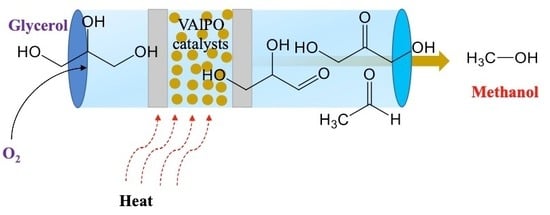
VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Catalytic Activity
3. Materials and Methods
3.1. Synthesis
3.2. Materials Characterization
3.3. Catalytic Reactions
3.4. Product Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gross, M.S.; Sánchez, B.S.; Querini, C.A. Glycerol oxidation in liquid phase: Highly stable Pt catalysts supported on ion exchange resins. Appl. Catal. A Gen. 2015, 501, 1–9. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Prati, L.; Hutchings, G.J. Glycerol Oxidation Using Gold-Containing Catalysts. Acc. Chem. Res. 2015, 48, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Katryniok, B.; Kimura, H.; Skrzynska, E.; Girardon, J.S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paul, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Tinoco, M.; Fernandez-Garcia, S.; Villa, A.; Gonzalez, J.M.; Blanco, G.; Hungria, A.B.; Jiang, L.; Prati, L.; Calvino, J.J.; Chen, X.W. Selective oxidation of glycerol on morphology controlled ceria nanomaterials. Catal. Sci. Technol. 2019, 9, 2328–2334. [Google Scholar] [CrossRef]
- Aroua, M.K.; Cognet, P. Editorial: From Glycerol to Value-Added Products. Front. Chem. 2020, 8, 69. [Google Scholar] [CrossRef]
- Farnetti, E.; Crotti, C. Selective oxidation of glycerol to formic acid catalyzed by iron salts. Catal. Commun. 2016, 84, 1–4. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Blanco-Bonilla, F.; Luna, D.; Bautista, F.M. Production of acrolein from glycerol in liquid phase on heterogeneous catalysts. Chem. Eng. J. 2015, 282, 179–186. [Google Scholar] [CrossRef]
- Witsuthammakul, A.; Sooknoi, T. Direct conversion of glycerol to acrylic acid via integrated dehydration-oxidation bed system. Appl. Catal. A-Gen. 2012, 413, 109–116. [Google Scholar] [CrossRef]
- Kim, M.; Lee, H. Highly Selective Production of Acrylic Acid from Glycerol via Two Steps Using Au/CeO2 Catalysts. Acs Sustain. Chem. Eng. 2017, 5, 11371–11376. [Google Scholar] [CrossRef]
- Lee, C.S.; Aroua, M.K.; Daud, W.A.W.; Cognet, P.; Peres, Y.; Ajeel, M.A. Selective Electrochemical Conversion of Glycerol to Glycolic Acid and Lactic Acid on a Mixed Carbon-Black Activated Carbon Electrode in a Single Compartment Electrochemical Cell. Front. Chem. 2019, 7, 110. [Google Scholar]
- Zhang, X.Q.; Zhou, D.; Wang, X.J.; Zhou, J.; Li, J.F.; Zhang, M.K.; Shen, Y.H.; Chu, H.B.; Qu, Y.Q. Overcoming the Deactivation of Pt/CNT by Introducing CeO2 for Selective Base-Free Glycerol-to-Glyceric Acid Oxidation. Acs Catal. 2020, 10, 3832–3837. [Google Scholar] [CrossRef]
- Liu, C.; Hirohara, M.; Maekawa, T.; Chang, R.; Hayashi, T.; Chiang, C.Y. Selective electro-oxidation of glycerol to dihydroxyacetone by a non-precious electrocatalyst - CuO. Appl. Catal. B-Environ. 2020, 265, 12. [Google Scholar] [CrossRef]
- Diguilio, E.; Galarza, E.D.; Domine, M.E.; Pierella, L.B.; Renzini, M.S. Tuning product selectivity in the catalytic oxidation of glycerol by employing metal-ZSM-11 materials. New J. Chem. 2020, 44, 4363–4375. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Burnham, S. The use of alkaline earth oxides as pH modifiers for selective glycerol oxidation over supported Au catalysts. Renew. Energy 2015, 78, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Kawanami, H.; Himeda, Y.; Laurenczy, G. Formic Acid as a Hydrogen Carrier for Fuel Cells Toward a Sustainable Energy System. Adv. Inorg. Chem. Inorg. React. Mech. 2017, 70, 395–427. [Google Scholar]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.T. Dealing with the surplus of glycerol production from biodiesel industry through catalytic upgrading to polyglycerols and other value-added products. Renew. Sustain. Energy Rev. 2014, 39, 327–341. [Google Scholar] [CrossRef]
- Wang, F.; Dubois, J.-L.; Ueda, W. Catalytic dehydration of glycerol over vanadium phosphate oxides in the presence of molecular oxygen. J. Catal. 2009, 268, 260–267. [Google Scholar] [CrossRef]
- Ning, X.; Li, Y.; Yu, H.; Peng, F.; Wang, H.; Yang, Y. Promoting role of bismuth and antimony on Pt catalysts for the selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2016, 335, 95–104. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Basiron, N.; Yehye, W.A.; Sudarsanam, P.; Bhargava, S.K. Nanoscale Pd-based catalysts for selective oxidation of glycerol with molecular oxygen: Structure–activity correlations. Polyhedron 2016, 120, 124–133. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Huang, Y.a.; Liu, X.; Zhang, F.; Ding, K.; Yang, X. Selective oxidation of glycerol with O2 catalyzed by low-cost CuNiAl hydrotalcites. J. Mol. Catal. A Chem. 2013, 379, 185–191. [Google Scholar] [CrossRef]
- Omata, K.; Matsumoto, K.; Murayama, T.; Ueda, W. Direct oxidative transformation of glycerol to acrylic acid over Nb-based complex metal oxide catalysts. Catal. Today 2016, 259, 205–212. [Google Scholar] [CrossRef]
- Yang, L.H.; Li, X.W.; Chen, P.; Hou, Z.Y. Selective oxidation of glycerol in a base-free aqueous solution: A short review. Chin. J. Catal. 2019, 40, 1020–1034. [Google Scholar] [CrossRef]
- Purushothaman, R.K.P.; van Haveren, J.; van Es, D.S.; Melian-Cabrera, I.; Meeldijk, J.D.; Heeres, H.J. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal. B-Environ. 2014, 147, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-T.; Yu, K.M.K.; Liao, F.; Young, N.; Nellist, P.; Dent, A.; Kroner, A.; Tsang, S.C.E. A non-syn-gas catalytic route to methanol production. Nat. Commun. 2012, 3, 1050. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.H.; Dummer, N.F.; Knight, D.W.; Jenkins, R.L.; Howard, M.; Moulijn, J.; Taylor, S.H.; Hutchings, G.J. Efficient green methanol synthesis from glycerol. Nat. Chem. 2015, 7, 1028–1032. [Google Scholar] [CrossRef] [Green Version]
- Paula, A.S.; Possato, L.G.; Ratero, D.R.; Contro, J.; Keinan-Adamsky, K.; Soares, R.R.; Goobes, G.; Martins, L.; Nery, J.G. One-step oxidehydration of glycerol to acrylic acid using ETS-10-like vanadosilicates. Microporous Mesoporous Mater. 2016, 232, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Vishnuvarthan, M.; Paterson, A.J.; Raja, R.; Piovano, A.; Bonino, F.; Gianotti, E.; Berlier, G. Spectroscopic investigation into the nature of the active sites for epoxidation reactions using vanadium-based aluminophosphate catalysts. Microporous Mesoporous Mater. 2011, 138, 167–175. [Google Scholar] [CrossRef]
- Lopez-Pedrajas, S.; Estevez, R.; Navarro, R.; Luna, D.; Bautista, F.M. Catalytic behaviour of mesoporous metal phosphates in the gas-phase glycerol transformation. J. Mol. Catal. A Chem. 2016, 421, 92–101. [Google Scholar] [CrossRef]
- Lopez-Pedrajas, S.; Estevez, R.; Schnee, J.; Gaigneaux, E.M.; Luna, D.; Bautista, F.M. Study of the gas-phase glycerol oxidehydration on systems based on transition metals (Co, Fe, V) and aluminium phosphate. Mol. Catal. 2018, 455, 68–77. [Google Scholar] [CrossRef]
- Lopez-Pedrajas, S.; Estevez, R.; Blanco-Bonilla, F.; Luna, D.; Bautista, F.M. Insight into the gas-phase glycerol dehydration on transition metal modified aluminium phosphates and zeolites. J. Chem. Technol. Biotechnol. 2017, 92, 2661–2672. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Zeng, D.; Yang, J.; Zhang, M.; Ye, C.; Deng, F. Crystallization of AlPO4-5 Aluminophosphate Molecular Sieve Prepared in Fluoride Medium: A Multinuclear Solid-State NMR Study. J. Phys. Chem. B 2007, 111, 7105–7113. [Google Scholar] [CrossRef] [PubMed]
- Rair, D.; Rochdi, A.; Majjane, A.; Jermoumi, T.; Chahine, A.; Touhami, M.E. Synthesis and study by FTIR, 31P NMR and electrochemical impedance spectroscopy of vanadium zinc phosphate glasses prepared by sol–gel route. J. Non-Cryst. Solids 2016, 432, 459–465. [Google Scholar] [CrossRef]
- Dzwigaj, S.; el Malki, E.M.; Peltre, M.-J.; Massiani, P.; Davidson, A.; Che, M. Effect of calcination/rehydration treatments on the environment of V in β zeolite. Top. Catal. 2000, 11, 379. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Louis, B.; Viswanathan, B.; Renken, A.; Varadarajan, T.K. Synthesis, characterisation and catalytic properties of vanadium substituted mesoporous aluminophosphates. Appl. Catal. A Gen. 2005, 282, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, J.; Zheng, M.; Li, X.; Pang, J.; Wang, C.; Zhang, T. Catalytic conversion of glucose to small polyols over a binary catalyst of vanadium modified beta zeolite and Ru/C. J. Energy Chem. 2019, 34, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Bonilla, F.; Lopez-Pedrajas, S.; Luna, D.; Marinas, J.M.; Bautista, F.M. Vanadium oxides supported on amorphous aluminum phosphate: Structural and chemical characterization and catalytic performance in the 2-propanol reaction. J. Mol. Catal. A Chem. 2016, 416, 105–116. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; de Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Florea, M.; Silvy, R.P.; Grange, P. Vanadium aluminium oxynitride catalysts for propane ammoxidation reaction: Effect of the V/Al ratio on the structure and catalytic behaviour. Appl. Catal. A Gen. 2005, 286, 1–10. [Google Scholar] [CrossRef]
- Xie, Q.; Li, Y.; Lv, Z.; Zhou, H.; Yang, X.; Chen, J.; Guo, H. Effective Adsorption and Removal of Phosphate from Aqueous Solutions and Eutrophic Water by Fe-based MOFs of MIL-101. Sci. Rep. 2017, 7, 3316. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.; Sun, Q.; Li, R.; Sham, T.-K.; Sun, X. Atomic layer deposition of lithium phosphates as solid-state electrolytes for all-solid-state microbatteries. Nanotechnology 2014, 25, 504007. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Neaţu, F.; Diculescu, V.C.; Trandafir, M.M.; Petrea, N.; Somacescu, S.; Krumeich, F.; Wennmacher, J.T.C.; Knorpp, A.J.; van Bokhoven, J.A.; et al. Undoped SnO2 as a Support for Ni Species to Boost Oxygen Generation through Alkaline Water Electrolysis. ACS Appl. Mater. Interfaces 2020, 12, 18407–18420. [Google Scholar] [CrossRef] [PubMed]
- Appapillai, A.T.; Mansour, A.N.; Cho, J.; Shao-Horn, Y. Microstructure of LiCoO2 with and without “AlPO4” Nanoparticle Coating: Combined STEM and XPS Studies. Chem. Mater. 2007, 19, 5748–5757. [Google Scholar] [CrossRef]
- Florea, M.; Marin, R.S.; Pălăşanu, F.M.; Neaţu, F.; Pârvulescu, V.I. Mesostructured vanadia–alumina catalysts for the synthesis of vitamin K3. Catal. Today 2015, 254, 29–35. [Google Scholar] [CrossRef]
- Yu, J.; Li, M.; Liu, Z.; Feng, Z.; Xin, Q.; Li, C. Comparative Study of the Vanadium Species in VAPO-5 and VAPSO-5 Molecular Sieves. J. Phys. Chem. B 2002, 106, 8937–8943. [Google Scholar] [CrossRef]
- Shvets, P.; Dikaya, O.; Maksimova, K.; Goikhman, A. A review of Raman spectroscopy of vanadium oxides. J. Raman Spectrosc. 2019, 50, 1226–1244. [Google Scholar] [CrossRef]
- Kumar, R.T.R.; Karunagaran, B.; Venkatachalam, S.; Mangalaraj, D.; Narayandass, S.K.; Kesavamoorthy, R. Influence of deposition temperature on the growth of vacuum evaporated V2O5 thin films. Mater. Lett. 2003, 57, 3820–3825. [Google Scholar] [CrossRef]
- Su, Q.; Pan, X.; Xie, E.; Wang, Y.; Qiu, J.; Liu, X. Influence of temperature on the microstructure of V2O5 film prepared by DC magnetron sputtering. Rare Met. 2006, 25, 82–87. [Google Scholar] [CrossRef]
- Zhao, X.T.; Yan, Y.Y.; Mao, L.; Fu, M.C.; Zhao, H.R.; Sun, L.S.; Xiao, Y.H.; Dong, G.J. A relationship between the V4+/V5+ ratio and the surface dispersion, surface acidity, and redox performance of V2O5-WO3/TiO2 SCR catalysts. Rsc Adv. 2018, 8, 31081–31093. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, S.; Watanabe, H.; Kizuka, T.; Nakagawa, Y.; Tomishige, K. Performance, structure and mechanism of Pd-Ag alloy catalyst for selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2013, 300, 205–216. [Google Scholar] [CrossRef]
- Mimura, N.; Hiyoshi, N.; Date, M.; Fujitani, T.; Dumeignil, F. Microscope Analysis of Au-Pd/TiO2 Glycerol Oxidation Catalysts Prepared by Deposition-Precipitation Method. Catal. Lett. 2014, 144, 2167–2175. [Google Scholar] [CrossRef]
- Tsukuda, E.; Sato, S.; Takahashi, R.; Sodesawa, T. Production of acrolein from glycerol over silica-supported heteropoly acids. Catal. Commun. 2007, 8, 1349–1353. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, H.P.; Liang, Y.; Xu, B.Q. Sustainable production of acrolein: Investigation of solid acid-base catalysts for gas-phase dehydration of glycerol. Green Chem. 2007, 9, 1130–1136. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A-Chem. 2009, 309, 71–78. [Google Scholar] [CrossRef]
- Heim, L.E.; Konnerth, H.; Prechtl, M.H.G. Future perspectives for formaldehyde: Pathways for reductive synthesis and energy storage. Green Chem. 2017, 19, 2347–2355. [Google Scholar] [CrossRef] [Green Version]
- Borah, P.; Datta, A.; Nguyen, K.T.; Zhao, Y.L. VOPO4 center dot 2H(2)O encapsulated in graphene oxide as a heterogeneous catalyst for selective hydroxylation of benzene to phenol. Green Chem. 2016, 18, 397–401. [Google Scholar] [CrossRef]
- Haider, M.H.; Dummer, N.F.; Zhang, D.; Miedziak, P.; Davies, T.E.; Taylor, S.H.; Willock, D.J.; Knight, D.W.; Chadwick, D.; Hutchings, G.J. Rubidium- and caesium-doped silicotungstic acid catalysts supported on alumina for the catalytic dehydration of glycerol to acrolein. J. Catal. 2012, 286, 206–213. [Google Scholar] [CrossRef]
- Haider, M.H.; D’Agostino, C.; Dummer, N.F.; Mantle, M.D.; Gladden, L.F.; Knight, D.W.; Willock, D.J.; Morgan, D.J.; Taylor, S.H.; Hutchings, G.J. The Effect of Grafting Zirconia and Ceria onto Alumina as a Support for Silicotungstic Acid for the Catalytic Dehydration of Glycerol to Acrolein. Chem. A Eur. J. 2014, 20, 1743–1752. [Google Scholar] [CrossRef]
- Chieregato, A.; Soriano, M.D.; Basile, F.; Liosi, G.; Zamora, S.; Concepción, P.; Cavani, F.; Nieto, J.M.L. One-pot glycerol oxidehydration to acrylic acid on multifunctional catalysts: Focus on the influence of the reaction parameters in respect to the catalytic performance. Appl. Catal. B Environ. 2014, 150–151, 37–46. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Dubois, J.-L.; Ueda, W. Catalytic Oxidative Dehydration of Glycerol over a Catalyst with Iron Oxide Domains Embedded in an Iron Orthovanadate Phase. ChemSusChem 2010, 3, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- van Ryneveld, E.; Mahomed, A.S.; van Heerden, P.S.; Green, M.J.; Friedrich, H.B. A catalytic route to lower alcohols from glycerol using Ni-supported catalysts. Green Chem. 2011, 13, 1819–1827. [Google Scholar] [CrossRef]
- Carr, A.G.; Shi, X.; Domene, C.; Leung, A.K.; Green, W.H. Methanol formation from the treatment of glycerol in supercritical water and with ethylsulfide. J. Supercrit. Fluids 2016, 117, 80–88. [Google Scholar] [CrossRef]
- Possato, L.G.; Acevedo, M.D.; Padró, C.L.; Briois, V.; Passos, A.R.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. Activation of Mo and V oxides supported on ZSM-5 zeolite catalysts followed by in situ XAS and XRD and their uses in oxydehydration of glycerol. Mol. Catal. 2020, 481, 110158. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
















| Catalyst | BET (m2 g−1) | Crystallite Size (nm) | Lattice Parameter | ||
|---|---|---|---|---|---|
| a | b | c | |||
| V5APO | 0.04 | 35.1 | 9.6930 | 8.7359 | 18.8351 |
| V7.5APO | 2.12 | 36.6 | 9.6930 | 8.7359 | 18.8351 |
| V10APO | 0.45 | 37.5 | 9.6930 | 8.7359 | 18.8351 |
| AlPO4 * | - | - | 9.6380 | 8.6640 | 18.2800 |
| Samples | V 2p | Al 2p | P 2p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BE (eV) | Atomic Ratio (%) (Vx+/Vtot) | BE (eV) | Atomic Ratio (%) (Vtot/Al) | BE (eV) | Atomic Ratio (%) (Vtot/P) | ||||
| XPS | Calc. | XPS | Calc. | ||||||
| V10AlPO | V3+ | 514.6 | 4.1 | 73.8 | 0.29 | 0.11 | 133.1 | 0.23 | 0.1 |
| V4+ | 516.1 | 60.1 | |||||||
| V5+ | 517.1 | 35.8 | |||||||
| V7.5AlPO | V3+ | 514.6 | 9.2 | 73.8 | 0.13 | 0.17 | 133.2 | 0.15 | 0.15 |
| V4+ | 516.1 | 81.3 | |||||||
| V5+ | 517.7 | 9.5 | |||||||
| V5AlPO | V3+ | - | - | 73.8 | 0.28 | 0.25 | 133.1 | 0.22 | 0.2 |
| V4+ | 516.1 | 71.0 | |||||||
| V5+ | 517.1 | 29.0 | |||||||
| NH3 Desorbed (μmol g−1) | ||||
|---|---|---|---|---|
| Catalyst | 200–250 | 350–450 | 500–700 | |
| Tdes range (°C) | ||||
| V5APO | 34.30 | 44.38 | ||
| V7.5APO | 38.18 | 3.53 | 21.8 | |
| V10APO | 62.44 | 19.19 | 18.36 | |
| Catalyst (Mass(g)) | O2 Flow (mL h−1) | Carrier gas Flow (mL h−1) | Glycerol in Water (%) | Feed Flow Rate (mL h−1) | P (bar) | T (°C) | Time (h) | C. (%) | S (%) MeOH | TOF (h−1) | STY (g MeOH kg−1 catalyst h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V7.5APO(0.2) | 240 | 1200 | 10 | 3.6 | - | 350 | 0.5 | 98.3 | 84.6 | 2.9 379 h | 531 | This study |
| CeO2 (0.5) | - | 6000 a | 10 | 1.0 | - | 340 | 3 | 97.0 | 60.0 | 0.5 | 51 | [27] |
| NiSiO2 (8.5) | - | 5300 b | 60 | 15.0 | 60 | 320 | ns | 99.9 | 68.5 c | 0.9 114 h | 25 | [62] |
| d | - | - | 10 | - | 300 e | 450 | 0.5 | 94.0 f | 45.0 g | - | - | [63] |
| V-ZSM-5 (0.2) | 360 | 1440 a | 10 | 3.0 | - | 320 | 1 | 100 | - | 0.1 42.0 h | 0 | [64] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, G.; Neațu, F.; Neațu, Ș.; Trandafir, M.M.; Florea, M. VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol. Catalysts 2020, 10, 728. https://doi.org/10.3390/catal10070728
Mitran G, Neațu F, Neațu Ș, Trandafir MM, Florea M. VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol. Catalysts. 2020; 10(7):728. https://doi.org/10.3390/catal10070728
Chicago/Turabian StyleMitran, Gheorghița, Florentina Neațu, Ștefan Neațu, Mihaela M. Trandafir, and Mihaela Florea. 2020. "VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol" Catalysts 10, no. 7: 728. https://doi.org/10.3390/catal10070728