; Xue, J.; Li, N.; Li, D.; Wang, R.; Dang, Y.; Hu, Z.; et al. Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 2018, 7, e1438111. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef]





| Primary | Secondary | Fluorophore | Dilution | Reference | Brand | IS Ch |
|---|---|---|---|---|---|---|
| * Mouse IgG2a anti-cytokeratin 7/8 | None | FITC | 1/100 | 130-060-301 | Miltenyi, Germany | 2 |
| Rabbit monoclonal [K21-F] to BRAF (mutatedV600E) | None | FITC | 1/200 | ab175637 | Abcam, UK | 2 |
| Mouse monoclonal [CAM5.2] anti-human cytokeratin (7/8) | None | PE-CF594 | 1/100 | 563615 | BD, USA | 4 |
| None | None | 7-AAD | 1/100 | 00-6993-50 | Invitrogen, USA | 5 |
| Rabbit polyclonal to PD-L1 | Goat anti-rabbit IgG | DyLight 405 | 1/100 1/100 | PA5-28115 35551 | Thermo Fisher, USA | 7 |
| Mouse monoclonal [2D1] anti-human CD45 APC CY7 | None | APC-Cy7 | 1/100 | 557833 | BD, USA | 12 |
| Patient Number | CK+ CTCs (N) | CTC Clusters (N) | BRAFV600E+ PD-L1+ (%) | BRAFV600E− PD-L1+ (%) | BRAFV600E+ PD-L1− (%) | BRAFV600E− PD-L1− (%) | PD-L1 Nuclear Location |
|---|---|---|---|---|---|---|---|
| HC 1 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| HC 2 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| HC 3 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| HC 4 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| HC 5 | 0 | 0 | 0 | 0 | 0 | 0 | N/A |
| 37 | 24 | 0 | 8.3 | 8.3 | 0.0 | 83.3 | Y |
| 33 | 54 | 1 | 3.7 | 11.1 | 0.0 | 85.2 | Y |
| 36 | 4 | 0 | 0.0 | 25.0 | 0.0 | 75.0 | N |
| 41 | 21 | 0 | 9.5 | 19.1 | 0.0 | 71.4 | N |
| 31 | 5 | 0 | 0.0 | 0.0 | 20.0 | 80.0 | N |
| 32 | 40 | 0 | 2.5 | 2.5 | 25.0 | 70.0 | N |
| 35 | 19 | 1 | 15.8 | 5.3 | 15.8 | 63.2 | N |
| 30 | 51 | 0 | 5.9 | 0.0 | 62.8 | 31.4 | N |
| 28 | 96 | 0 | 42.7 | 20.8 | 5.2 | 31.3 | N |
| 46 | 114 | 0 | 36.8 | 8.8 | 6.1 | 48.3 | Y |
| 34 | 34 | 0 | 29.4 | 11.8 | 26.5 | 32.4 | Y |
| 43 | 11 | 1 | 72.7 | 9.1 | 9.1 | 9.1 | Y |
| 42 | 1421 | 92 | 72.1 | 11.8 | 0.0 | 16.1 | Y |
| 39 | 11 | 0 | 63.6 | 0.0 | 9.1 | 27.3 | N |
| 38 | 174 | 7 | 73.0 | 0.0 | 0.0 | 27.0 | Y |
| 45 | 53 | 0 | 86.8 | 0.0 | 11.3 | 1.9 | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Rodríguez, A.J.; Molina-Vallejo, M.P.; Aznar-Peralta, I.; González Puga, C.; Cañas García, I.; González, E.; Lorente, J.A.; Serrano, M.J.; Garrido-Navas, M.C. Deep Phenotypic Characterisation of CTCs by Combination of Microfluidic Isolation (IsoFlux) and Imaging Flow Cytometry (ImageStream). Cancers 2021, 13, 6386. https://doi.org/10.3390/cancers13246386
Ruiz-Rodríguez AJ, Molina-Vallejo MP, Aznar-Peralta I, González Puga C, Cañas García I, González E, Lorente JA, Serrano MJ, Garrido-Navas MC. Deep Phenotypic Characterisation of CTCs by Combination of Microfluidic Isolation (IsoFlux) and Imaging Flow Cytometry (ImageStream). Cancers. 2021; 13(24):6386. https://doi.org/10.3390/cancers13246386
Chicago/Turabian StyleRuiz-Rodríguez, Antonio J., Maria P. Molina-Vallejo, Inés Aznar-Peralta, Cristina González Puga, Inés Cañas García, Encarna González, Jose A. Lorente, M. Jose Serrano, and M. Carmen Garrido-Navas. 2021. "Deep Phenotypic Characterisation of CTCs by Combination of Microfluidic Isolation (IsoFlux) and Imaging Flow Cytometry (ImageStream)" Cancers 13, no. 24: 6386. https://doi.org/10.3390/cancers13246386