Main Routes of Production of High-Value-Added 2,5-Furandincarboxylic Acid Using Heterogeneous Catalytic Systems
Abstract
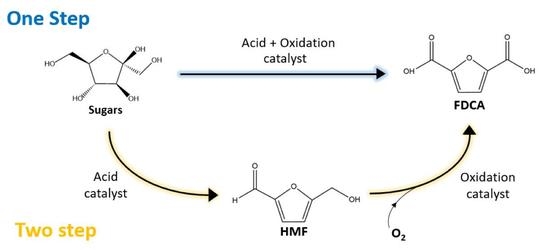
:1. Introduction
2. Catalytic Conversion of Sugars to FDCA in One Step
2.1. One-Step Production of FDCA by Physical Separation of the Byphasic System
2.2. One-Step Production of FDCA without Physical Separation of the Biphasic System
3. Catalytic Conversion of Fructose to FDCA in Two-Step
| Feed | Synthesis of HMF | HMF Yield (%) | Synthesis of FDCA | FDCA Yield (%) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst | Solvent | Temp. | Time | Catalyst | Solvent | Temp. | Oxidant | Time | ||||
| Fructose | HCl | [bmim]Cl | - | - | 89 | MnO2 | [bmim]Cl/H2O | 130 °C | O2 (10 bar) | 6 h | 85 | [16] |
| Fructose Huaia 2020 | ChCl/MeCN | 150 °C | 2 h | 90 | NiOx | H2O | 100 °C | NaClO, open pot | 2 h | 97 | [39] | |
| Fructose | HCl | Isopropanol | 120 °C | 3 h | 84 | Au/HT | H2O | 95 °C | O2 flow, Na2CO3 | 9 h | 98.8 | [17] |
| Fructose | HCl | GVL/H2O | 180 °C | 45 min | 70 | Pt/C | GVL/H2O | 110 °C | O2 (40 bar) | 16 h | 93 | [38] |
| Fructose | FDCA | GVL/H2O | 180 °C | 70 min | 70 | Pt/C | GVL/H2O | 110 °C | O2 (40 bar) | 16 h | 91 | [38] |
| Fructose | Amberlyst 15 | DMSO | 120 °C | 1 h | 97.1 | Pt/C | DMSO/H2O | 100 °C | O2 flow (30 mL/min) K2CO3 | 10 h | 91 | [45] |
| Fructose | Amberlyst 15 | DMSO | 120 °C | 2 h | - | Ru/C (NaHCO3) | DMSO/H2O | 130 °C | O2 (40 bar) | - | 89 | [46] |
| Fructose | Amberlyst 15 | TEAB/H2O | 95 °C | 30 min | 86 | Au8Pd2/HT | MIBK/H2O | 95 °C | O2 | 20 h | 78 | [28] |
| Fructose | Lewatit SPC 108 | H2O | 80 °C | 30 min | 80 | PtBi/C (5%/5%) | Water saturated MIBK | 80 °C | Air oxygen | 70 h | 25 | [27] |
| Fructose | CPN3 | Isopropanol | 140 °C | 3 h | 75.5 | Au/HT | H2O | 90 °C | O2 flow Na2CO3 | 4 h | 98 | [40] |
| Fructose | Fe3O4-RGO-SO3H | DMSO | 110 °C | 2 h | 81 | ZnFe1.65Ru0.35O4 | DMSO/H2O | 130 °C | O2 | 16 h | 70 | [47] |
| Fructose | Fe3O4@SiO2−SO3H | DMSO | 100 °C | 2 h | 93.1 | nano-Fe3O4-CoOx | DMSO | 80 °C | t-BuOOH | 15 h | 59.8 | [26] |
| Fructose | SiO2 gel | H2O | 88 °C | 8 h | 93 | Co(acac)3 | H2O | 160 °C | Air | 65 min | 46 | [27] |
| Fructose | SiO2 gel | H2O | 88 °C | 8 h | 93 | Co-gel | H2O | 160 °C | Air | 65 min | 72 | [29] |
| Fructose | Pd/CC | H2O | 140 °C | 9 h | 84 | Pd/CC | H2O | 140 °C | O2 | 30 h | 64 | [48] |
4. Conversion of Sugars into FDCA
4.1. Conversion of Glucose into FDCA
4.2. Conversion of Sucrose into FDCA
4.3. Conversion of Inulin into FDCA
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassan, A.M.; Al-Shalabi, E.W.; Ayoub, M.A. Updated perceptions on polymer-based enhanced oil recovery toward high-temperature high-salinity tolerance for successful field applications in carbonate reservoirs. Polymers 2022, 14, 2001. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis goes bio: From carbohydrates and sugar alcohols to platform chemicals. Angew. Chem.-Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Deng, J.; Xu, Q.; Zuo, Y.; Guo, Q.X.; Fu, Y. Catalytic conversion of furfural into a 2,5-furandicarboxylic acid-based polyester with total carbon utilization. ChemSusChem 2013, 6, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, E.; Yamaguchi, Y.; Kamata, K.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Hara, M. Effect of MnO2 crystal structure on aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. J. Am. Chem. Soc. 2019, 141, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Chen, J.; Yu, X.; Zhao, X.; Feng, Y.; Zuo, M.; Li, Z.; Yang, S.; Sun, Y.; Tang, X.; et al. Toward an integrated conversion of fructose for two-step production of 2,5-furandicarboxylic acid or furan-2,5-dimethylcarboxylate with air as oxidant. Chem. Eng. J. 2022, 450, 138172. [Google Scholar] [CrossRef]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Au-based catalysts: Optimization of active phase and metal-support interaction. Appl. Catal. B Environ. 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I; U.S. Department of Energy, Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2004; 76p. [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154–3171. [Google Scholar] [CrossRef]
- Elsevier Scopus—FDCA Search Results. Available online: https://www.scopus.com/results/results.uri?sort=plf-f&src=s&st1=fdca&sid=9d0126636fac6ba6aadb0e9986aa0118&sot=b&sdt=b&sl=9&s=ALL%28fdca%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Present (accessed on 12 May 2023).
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–555. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Chen, Z.; Qi, Z.; Wang, X. Enhanced formation of 5-HMF from glucose using a highly selective and stable SAPO-34 catalyst. Chem. Eng. J. 2017, 307, 877–883. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W.C. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-based products from Xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Sella Kapu, N.; Trajano, H.L. Review of hemicellulose hydrolysis in softwoods and bamboo nuwan. Biofuels Bioprod. Biorefining 2014, 8, 743. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Al Ghatta, A.; Hallett, J.P. High yield and isolation of 2,5-furandicarboxylic acid from HMF and sugars in ionic liquids, a new prospective for the establishment of a scalable and efficient catalytic route. Green Chem. 2022, 24, 3309–3313. [Google Scholar] [CrossRef]
- Yi, G.; Teong, S.P.; Li, X.; Zhang, Y. Purification of biomass-derived 5-hydroxymethylfurfural and its catalytic conversion to 2,5-furandicarboxylic acid. ChemSusChem 2014, 7, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Wang, Z.; Zhang, C.; Yuan, S.; Zhu, Y.; Wang, J. PVAm–PIP/PS composite membrane with high performance for CO2/N2 separation. AIChE J. 2012, 59, 215–228. [Google Scholar] [CrossRef]
- Pan, J.; Mao, Y.; Gao, H.; Xiong, Q.; Qiu, F.; Zhang, T.; Niu, X. Fabrication of hydrophobic polymer foams with double acid sites on surface of macropore for conversion of carbohydrate. Carbohydr. Polym. 2016, 143, 212–222. [Google Scholar] [CrossRef]
- Chen, P.; Yamaguchi, A.; Hiyoshi, N.; Mimura, N. Efficient Continuous dehydration of fructose to 5-hydroxymethylfurfural in ternary solvent system. Fuel 2023, 334, 126632. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Hu, Y.; Hu, C. Controlling the Reaction networks for efficient conversion of glucose into 5-hydroxymethylfurfural. ChemSusChem 2020, 13, 4812–4832. [Google Scholar] [CrossRef]
- Galaverna, R.; Breitkreitz, M.C.; Pastre, J.C. Conversion of D-fructose to 5-(hydroxymethyl)furfural: Evaluating batch and continuous flow conditions by design of experiments and in-line FTIR monitoring. ACS Sustain. Chem. Eng. 2018, 6, 4220–4230. [Google Scholar] [CrossRef]
- Aellig, C.; Hermans, I. Continuous D-fructose dehydration to 5-hydroxymethylfurfural under mild conditions. ChemSusChem 2012, 5, 1737–1742. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Josephson, T.R.; Nikolakis, V.; Caratzoulas, S. Origin of 5-hydroxymethylfurfural stability in water/dimethyl sulfoxide mixtures. ChemSusChem 2014, 7, 117–126. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933. [Google Scholar] [CrossRef]
- Cao, Z.; Fan, Z.; Chen, Y.; Li, M.; Shen, T.; Zhu, C.; Ying, H. Efficient preparation of 5-hydroxymethylfurfural from cellulose in a biphasic system over hafnyl phosphates. Appl. Catal. B Environ. 2019, 244, 170–177. [Google Scholar] [CrossRef]
- Kröger, M.; Prüße, U.; Vorlop, K.D. A new approach for the production of 2,5-furandicarboxylic acid by in situ oxidation of 5-hydroxymethylfurfural starting from fructose. Top. Catal. 2000, 13, 237–242. [Google Scholar] [CrossRef]
- Yi, G.; Teong, S.P.; Zhang, Y. The direct conversion of sugars into 2,5-furandicarboxylic acid in a triphasic system. ChemSusChem 2015, 8, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.L.; Schuchardt, U. Cooperative effect of cobalt acetylacetonate and silica in the catalytic cyclization and oxidation of fructose to 2,5-furandicarboxylic acid. Catal. Commun. 2003, 4, 83–86. [Google Scholar] [CrossRef]
- Yan, D.; Wang, G.; Gao, K.; Lu, X.; Xin, J.; Zhang, S. One-pot synthesis of 2,5-furandicarboxylic acid from fructose in ionic liquids. Ind. Eng. Chem. Res. 2018, 57, 1851–1858. [Google Scholar] [CrossRef]
- Yangyang, J.Z.; Zhou, F.; Ma, H.X.; Li, X.L.; Yuan, X.Z.; Liang, F.X.; Zhang, J. One step synthesis of 2, 5-furandicarboxylic acid from fructose catalyzed by Ce modified Ru/HAP. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2020, 48, 942–948. [Google Scholar] [CrossRef]
- Chai, Y.; Yang, H.; Bai, M.; Chen, A.; Peng, L.; Yan, B.; Zhao, D.; Qin, P.; Peng, C.; Wang, X. Direct production of 2, 5-furandicarboxylicacid from raw biomass by manganese dioxide catalysis cooperated with ultrasonic-assisted diluted acid pretreatment. Bioresour. Technol. 2021, 337, 125421. [Google Scholar] [CrossRef]
- Chai, Y.; Bai, M.; Chen, A.; Yuan, J.; Peng, C.; Zhao, D.; Yan, B.; Qin, P. Cr-Mn bimetallic functionalized USY zeolite monolithic catalyst for direct production of 2, 5-furandicarboxylic acid from raw biomass. Chem. Eng. J. 2022, 429, 132173. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Y.; Liao, Y.; Wang, H.; Liu, Q.; Ma, L.; Wang, C. Advances in understanding the humins: Formation, prevention and application. Appl. Energy Combust. Sci. 2022, 10, 100062. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, G. Mechanism insights into the aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid over MnO2 catalysts. J. Phys. Chem. C 2021, 125, 3818–3826. [Google Scholar] [CrossRef]
- Cong, H.; Yuan, H.; Tao, Z.; Bao, H.; Zhang, Z.; Jiang, Y.; Huang, D.; Liu, H.; Wang, T. Recent advances in catalytic conversion of biomass to 2,5-furandicarboxylic acid. Catalysts 2021, 11, 1113. [Google Scholar] [CrossRef]
- Deshan, A.D.K.; Atanda, L.; Moghaddam, L.; Rackemann, D.W.; Beltramini, J.; Doherty, W.O.S. Heterogeneous catalytic conversion of sugars into 2,5-furandicarboxylic acid. Front. Chem. 2020, 8, 659. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Hussain, A.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T. Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose. Sci. Adv. 2018, 4, eaap9722. [Google Scholar]
- Liu, H.; Li, W.; Zuo, M.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Fang, H.; Li, T.; Lin, L. Facile and efficient two-step formation of a renewable monomer 2,5-furandicarboxylic acid from carbohydrates over the NiOx catalyst. Ind. Eng. Chem. Res. 2020, 59, 4895–4904. [Google Scholar] [CrossRef]
- Teong, S.P.; Yi, G.; Cao, X.; Zhang, Y. Poly-benzylic ammonium chloride resins as solid catalysts for fructose dehydration. ChemSusChem 2014, 7, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, K.; Vrijburg, W.L.; Ouyang, X.; Iulian Dugulan, A.; Liu, Y.; Tiny Verhoeven, M.W.G.M.; Kosinov, N.A.; Pidko, E.A.; Hensen, E.J.M. Hydrogenation of levulinic acid to γ-valerolactone over Fe-Re/TiO2 catalysts. Appl. Catal. B Environ. 2020, 278, 119314. [Google Scholar] [CrossRef]
- Mushrif, S.H.; Caratzoulas, S.; Vlachos, D.G. Understanding solvent effects in the selective conversion of fructose to 5-hydroxymethyl-furfural: A molecular dynamics investigation. Phys. Chem. Chem. Phys. 2012, 14, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hu, Y.; Zhang, Y.; Zhang, Y.; Tang, D.; Zhu, L.; Hu, C. Solvent effects on degradative condensation side reactions of fructose in its initial conversion to 5-hydroxymethylfurfural. ChemSusChem 2020, 13, 501–512. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Liu, B. Catalytic conversion of fructose and 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid over a recyclable Fe3O4-CoOx magnetite nanocatalyst. ACS Sustain. Chem. Eng. 2015, 3, 406–412. [Google Scholar] [CrossRef]
- Chen, G.; Wu, L.; Fan, H.; Li, B.G. Highly efficient two-step synthesis of 2,5-furandicarboxylic acid from fructose without 5-hydroxymethylfurfural (Hmf) separation: In situ oxidation of Hmf in alkaline aqueous H2O/DMSO mixed solvent under mild conditions. Ind. Eng. Chem. Res. 2018, 57, 16172–16181. [Google Scholar] [CrossRef]
- Liu, H.; Cao, X.; Wang, T.; Wei, J.; Tang, X.; Zeng, X.; Sun, Y.; Lei, T.; Liu, S.; Lin, L. Efficient synthesis of bio-monomer 2,5-furandicarboxylic acid from concentrated 5-hydroxymethylfurfural or fructose in DMSO/H2O mixed solvent. J. Ind. Eng. Chem. 2019, 77, 209–214. [Google Scholar] [CrossRef]
- Yang, Z.; Qi, W.; Su, R.; He, Z. Selective synthesis of 2,5-diformylfuran and 2,5-furandicarboxylic acid from 5-hydroxymethylfurfural and fructose catalyzed by magnetically separable catalysts. Energy Fuels 2017, 31, 533–541. [Google Scholar] [CrossRef]
- Rathod, P.V.; Jadhav, V.H. Efficient method for synthesis of 2,5-furandicarboxylic acid from 5-hydroxymethylfurfural and fructose using Pd/CC catalyst under aqueous conditions. ACS Sustain. Chem. Eng. 2018, 6, 5766–5771. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; Macfarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, C.; Liu, X.; Xia, Q.; Wang, Y. Selective Oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over MnO:X-CeO2 composite catalysts. Green Chem. 2017, 19, 996–1004. [Google Scholar] [CrossRef]
- Hou, Q.; Laiq Ur Rehman, M.; Bai, X.; Qian, H.; Lai, R.; Xia, T.; Yu, G.; Tang, Y.; Xie, H.; Ju, M. Enhancing the reusability of hydroxyapatite by barium modification for efficient isomerization of glucose to fructose in ethanol. Fuel 2023, 338, 127308. [Google Scholar] [CrossRef]
- Zuo, M.; Jia, W.; Feng, Y.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Effective selectivity conversion of glucose to furan chemicals in the aqueous deep eutectic solvent. Renew. Energy 2021, 164, 23–33. [Google Scholar] [CrossRef]
- Pandey, S.; Dumont, M.J.; Orsat, V.; Rodrigue, D. Biobased 2,5-furandicarboxylic acid (FDCA) and its emerging copolyesters’ properties for packaging applications. Eur. Polym. J. 2021, 160, 110778. [Google Scholar] [CrossRef]
- Schade, O.R.; Dannecker, P.K.; Kalz, K.F.; Steinbach, D.; Meier, M.A.R.; Grunwaldt, J.D. Direct catalytic route to biomass-derived 2,5-furandicarboxylic acid and its use as monomer in a multicomponent polymerization. ACS Omega 2019, 4, 16972–16979. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; de Wolf, J. Inulin in Bread and other Cereal-Based Products; Woodhead Publishing Limited: Cambridge, UK, 2007; ISBN 9781845691776. [Google Scholar]
- Wrigstedt, P.; Keskiväli, J.; Perea-Buceta, J.E.; Repo, T. One-Pot Transformation of carbohydrates into valuable furan derivatives via 5-hydroxymethylfurfural. ChemCatChem 2017, 9, 4244–4255. [Google Scholar] [CrossRef]





| Feed | Catalyst | Catalyst Loading | Solvent | Physical Separation | T | P | Time | FDCA Yield (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Fructose * | SO3H − PtBi/C | 14 g − 3 g | Water − MIBK | Selective membrane | 80 °C | - | 7 days | 25 | [27] |
| Fructose * | Amberlyst 15 − AuPd/HT | 0.018 g − 0.25 g | TEAB − Water | Triphasic reaction | 95 °C | - | 20 h | 78 | [28] |
| Glucose * | Amberlyst 15 + CrCl3·6H2O − AuPd/HT | 0.018 g − 0.25 g | TEAB − Water | Triphasic reaction | 95 °C | - | 30 h | 50 | [28] |
| Fructose | Cobalt silica gel | 0.05 g | Water | - | 160 °C | 20 bar (air) | 65 min | 72 | [29] |
| Fructose | Amberlyst 15 + Fe0.6Zr0.4O2 | 0.01g + 0.01 g | [Bmim]Cl | - | 160 °C | 20 bar (O2) | 24 h | 46.4 | [30] |
| Fructose | Ce-Ru/HAP | 0.1 g | DMSO | - | 160 °C | 20 bar (O2) | 4 h | 34.2 | [31] |
| Rice straw | HCl + MnO2 | 10 mL + 1 g | Water + DMSO | - | 200 °C | - | - | 52.1 | [32] |
| Rice straw | Cr-Mn/USY | 0.7 g | Water + DMSO (Additive KHNO3) | - | 180 °C | - | - | 67 | [33] |
| Synthesis of HMF | HMF Yield (%) | Synthesis of FDCA | FDCA Yield (%) | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feed | Catalyst | Solvent | Temp. | Time | Catalyst | Solvent | Temp. | Oxidant | Time | |||
| Glucose | CrCl3 · 6H2O | [bmim]Cl | 120 °C | 30 min | 55 | MnO2 | [bmim]Cl (20% H2O) | 120 °C | O2 (10 bar) | 6 h | 50 | [16] |
| Glucose | AlCl3 | EtAc/H2O | 120 °C | 12 h | 52.9 | Pt/C | NaHCO3/H2O | 120 °C | O2 (20 bar) | 2 h | 43.2 | [52] |
| Sucrose | H2SO4 | H2O | 200 °C | 10 min | 34 | Au/ZrO2 | H2O/NaOH | 100 °C | Synthetic air | 5 h | 71 | [53] |
| Inulin | H2SO4 | MeCN (KBr) | 150 °C | 4 min | >99 | Pt/C | NaHCO3 | 70 °C | O2 (8 bar) | 10 h | 70 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, A.; Barredo, A.; Viar, N.; Requies, J. Main Routes of Production of High-Value-Added 2,5-Furandincarboxylic Acid Using Heterogeneous Catalytic Systems. Catalysts 2023, 13, 880. https://doi.org/10.3390/catal13050880
Bueno A, Barredo A, Viar N, Requies J. Main Routes of Production of High-Value-Added 2,5-Furandincarboxylic Acid Using Heterogeneous Catalytic Systems. Catalysts. 2023; 13(5):880. https://doi.org/10.3390/catal13050880
Chicago/Turabian StyleBueno, Ane, Asier Barredo, Nerea Viar, and Jesus Requies. 2023. "Main Routes of Production of High-Value-Added 2,5-Furandincarboxylic Acid Using Heterogeneous Catalytic Systems" Catalysts 13, no. 5: 880. https://doi.org/10.3390/catal13050880