Ultrasound-Assisted Preparation of Mo/ZSM-5 Zeolite Catalyst for Non-Oxidative Methane Dehydroaromatization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of Ultrasonic Irradiation on Physico-Chemical Properties
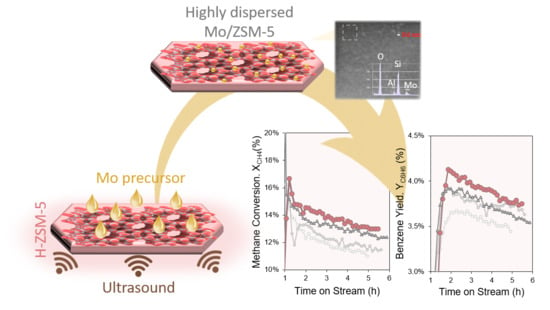
2.2. Influence of Ultrasonic Irradiation on the Catalytic Activity
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Catalysts Characterization
3.4. Catalysts Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collet, T.S.; Johnson, A.H.; Knapp, C.C.; Boswell, R. Natural gas hydrates: A review, natural gas hydrates—Energy resource potential and associated geological hazards. AAPG Memoir 2009, 89, 146–219. [Google Scholar]
- BP Statistical Review of World Energy 2017 (2016). Available online: https://es.slideshare.net/BP_plc/bp-statistical-review-of-world-energy-2017-76902409 (accessed on 31 January 2021).
- Horn, R.; Schloegl, R. Methane activation by heterogeneous catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.S.; Thybaut, J.W.; Tangstad, E.; Stocker, M.W.; Marin, G.B. Methane aromatisation based upon elementary steps: Kinetic and catalyst descriptors. Micro. Meso. Mat. 2012, 164, 302–312. [Google Scholar] [CrossRef]
- Karakaya, C.; Morejudo, S.H.; Zhu, H.; Kee, R.J. Catalytic chemistry for methane dehydroaromatization (MDA) on a bifunctional Mo/HZSM-5 catalyst in a packed bed. Ind. Eng. Chem. Res. 2016, 55, 9895–9906. [Google Scholar] [CrossRef]
- Taifan, W.; Baltrusaitis, J. CH4 conversion to value added products: Potential, limitations and extensions of a single step heterogeneous catalysis. Appl. Catal. B Environ. 2016, 198, 525–547. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, S.K.; Hwang, A.; Kim, S.; Hong, D.; Kwak, G.; Jun, K.; Kim, Y.T. Non-oxidative dehydroaromatization of methane over Mo/H-ZSM-5 catalysts: A detailed analysis of the reaction-regeneration cycle. Appl. Catal. B Environ. 2019, 241, 305–318. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Zhu, X.; Hensen, E.J.M. Activation of Mo/HZSM-5 for methane aromatization. Chin. J. Catal. 2015, 36, 829–837. [Google Scholar] [CrossRef]
- Olivos-Suarez, A.I.; Szecsenyi, A.; Hensen, E.J.M.; Ruiz-Martinez, J.; Pidko, E.A.; Gascon, J. Strategies for the direct catalytic valorization of methane using heterogeneous catalysis: Challenges and opportunities. ACS Catal. 2016, 6, 2965–2981. [Google Scholar] [CrossRef]
- Kosinov, N.; Uslamin, E.A.; Meng, L.; Parastaev, A.; Liu, Y.; Hensen, E.J.M. Reversible nature of coke formation on Mo/ZSM-5 methane dehydroaromatization catalysts. Angew. Chem. Int. Edit. 2019, 58, 7068–7072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismagilov, Z.R.; Matus, E.V.; Tsikoza, L.T. Direct conversion of methane on Mo/ZSM-5 catalysts to produce benzene and hydrogen: Achievements and perspectives. Energy Environ. Sci. 2008, 1, 526–541. [Google Scholar] [CrossRef]
- Ma, S.; Guo, X.; Zhao, L.; Scott, S.; Bao, X. Recent progress in methane dehydroaromatization: From laboratory curiosities to promising technology. J. Energy Chem. 2013, 22, 1–20. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, X.; Lin, L. Direct conversion of methane under nonoxidative conditions. J. Catal. 2003, 216, 386–395. [Google Scholar] [CrossRef]
- Wang, L.; Tao, L.; Xie, L.; Xu, G.; Huang, J.; Xu, Y. Dehydrogenation and aromatization of methane under non-oxidizing conditions. Catal. Lett. 1993, 21, 35–41. [Google Scholar] [CrossRef]
- Sun, K.; Ginosar, D.M.; He, T.; Zhang, Y.; Fan, M.; Chen, R. Progress in Nonoxidative Dehydroaromatization of Methane in the Last 6 Years. Ind. Eng. Chem. Res. 2018, 57, 1768–1789. [Google Scholar] [CrossRef]
- Spivey, J.; Hutchings, G. Catalytic aromatization of methane. Chem. Soc. Rev. 2014, 43, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Schwach, P.; Pan, X.; Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef]
- Vollmer, I.; Li, G.; Yarulina, I.; Kosinov, N.; Hensen, E.J.; Houben, K.; Mance, D.; Baldus, M.; Gascon, J.; Kapteijn, F. Relevance of the Mo-precursor state in H-ZSM-5 for methane dehydroaromatization. Catal. Sci. Technol. 2018, 8, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, I.; Mondal, A.; Yarulina, I.; Abou-Hamad, E.; Kapteijn, F.; Gascon, J. Quantifying the impact of dispersion, acidity and porosity of Mo/HZSM-5 on the performance in methane dehydroaromatization. Appl. Catal. A 2019, 574, 144–150. [Google Scholar] [CrossRef]
- Tempelman, C.H.; Hensen, E.J. On the deactivation of Mo/HZSM-5 in the methane dehydroaromatization reaction. Appl. Catal B 2015, 176, 731–739. [Google Scholar] [CrossRef]
- Vollmer, I.; Linden, B.; Ould-Chikh, S.; Aguilar-Tapia, A.; Yarulina, I.; Abou-Hamad, E.; Sneider, Y.G.; Olivos Suarez, A.I.; Hazemann, J.-L.; Kapteijn, F.; et al. On the dynamic nature of Mo sites for methane dehydroaromatization. Chem. Sci. 2018, 9, 4801–4807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.; Mäki-Arvela, P.; Hajek, J.; Salmi, T.; Murzin, D.Y.; Heikkilä, T.; Laine, E.; Laukkanen, P.; Väyrynen, J. Physico-chemical and catalytic properties of Ru–MCM-41 mesoporous molecular sieve catalyst: Influence of Ru modification methods. Microporous Mesoporous Mater. 2004, 69, 173–179. [Google Scholar] [CrossRef]
- Kumar, N.; Masloboischikova, O.V.; Kustov, L.M.; Heikkilä, T.; Salmi, T.; Murzin, D.Y. Synthesis of Pt modified ZSM-5 and beta zeolite catalysts: Influence of ultrasonic irradiation and preparation methods on physico-chemical and catalytic properties in pentane isomerization. Ultrason. Sonochem. 2007, 14, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kosinov, N.; Coumands, F.J.; Uslamin, E.A.; Wijpkema, A.S.; Mezari, B.; Hensen, E.J. Methane Dehydroaromatization by Mo/HZSM-5: Mono- or Bifunctional Catalysis? ACS Catal. 2017, 7, 520–529. [Google Scholar] [CrossRef]
- Sun, C.; Yao, S.; Shen, W.; Lin, L. Highly Dispersed Molybdenum Oxide Supported on HZSM-5 for Methane Dehydroaromatization. Catal. Lett. 2008, 122, 84–90. [Google Scholar] [CrossRef]
- Song, Y.; Sun, C.; Shen, W.; Lin, L. Hydrothermal post-synthesis of HZSM-5 zeolite to enhance the coke-resistance of Mo/HZSM-5 catalyst for methane dehydroaromatization reaction: Reconstruction of pore structure and modification of acidity. Appl. Catal. A 2007, 317, 266–274. [Google Scholar] [CrossRef]
- Julian, I.; Hueso, J.L.; Lara, N.; Solé-Daura, A.; Poblet, J.M.; Mitchell, S.G.; Mallada, R.; Santamaria, J. Polyoxometalates as alternative Mo precursors for methane dehydroaromatization on Mo/ZSM-5 and Mo/MCM-22 catalysts. Catal. Sci. Technol. 2019, 9, 5927–5942. [Google Scholar] [CrossRef] [Green Version]
- Julian, I.; Roedern, M.B.; Hueso, J.L.; Irusta, S.; Baden, A.K.; Mallada, R.; David, Z.; Santamaria, J. Supercritical solvothermal synthesis under reducing conditions to increase stability and durability of Mo/ZSM-5 catalysts in methane dehydroaromatization. Appl. Catal. B 2020, 263, 118360. [Google Scholar] [CrossRef]
- Dantsin, G.; Suslick, K.S. Sonochemical Preparation of a Nanostructured Bifunctional Catalyst. J. Am. Chem. Soc. 2000, 122, 5214–5215. [Google Scholar] [CrossRef]
- Woo, J.-M.; Seo, J.Y.; Kim, H.; Lee, D.-H.; Park, Y.C.; Yi, C.-K.; Park, Y.S.; Moon, J.-H. CuY zeolite catalysts prepared by ultrasonication-assisted ion-exchange for oxidative carbonylation of methanol to dimethyl carbonate. Ultrason. Sonochem. 2018, 44, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Vafaeian, Y.; Haghighi, M.; Aghamohammadi, S. Ultrasound assisted dispersion of different amount of Ni over ZSM-5 used as nanostructured catalyst for hydrogen production via CO2 reforming of methane. Energy Conver. Manag. 2013, 76, 1093–1103. [Google Scholar] [CrossRef]
- Erten-Kaya, Y.; Cakicioglu-Ozkan, F. Effect of ultrasound on the kinetics of cation exchange in NaX zeolite. Ultrason. Sonochem. 2012, 19, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerzeliev, I.M.; Ostroumova, V.A.; Baskhanova, M.N. Enhancement of Ion Exchange in a FAU Type Zeolite during the Synthesis of an Active and Selective Catalyst for Isobutane Alkylation with Butylenes. Pet. Chem. 2018, 58, 676–680. [Google Scholar] [CrossRef]
- Velebna, K.; Horňáček, M.; Jorík, V.; Hudec, P.; Čaplovičová, M.; Čaplovič, L. The influence of molybdenum loading on activity of ZSM-5 zeolite in dehydroaromatization of methane. Microporous Mesoporous Mater. 2015, 212, 146–155. [Google Scholar] [CrossRef]
- Li, W.; Meitzner, G.D.; Borry, R.W., III; Iglesias, E. Raman and X-ray Absorption Studies of Mo Species in Mo/H-ZSM5 Catalysts for Non-Oxidative CH4 Reactions. J. Catal. 2000, 191, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Rzhevskii, A.M.; Choi, P.; Ribeiro, F.H.; Gulotty, R.J.; Olken, M.M. Monitoring of molybdenum H-ZSM5 catalyst preparation by in situ ultraviolet Raman spectroscopy. Catal. Lett. 2001, 73, 187–191. [Google Scholar] [CrossRef]
- Tan, P. The catalytic performance of Mo-impregnated HZSM-5 zeolite in CH4 aromatization: Strong influence of Mo loading and pretreatment conditions. Catal. Commun. 2018, 103, 101–104. [Google Scholar] [CrossRef]
- Martinez, A.; Penis, E. Non-oxidative methane dehydroaromatization on Mo/HZSm-5 catalysts: Tuning the acidit and catalytic properties through partial exchange of zeolite protons with alkali and alkaline-earth cations. Appl. Catal. A Gen. 2016, 515, 32–44. [Google Scholar] [CrossRef]
- Liu, H.; Bao, X.; Xu, Y. Methane dehydroaromatization under nonoxidative conditions over Mo/HZSM-5 catalysts: Identification and preparation of the Mo active species. J. Catal. 2006, 239, 441–450. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, C.; Zhang, Y.; Kan, Q. Facile synthesis and its high catalytic performance of hierarchical ZSM-5 zeolite from economical bulk silicon oxides. Micro. Meso. Mat. 2018, 260, 116–124. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, J.; Wang, J.; Zhang, Z. Mo-based zeolite catalysts and oxygen-free methane aromatization. Prog. Chem. 2011, 23, 90–106. [Google Scholar]
- Julian, I.; Ramirez, H.; Hueso, J.L.; Mallada, R.; Santamaria, J. Non-oxidative methane conversion in microwave-assisted structure reactors. Chem. Eng. J. 2019, 377, 119764. [Google Scholar] [CrossRef]











| Sample | Theoretical wt. % Mo | wt. % Mo a | Surface Area b (m2·g−1) | Pore Volume c (cm3·g−1) |
|---|---|---|---|---|
| H-ZSM-5 | - | - | 351 | 0.130 |
| 5 wt. %—US | 5.44 | 5.35 | 333 | 0.122 |
| 5 wt. %—No US | 5.44 | 5.35 | 338 | 0.120 |
| 6 wt. %—US | 6.50 | 6.39 | 310 | 0.113 |
| 6 wt. %—No US | 6.50 | 6.39 | 315 | 0.118 |
| Sample | Mo (wt. %) SEM-EDX | Si/Al SEM-EDX | Si/Al XPS | Mo/(Si + Al) SEM-EDX | Mo/(Si + Al) XPS |
|---|---|---|---|---|---|
| 5 wt. %—US | 5.0 ± 0.7 | 13.7 | 14.1 | 0.03 | 0.15 |
| 5 wt. %—No US | 5.2 ± 0.7 | 14.1 | 11.9 | 0.03 | 0.22 |
| 6 wt. %—US | 5.8 ± 0.8 | 14.3 | 14.1 | 0.04 | 0.16 |
| 6 wt. %—No US | 5.9 ± 0.7 | 14.3 | 12.5 | 0.04 | 0.17 |
| Sample | XCH4-4h (%) | YC2+ (%) | YC6H6-4h (%) |
|---|---|---|---|
| 5 wt. %—US | 12.0 | 7.8 | 3.5 |
| 5 wt. %—No US | 11.4 | 7.7 | 3.7 |
| 6 wt. %—US | 13.2 | 7.9 | 3.8 |
| 6 wt. %—No US | 13.0 | 7.6 | 3.7 |
| Reference | XCH4 (5h) (%) | YC2+ (5h) (%) |
|---|---|---|
| Julian et al. [28] | 12.7 | 9.0 |
| Han et al. [7] | 8.1 | 7.5 |
| Tan [37] | 10.5 | 8.4 |
| Martinez and Penis [38] | 8.2 | 6.0 |
| Liu et al. [39] | 11.2 | 6.9 |
| Song et al. [26] | 11.7 | 8.7 |
| Liu et al. [40] | 13.3 | 9.4 |
| Xu et al. [41] | 5.5 | 5.0 |
| 6 wt.%—US (This work) | 13.0 | 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Mendoza, H.; Valdez Lancinha Pereira, M.; Van Gerven, T.; Lutz, C.; Julian, I. Ultrasound-Assisted Preparation of Mo/ZSM-5 Zeolite Catalyst for Non-Oxidative Methane Dehydroaromatization. Catalysts 2021, 11, 313. https://doi.org/10.3390/catal11030313
Ramirez-Mendoza H, Valdez Lancinha Pereira M, Van Gerven T, Lutz C, Julian I. Ultrasound-Assisted Preparation of Mo/ZSM-5 Zeolite Catalyst for Non-Oxidative Methane Dehydroaromatization. Catalysts. 2021; 11(3):313. https://doi.org/10.3390/catal11030313
Chicago/Turabian StyleRamirez-Mendoza, Heidy, Mafalda Valdez Lancinha Pereira, Tom Van Gerven, Cécile Lutz, and Ignacio Julian. 2021. "Ultrasound-Assisted Preparation of Mo/ZSM-5 Zeolite Catalyst for Non-Oxidative Methane Dehydroaromatization" Catalysts 11, no. 3: 313. https://doi.org/10.3390/catal11030313