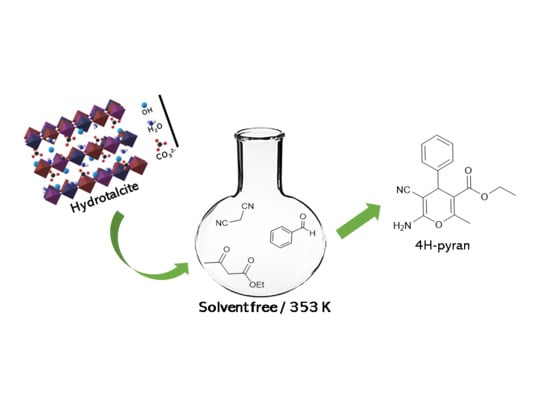
Ternary Hydrotalcites in the Multicomponent Synthesis of 4H-Pyrans
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Catalytic Activity
2.3. Reusability
3. Materials and Methods
3.1. Synthesis of Hydrotalcites
3.2. Characterization of Hydrotalcite
3.3. Catalytic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chagas, L.H.; De Carvalho, G.S.G.; Do Carmo, W.R.; San Gil, R.A.S.; Chiaro, S.S.X.; Leitão, A.A.; Diniz, R.; De Sena, L.A.; Achete, C.A. MgCoAl and NiCoAl LDHs Synthesized by the Hydrothermal Urea Hydrolysis Method: Structural Characterization and Thermal Decomposition. Mater. Res. Bull. 2015, 64, 207–215. [Google Scholar] [CrossRef]
- Nguyen, H.K.D.; Nguyen, T.D.; Vu, A.T.; Nguyen, T.T. Preparation of Acid–Base Bi-Functional Hydrotalcite Based Catalyst for Converting Vietnamese Coconut Oil to Green Hydrocarbons. Chem. Pap. 2017, 71, 961–970. [Google Scholar] [CrossRef]
- Lee, G.; Kang, J.Y.; Yan, N.; Suh, Y.-W.; Jung, J.C. Simple Preparation Method for Mg–Al Hydrotalcites as Base Catalysts. J. Mol. Catal. A Chem. 2016, 423, 347–355. [Google Scholar] [CrossRef]
- Pagadala, R.; Maddila, S.; Dasireddy, V.D.B.C.; Jonnalagadda, S.B. Zn-VCO3 Hydrotalcite: A Highly Efficient and Reusable Heterogeneous Catalyst for the Hantzsch Dihydropyridine Reaction. Catal. Commun. 2014, 45, 148–152. [Google Scholar] [CrossRef]
- Kolb, J.; Beck, B.; Dömling, A. Simultaneous Assembly of the β-Lactam and Thiazole Moiety by a New Multicomponent Reaction. Tetrahedron Lett. 2002, 43, 6897–6901. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent Reactions: Advanced Tools for Sustainable Organic Synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Kshirsagar, S.W.; Patil, N.R.; Samant, S.D. One-Pot Synthesis of 2-Amino-5-Nitro-4,6-Diarylcyclohex-1-Ene-1,3,3-Tricarbo-Nitriles by Condensation of Aldehyde, Malononitrile, and Nitromethane in the Presence of Mg–Al HT under Solvent-Free Condition. Tetrahedron Lett. 2010, 51, 2924–2927. [Google Scholar] [CrossRef]
- Surpur, M.P.; Kshirsagar, S.; Samant, S.D. Exploitation of the Catalytic Efficacy of Mg/Al Hydrotalcite for the Rapid Synthesis of 2-Aminochromene Derivatives Via a Multicomponent Strategy in the Presence of Microwaves. Tetrahedron Lett. 2009, 50, 719–722. [Google Scholar] [CrossRef]
- Azarifar, D.; Tadayoni, M.; Ghaemi, M. γ-Fe2O3@Cu3Al-LDH-TUD as a New Amphoteric, Highly Efficient and Recyclable Heterogeneous Catalyst for the Solvent-free Synthesis of Dihydropyrano[3,2-c]Pyrazoles and Dihydropyrano[3,2-c]Chromens. Appl. Organomet. Chem. 2018, 32, e4293. [Google Scholar] [CrossRef]
- Reddy, T.N.; Ravinder, M.; Bikshapathi, R.; Sujitha, P.; Kumar, C.G.; Rao, V.J. Design, Synthesis, and Biological Evaluation of 4-H Pyran Derivatives as Antimicrobial and Anticancer Agents. Med. Chem. Res. 2017, 26, 2832–2844. [Google Scholar] [CrossRef]
- Udhaya Kumar, C.; Pillai, M.V.; Gokula Krishnan, K.; Ramalingan, C. Synthesis, Experimental and Theoretical Studies on Highly Functionalized Novel 4H-Pyran-3-Carboxylate. J. Mol. Struct. 2019, 1175, 587–592. [Google Scholar] [CrossRef]
- Aytemir, M.D.; Çaliş, Ü.; Özalp, M. Synthesis and Evaluation of Anticonvulsant and Antimicrobial Activities of 3-Hydroxy-6-methyl-2-substituted 4H-Pyran-4-one Derivatives. Arch. der Pharm. 2004, 337, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Horn, A.; Khatri, H.; Sereda, G. A Green One-Pot Multicomponent Synthesis of 4H-Pyrans and Polysubstituted Aniline Derivatives of Biological, Pharmacological, and Optical Applications Using Silica Nanoparticles as Reusable Catalyst. Tetrahedron Lett. 2011, 52, 1878–1881. [Google Scholar] [CrossRef]
- Kshirsagar, S.W.; Patil, N.R.; Samant, S.D. Mg-Al Hydrotalcite as a First Heterogeneous Basic Catalyst for the Synthesis of 4H-Pyrano[2,3-c]pyrazoles Through a Four-Component Reaction. Synth. Commun. 2011, 41, 1320–1325. [Google Scholar] [CrossRef]
- Seshu Babu, N.; Pasha, N.; Venkateswara Rao, K.T.; Sai Prasad, P.S.; Lingaiah, N. A Heterogeneous Strong Basic Mg/La Mixed Oxide Catalyst for Efficient Synthesis of Polyfunctionalized Pyrans. Tetrahedron Lett. 2008, 49, 2730–2733. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zhang, Y.; Xu, Y.; Shang, S.; Yin, Y. Ni–Co Catalyst Derived from Layered Double Hydroxides for Dry Reforming of Methane. Int. J. Hydrogen Energy 2015, 40, 16115–16126. [Google Scholar] [CrossRef]
- van Dijk, H.A.J.; Walspurger, S.; Cobden, P.D.; van den Brink, R.W.; de Vos, F.G. Testing of hydrotalcite-Based Sorbents for CO2 and H2S Capture for Use in Sorption Enhanced Water Gas Shift. Int. J. Greenh. Gas Control 2011, 5, 505–511. [Google Scholar] [CrossRef]
- Perez-Lopez, O.W.; Senger, A.; Marcilio, N.R.; Lansarin, M.A. Effect of Composition and Thermal Pretreatment on Properties of Ni–Mg–Al Catalysts for CO2 Reforming of Methane. Appl. Catal. A Gen. 2006, 303, 234–244. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Wei, W.; Sun, Y. Partial Oxidation of Methane Over Ni/Mg/Al/La Mixed Oxides Prepared from Layered Double Hydrotalcites. Int. J. Hydrogen Energy 2010, 35, 11776–11786. [Google Scholar] [CrossRef]
- Kaneda, K.; Mizugaki, T. Design of High-Performance Heterogeneous Catalysts Using Hydrotalcite for Selective Organic Transformations. Green Chem. 2019, 21, 1361–1389. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Xu, Y.; Ye, T.; Wang, R.; Yang, Z.; Jia, Z.; Ju, S. Cu/Mg/Al Hydrotalcite-Like Hydroxide Catalysts for O-Phenylphenol Synthesis. Appl. Clay Sci. 2016, 126, 207–214. [Google Scholar] [CrossRef]
- Wierzbicki, D.; Baran, R.; Dębek, R.; Motak, M.; Grzybek, T.; Gálvez, M.E.; Da Costa, P. The Influence of Nickel Content on the Performance of Hydrotalcite-Derived Catalysts in CO2 Methanation Reaction. Int. J. Hydrogen Energy 2017, 42, 23548–23555. [Google Scholar] [CrossRef]
- Dębek, R.; Zubek, K.; Motak, M.; Da Costa, P.; Grzybek, T. Effect of Nickel Incorporation into Hydrotalcite-Based Catalyst Systems for Dry Reforming of Methane. Res. Chem. Intermed. 2015, 41, 9485–9495. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Qu, W.; Wang, B.; Tian, Z.; Ma, H.; Xu, R. The Application of Zr Incorporated Zn-Al Dehydrated Hydrotalcites as Solid Base in Transesterification. Catal. Today 2014, 234, 161–166. [Google Scholar] [CrossRef]
- Zhou, W.; Tao, Q.; Pan, J.; Liu, J.; Qian, J.; He, M.; Chen, Q. Effect of Basicity on the Catalytic Properties of Ni-Containing Hydrotalcites in the Aerobic Oxidation of Alcohol. J. Mol. Catal. A Chem. 2016, 425, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Rives, V. Characterisation of Layered Double Hydroxides and Their Decomposition Products. Mater. Chem. Phys. 2002, 75, 19–25. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Promotion Effect of Zirconia on Mg(Ni,Al)O Mixed Oxides Derived from Hydrotalcites in CO2 Methane Reforming. Appl. Catal. B Environ. 2018, 223, 36–46. [Google Scholar] [CrossRef]
- Kawabata, T.; Shinozuka, Y.; Ohishi, Y.; Shishido, T.; Takaki, K.; Takehira, K. Nickel Containing Mg-Al Hydrotalcite-Type Anionic Clay Catalyst for the Oxidation of Alcohols with Molecular Oxygen. J. Mol. Catal. A Chem. 2005, 236, 206–215. [Google Scholar] [CrossRef]
- Qi, Y.; Cheng, Z.; Zhou, Z. Steam Reforming of Methane over Ni Catalysts Prepared from Hydrotalcite-Type Precursors: Catalytic Activity and Reaction Kinetics. Chin. J. Chem. Eng. 2015, 23, 76–85. [Google Scholar] [CrossRef]
- Seftel, E.M.; Popovici, E.; Mertens, M.; Van Tendeloo, G.; Cool, P.; Vansant, E.F. The Influence of the Cationic Ratio on the Incorporation of Ti4+ in the Brucite-Like Sheets of Layered Double Hydroxides. Microporous Mesoporous Mater. 2008, 111, 12–17. [Google Scholar] [CrossRef]
- Navarro, R.M.; Guil-Lopez, R.; Fierro, J.L.G.; Mota, N.; Jiménez, S.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Catalytic Fast Pyrolysis of Biomass over Mg-Al Mixed Oxides Derived from Hydrotalcite-Like Precursors: Influence of Mg/Al Ratio. J. Anal. Appl. Pyrolysis 2018, 134, 362–370. [Google Scholar] [CrossRef]
- Gomes, J.F.P.; Puna, J.F.B.; Gonçalves, L.M.; Bordado, J.C.M. Study on the Use of MgAl Hydrotalcites as Solid Heterogeneous Catalysts for Biodiesel Production. Energy 2011, 36, 6770–6778. [Google Scholar] [CrossRef]
- Liu, Y.; Suzuki, K.; Hamakawa, S.; Hayakawa, T.; Murata, K.; Ishii, T.; Kumagai, M. Highly Active Methanol Decomposition Catalyst Derived from Pd-Hydrotalcite Dispersed on Mesoporous Silica. Catal. Lett. 2000, 66, 205. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, X.; Chen, N.; Wang, K.; Kang, L.; Liu, Z.-H. Oxidizing Synthesis of Ni2+–Mn3+ Layered Double Hydroxide with Good Crystallinity. Mater. Res. Bull. 2011, 46, 1843–1847. [Google Scholar] [CrossRef]
- Shekoohi, K.; Hosseini, F.S.; Haghighi, A.H.; Sahrayian, A. Synthesis of Some Mg/Co-Al Type Nano Hydrotalcites and Characterization. MethodsX 2017, 4, 86–94. [Google Scholar] [CrossRef]
- Costa, D.G.; Rocha, A.B.; Diniz, R.; Souza, W.F.; Chiaro, S.S.X.; Leitão, A.A. Structural Model Proposition and Thermodynamic and Vibrational Analysis of Hydrotalcite-Like Compounds by DFT Calculations. J. Phys. Chem. C 2010, 114, 14133–14140. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Kaichev, V.V. Mixed Layered Ni–Mn–Co Hydroxides: Crystal Structure, Electronic State of Ions, and Thermal Decomposition. J. Power Sources 2007, 174, 735–740. [Google Scholar] [CrossRef]
- Nishimura, S.; Takagaki, A.; Ebitani, K. Characterization, synthesis and catalysis of hydrotalcite-related materials for highly efficient materials transformations. Green Chem. 2013, 15, 2026–2042. [Google Scholar] [CrossRef]
- Faramawy, S.; Zaki, T.; Sakr, A.A.E.; Saber, O.; Aboul-Gheit, A.K.; Hassan, S.A. The Activity of Mg-Al Layered Double Hydroxides Intercalated with Nitrogen-Containing Anions Towards the Removal of Carbon Dioxide from Natural Gas. J. Nat. Gas Sci. Eng. 2018, 54, 72–82. [Google Scholar] [CrossRef]
- Soares Dias, A.P.; Bernardo, J.; Felizardo, P.; Neiva Correia, M.J. Biodiesel Production over Thermal Activated Cerium Modified Mg-Al Hydrotalcites. Energy 2012, 41, 344–353. [Google Scholar] [CrossRef]
- Azath, I.A.; Puthiaraj, P.; Pitchumani, K. One-Pot Multicomponent Solvent-Free Synthesis of 2-Amino-4H-benzo[b]pyrans Catalyzed by Per-6-amino-β-cyclodextrin. ACS Sustain. Chem. Eng. 2013, 1, 174–179. [Google Scholar] [CrossRef]
- Khurana, J.M.; Chaudhary, A. Efficient and Green Synthesis of 4H-Pyrans and 4H-Pyrano[2,3-c] Pyrazoles Catalyzed by Task-Specific Ionic Liquid [bmim]OH under Solvent-Free Conditions. Green Chem. Lett. Rev. 2012, 5, 633–638. [Google Scholar] [CrossRef]
- Tanaka, K.; Toda, F. Solvent-Free Organic Synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef] [PubMed]
- Malviya, J.; Kala, S.; Sharma, L.K.; Singh, R.K.P. Efficient Three-Component One-Pot Synthesis of 4H-Pyrans. Russ. J. Org. Chem. 2019, 55, 686–693. [Google Scholar] [CrossRef]
- Van Laar, F.M.P.R.; De Vos, D.E.; Pierard, F.; Kirsch-De Mesmaeker, A.; Fiermans, L.; Jacobs, P.A. Generation of Singlet Molecular Oxygen from H2O2 with Molybdate-Exchanged Layered Double Hydroxides: Effects of Catalyst Composition and Reaction Conditions. J. Catal. 2001, 197, 139–150. [Google Scholar] [CrossRef]
- Nope, E.; Martínez, J.; Rojas, H.; Sathicq, Á.; Romanelli, G. Synthesis of Mesoporous Ca-MCM Catalysts and Their Use in Suitable Multicomponent Synthesis of Polyfunctionalized Pyrans. Res. Chem. Intermed. 2016, 43, 2103–2118. [Google Scholar] [CrossRef]
- D’Alessandro, O.; Sathicq, Á.G.; Sambeth, J.E.; Thomas, H.J.; Romanelli, G.P. A Study of the Temperature Effect on Hantzsch Reaction Selectivity Using Mn and Ce Oxides under Solvent-Free Conditions. Catal. Commun. 2015, 60, 65–69. [Google Scholar] [CrossRef]
- Haas, C.P.; Tallarek, U. Kinetics Studies on a Multicomponent Knoevenagel−Michael Domino Reaction by an Automated Flow Reactor. Chem. Open 2019, 8, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Ladage, S.; Ravishankar, L. Mg-Al Hydrotalcite Catalyzed Efficient One-Pot Synthesis of 4-H-Benzo [b] Pyrans, 2-Aryl Benzimidazole and 2-Aryl-4, 5-Dihydro-1H-Imidazole Derivatives. Chemistry 2015, 1, 5–9. [Google Scholar]
- Mokhtar, M.; Saleh, T.S.; Basahel, S.N. Mg–Al Hydrotalcites as Efficient Catalysts for Aza-Michael Addition Reaction: A Green Protocol. J. Mol. Catal. A Chem. 2012, 353–354, 122–131. [Google Scholar] [CrossRef]
- Pavel, O.D.; Tichit, D.; Marcu, I.-C. Acido-Basic and Catalytic Properties of Transition-Metal Containing Mg–Al Hydrotalcites and Their Corresponding Mixed Oxides. Appl. Clay Sci. 2012, 61, 52–58. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Yi, H.H.; Tang, X.L.; Kang, D.J.; Yu, Q.J.; Gao, F.Y.; Wang, J.G.; Huang, Y.H.; Yang, Z.Y. Mechanism of Activity Enhancement of the Ni Based Hydrotalcite-Derived Materials in Carbonyl Sulfide Removal. Mater. Chem. Phys. 2018, 205, 35–43. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Liu, C.; Cheng, T.; Wei, W.; Sun, Y. Transition Metal-Modified Mesoporous Mg-Al Mixed Oxides: Stable Base Catalysts for the Synthesis of Diethyl Carbonate from Ethyl Carbamate and Ethanol. Appl. Catal. A Gen. 2015, 505, 478–486. [Google Scholar] [CrossRef]
- Mancipe, S.; Tzompantzi, F.; Rojas, H.; Gómez, R. Photocatalytic Degradation of Phenol Using MgAlSn Hydrotalcite-Like Compounds. Appl. Clay Sci. 2016, 129, 71–78. [Google Scholar] [CrossRef]
- Prihod’ko, R.; Sychev, M.; Kolomitsyn, I.; Stobbelaar, P.J.; Hensen, E.J.M.; van Santen, R.A. Layered Double Hydroxides as Catalysts for Aromatic Nitrile Hydrolysis. Microporous Mesoporous Mater. 2002, 56, 241–255. [Google Scholar] [CrossRef]










| Catalyst | Lattice Parameters | d (Å) | |
|---|---|---|---|
| a (Å) | c (Å) | ||
| LDH | 3.07 | 23.23 | 7.69 |
| LDH–Ni | 3.07 | 23.36 | 7.72 |
| LDH–Co | 3.07 | 23.43 | 7.78 |
| Catalyst | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Basicity a (mmol/g) |
|---|---|---|---|---|
| LDH–Mg | 17 | 0.11 | 8 | 0.016 |
| LDH–Ni | 42 | 0.21 | 9 | 0.321 |
| LDH–Co | 28 | 0.13 | 8.5 | 0.163 |
| Solvent | Yield to 4H-Pyran (%) | Time (h) |
|---|---|---|
| Water | 21 | 48 |
| Ethanol | 25 | 48 |
| Ethyl acetate | 27 | 48 |
| Solvent-free | 30 | 8 |
| Yield (%) | ||||
|---|---|---|---|---|
| Catalyst | Room Temperature | 353 K | ||
| Intermediate | 4H-Pyran | Intermediate | 4H-Pyran | |
| LDH–Mg | 40 | 23 | 43 | 34 |
| LDH–Ni | 28 | 39 | 20 | 58 |
| LDH–Co | 25 | 28 | 18 | 40 |
| LDH–Ni | Selectivity (%) | |||
|---|---|---|---|---|
| Temperature | 293 K | 333 K | 353 K | 393 K |
| Intermediate | 30 | 7 | 2 | 6 |
| 4H-pyran | 59 | 89 | 90 | 89 |
| Reaction | Cycle 1 | Cycle 2 | Cycle 3 | |
|---|---|---|---|---|
| Yield of 4H-pyran (%) | 97 | 94 | 92 | 87 |
| Yield of intermediate | 3 | 6 | 8 | 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nope, E.; Sathicq, Á.G.; Martínez, J.J.; Rojas, H.A.; Luque, R.; Romanelli, G.P. Ternary Hydrotalcites in the Multicomponent Synthesis of 4H-Pyrans. Catalysts 2020, 10, 70. https://doi.org/10.3390/catal10010070
Nope E, Sathicq ÁG, Martínez JJ, Rojas HA, Luque R, Romanelli GP. Ternary Hydrotalcites in the Multicomponent Synthesis of 4H-Pyrans. Catalysts. 2020; 10(1):70. https://doi.org/10.3390/catal10010070
Chicago/Turabian StyleNope, Eliana, Ángel G. Sathicq, José J. Martínez, Hugo A. Rojas, Rafael Luque, and Gustavo P. Romanelli. 2020. "Ternary Hydrotalcites in the Multicomponent Synthesis of 4H-Pyrans" Catalysts 10, no. 1: 70. https://doi.org/10.3390/catal10010070