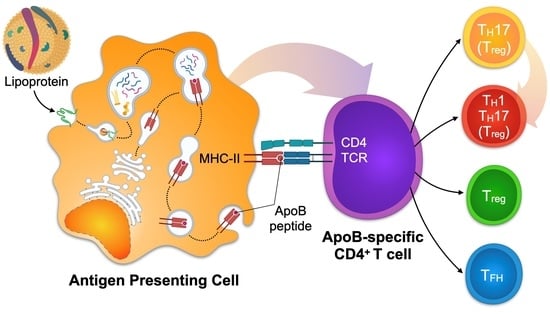
ApoB-Specific CD4+ T Cells in Mouse and Human Atherosclerosis
Abstract
:1. Atherosclerosis Is an Immune-Driven, Chronic Inflammatory Disease
2. Frequencies, Immune Phenotypes, and Roles of T-Helper Cells in Atherosclerosis
2.1. TH1 Cells
2.2. T Regulatory Cells
2.3. TH17 Cells
2.4. TH2 Cells
2.5. Follicular-Helper T Cells (TFH)
2.6. CD4+ Cytotoxic Lymphocytes (CTL)
2.7. Other Types of T Cell Immunity
2.8. Multi-TH Committed CD4+ T Cells in the Atherosclerotic Plaque
3. Evidence for Autoimmunity in Atherosclerosis
4. ApoB-Specific CD4+ T Cells in Mice and Humans
4.1. Mechanisms of CD4+ T Cell Activation
4.2. Detection of ApoB-Specific CD4+ T Cells in Humans by Functional Restimulation
4.3. Detection of ApoB-Specific CD4+ T Cells in Mice and Humans by Tetramers of MHC-II
4.4. Cloning of CD4+ T Cell Lines and TCR-Transgenic Mice
5. Function of ApoB-Specific CD4+ T Cells
6. Clinical Translation and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Kruk, M.E.; Gage, A.D.; Joseph, N.T.; Danaei, G.; Garcia-Saiso, S.; Salomon, J.A. Mortality due to low-quality health systems in the universal health coverage era: A systematic analysis of amenable deaths in 137 countries. Lancet 2018. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Marchini, T.; Zirlik, A.; Wolf, D. Pathogenic Role of Air Pollution Particulate Matter in Cardiometabolic Disease: Evidence from Mice and Humans. Antioxid Redox Signal 2020, 33, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Colantonio, L.D.; Bittner, V.; Reynolds, K.; Levitan, E.B.; Rosenson, R.S.; Banach, M.; Kent, S.T.; Derose, S.F.; Zhou, H.; Safford, M.M.; et al. Association of Serum Lipids and Coronary Heart Disease in Contemporary Observational Studies. Circulation 2016, 133, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Hansson, G.K. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1594–1607. [Google Scholar] [CrossRef]
- Ross, R. The pathogenesis of atherosclerosis--an update. N. Eng. J. Med. 1986, 314, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Zirlik, A.; Ley, K. Beyond vascular inflammation--recent advances in understanding atherosclerosis. Cell Mol. Life Sci. 2015, 72, 3853–3869. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Maatta, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Libby, P.; Aikawa, E.; Alcaide, P.; Luscinskas, F.W.; Weissleder, R.; Pittet, M.J. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 2007, 117, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahrendorf, M. Myeloid cell contributions to cardiovascular health and disease. Nat. Med. 2018, 24, 711–720. [Google Scholar] [CrossRef]
- Winkels, H.; Ehinger, E.; Vassallo, M.; Buscher, K.; Dinh, H.Q.; Kobiyama, K.; Hamers, A.A.J.; Cochain, C.; Vafadarnejad, E.; Saliba, A.E.; et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ. Res. 2018, 122, 1675–1688. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah, G.A.; Wei, G.S.; Sorlie, P.D.; Fine, L.J.; Rosenberg, Y.; Kaufmann, P.G.; Mussolino, M.E.; Hsu, L.L.; Addou, E.; Engelgau, M.M.; et al. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circ. Res. 2017, 120, 366–380. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Eng. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Eng. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Eng. J. Med. 2020. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef]
- Stemme, S.; Faber, B.; Holm, J.; Wiklund, O.; Witztum, J.L.; Hansson, G.K. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 1995, 92, 3893–3897. [Google Scholar] [CrossRef] [Green Version]
- Tsiantoulas, D.; Diehl, C.J.; Witztum, J.L.; Binder, C.J. B cells and humoral immunity in atherosclerosis. Circ. Res. 2014, 114, 1743–1756. [Google Scholar] [CrossRef]
- Chou, M.Y.; Fogelstrand, L.; Hartvigsen, K.; Hansen, L.F.; Woelkers, D.; Shaw, P.X.; Choi, J.; Perkmann, T.; Backhed, F.; Miller, Y.I.; et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 2009, 119, 1335–1349. [Google Scholar] [CrossRef] [Green Version]
- Winkels, H.; Wolf, D. Heterogeneity of T Cells in Atherosclerosis Defined by Single-Cell RNA-Sequencing and Cytometry by Time of Flight. Arter. Thromb. Vasc. Biol. 2020. [Google Scholar] [CrossRef]
- Jonasson, L.; Holm, J.; Skalli, O.; Bondjers, G.; Hansson, G.K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 1986, 6, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Jonasson, L. The discovery of cellular immunity in the atherosclerotic plaque. Arter. Thromb. Vasc. Biol. 2009, 29, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, M.A.; Prange, K.H.; Slenders, L.; Ord, T.; Elbersen, D.; Boltjes, A.; de Jager, S.C.; Asselbergs, F.W.; de Borst, G.J.; Aavik, E.; et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Jaroslav, P.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018. [Google Scholar] [CrossRef]
- Cole, J.E.; Park, I.; Ahern, D.J.; Kassiteridi, C.; Danso Abeam, D.; Goddard, M.E.; Green, P.; Maffia, P.; Monaco, C. Immune cell census in murine atherosclerosis: Cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res. 2018, 114, 1360–1371. [Google Scholar] [CrossRef] [Green Version]
- Taniuchi, I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu. Rev. Immunol. 2018, 36, 579–601. [Google Scholar] [CrossRef]
- Peaper, D.R.; Cresswell, P. Regulation of MHC class I assembly and peptide binding. Annu. Rev. Cell Dev. Biol. 2008, 24, 343–368. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.; Paul, P.; Bakke, O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Frostegard, J.; Ulfgren, A.K.; Nyberg, P.; Hedin, U.; Swedenborg, J.; Andersson, U.; Hansson, G.K. Cytokine expression in advanced human atherosclerotic plaques: Dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 1999, 145, 33–43. [Google Scholar] [CrossRef]
- Van Dijk, R.A.; Duinisveld, A.J.; Schaapherder, A.F.; Mulder-Stapel, A.; Hamming, J.F.; Kuiper, J.; de Boer, O.J.; van der Wal, A.C.; Kolodgie, F.D.; Virmani, R.; et al. A change in inflammatory footprint precedes plaque instability: A systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, M.J.; Filipowicz, A.R.; Waseem, T.C.; McGary, C.M.; Crow, K.J.; Magilnick, N.; Boldin, M.; Lundberg, P.S.; Galkina, E.V. Atherosclerosis-Driven Treg Plasticity Results in Formation of a Dysfunctional Subset of Plastic IFNgamma+ Th1/Tregs. Circ. Res. 2016, 119, 1190–1203. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Koltsova, E.K.; Garcia, Z.; Chodaczek, G.; Landau, M.; McArdle, S.; Scott, S.R.; von Vietinghoff, S.; Galkina, E.; Miller, Y.I.; Acton, S.T.; et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J. Clin. Invest. 2012, 122, 3114–3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; McArdle, S.; Gholami, A.; Kimura, T.; Wolf, D.; Gerhardt, T.; Miller, J.; Weber, C.; Ley, K. CCR5+T-bet+FoxP3+ Effector CD4 T Cells Drive Atherosclerosis. Circ. Res. 2016, 118, 1540–1552. [Google Scholar] [CrossRef] [Green Version]
- Niwa, T.; Wada, H.; Ohashi, H.; Iwamoto, N.; Ohta, H.; Kirii, H.; Fujii, H.; Saito, K.; Seishima, M. Interferon-gamma produced by bone marrow-derived cells attenuates atherosclerotic lesion formation in LDLR-deficient mice. J. Atheroscler. Thromb. 2004, 11, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Buono, C.; Come, C.E.; Stavrakis, G.; Maguire, G.F.; Connelly, P.W.; Lichtman, A.H. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arter. Thromb. Vasc. Biol. 2003, 23, 454–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Pablo, A.M.; Jiang, X.; Wang, N.; Tall, A.R.; Schindler, C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 1997, 99, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Buono, C.; Binder, C.J.; Stavrakis, G.; Witztum, J.L.; Glimcher, L.H.; Lichtman, A.H. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl. Acad. Sci. USA 2005, 102, 1596–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitman, S.C.; Ravisankar, P.; Elam, H.; Daugherty, A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am. J. Pathol. 2000, 157, 1819–1824. [Google Scholar] [CrossRef]
- Foks, A.C.; Lichtman, A.H.; Kuiper, J. Treating atherosclerosis with regulatory T cells. Arter. Thromb. Vasc. Biol. 2015, 35, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004, 22, 531–562. [Google Scholar] [CrossRef]
- De Boer, O.J.; van der Meer, J.J.; Teeling, P.; van der Loos, C.M.; van der Wal, A.C. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE 2007, 2, e779. [Google Scholar] [CrossRef] [Green Version]
- Wolf, D.; Gerhardt, T.; Winkels, H.; Michel, N.A.; Pramod, A.B.; Ghosheh, Y.; Brunel, S.; Buscher, K.; Miller, J.; McArdle, S.; et al. Pathogenic Autoimmunity in Atherosclerosis Evolves From Initially Protective Apolipoprotein B100-Reactive CD4(+) T-Regulatory Cells. Circulation 2020, 142, 1279–1293. [Google Scholar] [CrossRef]
- Gaddis, D.E.; Padgett, L.E.; Wu, R.; McSkimming, C.; Romines, V.; Taylor, A.M.; McNamara, C.A.; Kronenberg, M.; Crotty, S.; Thomas, M.J.; et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat. Commun 2018, 9, 1095. [Google Scholar] [CrossRef] [PubMed]
- Pitoiset, F.; Barbie, M.; Monneret, G.; Braudeau, C.; Pochard, P.; Pellegrin, I.; Trauet, J.; Labalette, M.; Klatzmann, D.; Rosenzwajg, M. A standardized flow cytometry procedure for the monitoring of regulatory T cells in clinical trials. Cytom. Part B Clin. Cytom. 2018, 94, 621–626. [Google Scholar] [CrossRef]
- Shami, A.; Atzler, D.; Bosmans, L.A.; Winkels, H.; Meiler, S.; Lacy, M.; van Tiel, C.; Ta Megens, R.; Nitz, K.; Baardman, J.; et al. Glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) drives atherosclerosis in mice and is associated with an unstable plaque phenotype and cerebrovascular events in humans. Eur. Heart J. 2020, 41, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Meiler, S.; Smeets, E.; Winkels, H.; Shami, A.; Pascutti, M.F.; Nolte, M.A.; Beckers, L.; Weber, C.; Gerdes, N.; Lutgens, E. Constitutive GITR Activation Reduces Atherosclerosis by Promoting Regulatory CD4+ T-Cell Responses-Brief Report. Arter. Thromb. Vasc. Biol. 2016, 36, 1748–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait-Oufella, H.; Salomon, B.L.; Potteaux, S.; Robertson, A.K.; Gourdy, P.; Zoll, J.; Merval, R.; Esposito, B.; Cohen, J.L.; Fisson, S.; et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006, 12, 178–180. [Google Scholar] [CrossRef]
- Klingenberg, R.; Gerdes, N.; Badeau, R.M.; Gistera, A.; Strodthoff, D.; Ketelhuth, D.F.; Lundberg, A.M.; Rudling, M.; Nilsson, S.K.; Olivecrona, G.; et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 2013, 123, 1323–1334. [Google Scholar] [CrossRef]
- Sharma, M.; Schlegel, M.P.; Afonso, M.S.; Brown, E.J.; Rahman, K.; Weinstock, A.; Sansbury, B.E.; Corr, E.M.; van Solingen, C.; Koelwyn, G.J.; et al. Regulatory T Cells License Macrophage Pro-Resolving Functions During Atherosclerosis Regression. Circ. Res. 2020, 127, 335–353. [Google Scholar] [CrossRef]
- Mor, A.; Luboshits, G.; Planer, D.; Keren, G.; George, J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur. Heart J. 2006, 27, 2530–2537. [Google Scholar] [CrossRef] [Green Version]
- Wigren, M.; Bjorkbacka, H.; Andersson, L.; Ljungcrantz, I.; Fredrikson, G.N.; Persson, M.; Bryngelsson, C.; Hedblad, B.; Nilsson, J. Low levels of circulating CD4+FoxP3+ T cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arter. Thromb. Vasc. Biol. 2012, 32, 2000–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mailer, R.K.W.; Gistera, A.; Polyzos, K.A.; Ketelhuth, D.F.J.; Hansson, G.K. Hypercholesterolemia Enhances T Cell Receptor Signaling and Increases the Regulatory T Cell Population. Sci. Rep. 2017, 7, 15655. [Google Scholar] [CrossRef] [Green Version]
- Maganto-Garcia, E.; Tarrio, M.L.; Grabie, N.; Bu, D.X.; Lichtman, A.H. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation 2011, 124, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Mailer, R.K.W.; Gistera, A.; Polyzos, K.A.; Ketelhuth, D.F.J.; Hansson, G.K. Hypercholesterolemia Induces Differentiation of Regulatory T Cells in the Liver. Circ. Res. 2017, 120, 1740–1753. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Miossec, P.; Kolls, J.K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 2012, 11, 763–776. [Google Scholar] [CrossRef]
- Erbel, C.; Akhavanpoor, M.; Okuyucu, D.; Wangler, S.; Dietz, A.; Zhao, L.; Stellos, K.; Little, K.M.; Lasitschka, F.; Doesch, A.; et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J. Immunol. 2014, 193, 4344–4355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbel, C.; Chen, L.; Bea, F.; Wangler, S.; Celik, S.; Lasitschka, F.; Wang, Y.; Bockler, D.; Katus, H.A.; Dengler, T.J. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 2009, 183, 8167–8175. [Google Scholar] [CrossRef] [Green Version]
- Butcher, M.J.; Gjurich, B.N.; Phillips, T.; Galkina, E.V. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 2012, 110, 675–687. [Google Scholar] [CrossRef] [Green Version]
- Usui, F.; Kimura, H.; Ohshiro, T.; Tatsumi, K.; Kawashima, A.; Nishiyama, A.; Iwakura, Y.; Ishibashi, S.; Takahashi, M. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem Biophys Res. Commun. 2012, 420, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yuan, X.; Deng, L.; Xu, W.; Zheng, Y.; Yue, C.; Zhang, G.; Xie, F.; Yang, Y.H.; Gantier, M.P.; et al. Imbalanced frequencies of Th17 and Treg cells in acute coronary syndromes are mediated by IL-6-STAT3 signaling. PLoS ONE 2013, 8, e72804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, X.; Yu, X.; Ding, Y.J.; Fu, Q.Q.; Xie, J.J.; Tang, T.T.; Yao, R.; Chen, Y.; Liao, Y.H. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 2008, 127, 89–97. [Google Scholar] [CrossRef]
- Danzaki, K.; Matsui, Y.; Ikesue, M.; Ohta, D.; Ito, K.; Kanayama, M.; Kurotaki, D.; Morimoto, J.; Iwakura, Y.; Yagita, H.; et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arter. Thromb. Vasc. Biol. 2012, 32, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Brauner, S.; Jiang, X.; Thorlacius, G.E.; Lundberg, A.M.; Ostberg, T.; Yan, Z.Q.; Kuchroo, V.K.; Hansson, G.K.; Wahren-Herlenius, M. Augmented Th17 differentiation in Trim21 deficiency promotes a stable phenotype of atherosclerotic plaques with high collagen content. Cardiovasc Res. 2018, 114, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Gistera, A.; Robertson, A.K.; Andersson, J.; Ketelhuth, D.F.; Ovchinnikova, O.; Nilsson, S.K.; Lundberg, A.M.; Li, M.O.; Flavell, R.A.; Hansson, G.K. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci. Transl. Med. 2013, 5, 196ra100. [Google Scholar] [CrossRef]
- Taleb, S.; Romain, M.; Ramkhelawon, B.; Uyttenhove, C.; Pasterkamp, G.; Herbin, O.; Esposito, B.; Perez, N.; Yasukawa, H.; Van Snick, J.; et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009, 206, 2067–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.; Prasad, K.M.; Butcher, M.; Dobrian, A.; Kolls, J.K.; Ley, K.; Galkina, E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 2010, 121, 1746–1755. [Google Scholar] [CrossRef]
- Simon, T.; Taleb, S.; Danchin, N.; Laurans, L.; Rousseau, B.; Cattan, S.; Montely, J.M.; Dubourg, O.; Tedgui, A.; Kotti, S.; et al. Circulating levels of interleukin-17 and cardiovascular outcomes in patients with acute myocardial infarction. Eur. Heart J. 2013, 34, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhur, M.S.; Funt, S.A.; Li, L.; Vinh, A.; Chen, W.; Lob, H.E.; Iwakura, Y.; Blinder, Y.; Rahman, A.; Quyyumi, A.A.; et al. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arter. Thromb. Vasc. Biol. 2011, 31, 1565–1572. [Google Scholar] [CrossRef] [Green Version]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.F.; Ranjbaran, H.; Gallo, A.; Sokol, S.I.; Pfau, S.; Pober, J.S.; Tellides, G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- King, V.L.; Szilvassy, S.J.; Daugherty, A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arter. Thromb. Vasc. Biol. 2002, 22, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Cardilo-Reis, L.; Gruber, S.; Schreier, S.M.; Drechsler, M.; Papac-Milicevic, N.; Weber, C.; Wagner, O.; Stangl, H.; Soehnlein, O.; Binder, C.J. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 2012, 4, 1072–1086. [Google Scholar] [CrossRef]
- King, V.L.; Cassis, L.A.; Daugherty, A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am. J. Pathol. 2007, 171, 2040–2047. [Google Scholar] [CrossRef] [Green Version]
- Davenport, P.; Tipping, P.G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2003, 163, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Engelbertsen, D.; Andersson, L.; Ljungcrantz, I.; Wigren, M.; Hedblad, B.; Nilsson, J.; Bjorkbacka, H. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arter. Thromb. Vasc. Biol. 2013, 33, 637–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silveira, A.; McLeod, O.; Strawbridge, R.J.; Gertow, K.; Sennblad, B.; Baldassarre, D.; Veglia, F.; Deleskog, A.; Persson, J.; Leander, K.; et al. Plasma IL-5 concentration and subclinical carotid atherosclerosis. Atherosclerosis 2015, 239, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.M.; Xu, D.; Asquith, D.L.; Denby, L.; Li, Y.; Sattar, N.; Baker, A.H.; McInnes, I.B.; Liew, F.Y. IL-33 reduces the development of atherosclerosis. J. Exp. Med. 2008, 205, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Ryu, H.; Lim, H.; Choi, G.; Park, Y.J.; Cho, M.; Na, H.; Ahn, C.W.; Kim, Y.C.; Kim, W.U.; Lee, S.H.; et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat. Immunol. 2018, 19, 583–593. [Google Scholar] [CrossRef]
- Nus, M.; Sage, A.P.; Lu, Y.; Masters, L.; Lam, B.Y.H.; Newland, S.; Weller, S.; Tsiantoulas, D.; Raffort, J.; Marcus, D.; et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med. 2017, 23, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Clement, M.; Guedj, K.; Andreata, F.; Morvan, M.; Bey, L.; Khallou-Laschet, J.; Gaston, A.T.; Delbosc, S.; Alsac, J.M.; Bruneval, P.; et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation 2015, 131, 560–570. [Google Scholar] [CrossRef] [Green Version]
- Ghamar Talepoor, A.; Khosropanah, S.; Doroudchi, M. Functional subsets of circulating follicular helper T cells in patients with atherosclerosis. Physiol Rep. 2020, 8, e14637. [Google Scholar] [CrossRef] [PubMed]
- Gistera, A.; Klement, M.L.; Polyzos, K.A.; Mailer, R.K.W.; Duhlin, A.; Karlsson, M.C.I.; Ketelhuth, D.F.J.; Hansson, G.K. Low-Density Lipoprotein-Reactive T Cells Regulate Plasma Cholesterol Levels and Development of Atherosclerosis in Humanized Hypercholesterolemic Mice. Circulation 2018, 138, 2513–2526. [Google Scholar] [CrossRef]
- Dumitriu, I.E. The life (and death) of CD4+ CD28(null) T cells in inflammatory diseases. Immunology 2015, 146, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acuto, O.; Michel, F. CD28-mediated co-stimulation: A quantitative support for TCR signalling. Nat. Rev. Immunol. 2003, 3, 939–951. [Google Scholar] [CrossRef]
- Kyaw, T.; Peter, K.; Li, Y.; Tipping, P.; Toh, B.H.; Bobik, A. Cytotoxic lymphocytes and atherosclerosis: Significance, mechanisms and therapeutic challenges. Br. J. Pharmacol. 2017, 174, 3956–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumitriu, I.E.; Baruah, P.; Finlayson, C.J.; Loftus, I.M.; Antunes, R.F.; Lim, P.; Bunce, N.; Kaski, J.C. High levels of costimulatory receptors OX40 and 4-1BB characterize CD4+CD28null T cells in patients with acute coronary syndrome. Circ. Res. 2012, 110, 857–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020. [Google Scholar] [CrossRef]
- Liuzzo, G.; Goronzy, J.J.; Yang, H.; Kopecky, S.L.; Holmes, D.R.; Frye, R.L.; Weyand, C.M. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 2000, 101, 2883–2888. [Google Scholar] [CrossRef] [Green Version]
- Liuzzo, G.; Kopecky, S.L.; Frye, R.L.; O’Fallon, W.M.; Maseri, A.; Goronzy, J.J.; Weyand, C.M. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 1999, 100, 2135–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, T.; Schulte, S.; Warrington, K.J.; Kopecky, S.L.; Frye, R.L.; Goronzy, J.J.; Weyand, C.M. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation 2002, 105, 570–575. [Google Scholar] [CrossRef] [Green Version]
- Zal, B.; Kaski, J.C.; Arno, G.; Akiyu, J.P.; Xu, Q.; Cole, D.; Whelan, M.; Russell, N.; Madrigal, J.A.; Dodi, I.A.; et al. Heat-shock protein 60-reactive CD4+CD28null T cells in patients with acute coronary syndromes. Circulation 2004, 109, 1230–1235. [Google Scholar] [CrossRef] [Green Version]
- Zal, B.; Kaski, J.C.; Akiyu, J.P.; Cole, D.; Arno, G.; Poloniecki, J.; Madrigal, A.; Dodi, A.; Baboonian, C. Differential pathways govern CD4+ CD28- T cell proinflammatory and effector responses in patients with coronary artery disease. J. Immunol. 2008, 181, 5233–5241. [Google Scholar] [CrossRef]
- Li, H.; van der Leun, A.M.; Yofe, I.; Lubling, Y.; Gelbard-Solodkin, D.; van Akkooi, A.C.J.; van den Braber, M.; Rozeman, E.A.; Haanen, J.; Blank, C.U.; et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 2019, 176, 775–789 e718. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H. Th9 cells: Differentiation and disease. Immunol. Rev. 2013, 252, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.T.; Gao, Y.; Lazarevic, V. Transcriptional regulation of CD4(+) TH cells that mediate tissue inflammation. J. Leukoc. Biol. 2018, 104, 1069–1085. [Google Scholar] [CrossRef]
- Gregersen, I.; Skjelland, M.; Holm, S.; Holven, K.B.; Krogh-Sorensen, K.; Russell, D.; Askevold, E.T.; Dahl, C.P.; Orn, S.; Gullestad, L.; et al. Increased systemic and local interleukin 9 levels in patients with carotid and coronary atherosclerosis. PLoS ONE 2013, 8, e72769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.Z.; Wu, B.W.; Lu, Z.D.; Huang, Y.; Shi, Y.; Liu, H.; Liu, L.; Zeng, Q.T.; Wang, X.; Ji, Q.W. Circulating Th22 and Th9 levels in patients with acute coronary syndrome. Mediat. Inflamm. 2013, 2013, 635672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, T.; Nie, D.; Wen, S.; Jia, C.; Zhu, Z.; Xia, N.; Nie, S.; Zhou, S.; Jiao, J.; et al. IL-9 aggravates the development of atherosclerosis in ApoE−/− mice. Cardiovasc Res. 2015, 106, 453–464. [Google Scholar] [CrossRef]
- Azizi, G.; Yazdani, R.; Mirshafiey, A. Th22 cells in autoimmunity: A review of current knowledge. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 108–117. [Google Scholar]
- Shi, L.; Ji, Q.; Liu, L.; Shi, Y.; Lu, Z.; Ye, J.; Zeng, T.; Xue, Y.; Yang, Z.; Liu, Y.; et al. IL-22 produced by Th22 cells aggravates atherosclerosis development in ApoE(−/−) mice by enhancing DC-induced Th17 cell proliferation. J. Cell Mol. Med. 2020, 24, 3064–3078. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, T.; Wang, X.Q.; Du, R.Z.; Zhang, K.N.; Liu, X.G.; Ma, D.X.; Yu, S.; Su, G.H.; Li, Z.H.; et al. Elevated frequencies of circulating Th22 cell in addition to Th17 cell and Th17/Th1 cell in patients with acute coronary syndrome. PLoS ONE 2013, 8, e71466. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobryshev, Y.V.; Lord, R.S. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J. Histochem. Cytochem. 2005, 53, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getz, G.S.; Reardon, C.A. Natural killer T cells in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 304–314. [Google Scholar] [CrossRef]
- Harty, J.T.; Tvinnereim, A.R.; White, D.W. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 2000, 18, 275–308. [Google Scholar] [CrossRef]
- Schafer, S.; Zernecke, A. CD8(+) T Cells in Atherosclerosis. Cells 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- DuPage, M.; Bluestone, J.A. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat. Rev. Immunol. 2016, 16, 149–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.J.; Makings, J.; Ley, K. Regulatory T Cell Stability and Plasticity in Atherosclerosis. Cells 2020, 9, 2665. [Google Scholar] [CrossRef]
- Kimura, T.; Kobiyama, K.; Winkels, H.; Tse, K.; Miller, J.; Vassallo, M.; Wolf, D.; Ryden, C.; Orecchioni, M.; Dileepan, T.; et al. Regulatory CD4(+) T Cells Recognize MHC-II-Restricted Peptide Epitopes of Apolipoprotein B. Circulation 2018, 138, 1130–1143. [Google Scholar] [CrossRef]
- Tan, T.G.; Mathis, D.; Benoist, C. Singular role for T-BET+CXCR3+ regulatory T cells in protection from autoimmune diabetes. Proc. Natl. Acad. Sci. USA 2016, 113, 14103–14108. [Google Scholar] [CrossRef] [Green Version]
- Tartar, D.M.; VanMorlan, A.M.; Wan, X.; Guloglu, F.B.; Jain, R.; Haymaker, C.L.; Ellis, J.S.; Hoeman, C.M.; Cascio, J.A.; Dhakal, M.; et al. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J. Immunol. 2010, 184, 3377–3385. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, A.; Di Giovangiulio, M.; Stolfi, C.; Franze, E.; Fehling, H.J.; Carsetti, R.; Giorda, E.; Colantoni, A.; Ortenzi, A.; Rugge, M.; et al. RORgammat-Expressing Tregs Drive the Growth of Colitis-Associated Colorectal Cancer by Controlling IL6 in Dendritic Cells. Cancer Immunol. Res. 2018, 6, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M.; et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science 2015, 349, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Di Giovangiulio, M.; Rizzo, A.; Franze, E.; Caprioli, F.; Facciotti, F.; Onali, S.; Favale, A.; Stolfi, C.; Fehling, H.J.; Monteleone, G.; et al. Tbet Expression in Regulatory T Cells Is Required to Initiate Th1-Mediated Colitis. Front. Immunol. 2019, 10, 2158. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Bucktrout, S.L.; Martinez-Llordella, M.; Zhou, X.; Anthony, B.; Rosenthal, W.; Luche, H.; Fehling, H.J.; Bluestone, J.A. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 2013, 39, 949–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Korn, T.; Reddy, J.; Gao, W.; Bettelli, E.; Awasthi, A.; Petersen, T.R.; Backstrom, B.T.; Sobel, R.A.; Wucherpfennig, K.W.; Strom, T.B.; et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat. Med. 2007, 13, 423–431. [Google Scholar] [CrossRef]
- Ranjbaran, H.; Sokol, S.I.; Gallo, A.; Eid, R.E.; Iakimov, A.O.; D’Alessio, A.; Kapoor, J.R.; Akhtar, S.; Howes, C.J.; Aslan, M.; et al. An inflammatory pathway of IFN-gamma production in coronary atherosclerosis. J. Immunol. 2007, 178, 592–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F.J.; Ott, I.; Gawaz, M.; Richardt, G.; Holzapfel, H.; Jochum, M.; Schomig, A. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction. Circulation 1995, 92, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Okba, A.M.; Abd El Raouf Raafat, M.; Nazmy Farres, M.; Abd El Nour Melek, N.; Amin, M.M.; Gendy, N.N. Expanded peripheral CD4(+)CD28(null) T cells and its association with atherosclerotic changes in patients with end stage renal disease on hemodialysis. Hum. Immunol. 2019, 80, 748–754. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Remedios, K.A.; Abbas, A.K. Mechanisms of human autoimmunity. J. Clin. Invest. 2015, 125, 2228–2233. [Google Scholar] [CrossRef] [Green Version]
- Adler, R. Janeway’s immunobiology. Choice Curr. Rev. Acad. Libr. 2008, 45, 1793–1794. [Google Scholar]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ’T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Bu, D.X.; Tarrio, M.; Maganto-Garcia, E.; Stavrakis, G.; Tajima, G.; Lederer, J.; Jarolim, P.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Kusters, P.J.H.; Lutgens, E.; Seijkens, T.T.P. Exploring immune checkpoints as potential therapeutic targets in atherosclerosis. Cardiovasc Res. 2018, 114, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, A.; Ketelhuth, D.F.; Strodthoff, D.; Wurm, M.; Hansson, E.M.; Nicoletti, A.; Paulsson-Berne, G.; Hansson, G.K. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J. Exp. Med. 2010, 207, 1081–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutgens, E.; de Muinck, E.D.; Kitslaar, P.J.; Tordoir, J.H.; Wellens, H.J.; Daemen, M.J. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999, 41, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Zernecke, A.; Winkels, H.; Cochain, C.; Williams, J.W.; Wolf, D.; Soehnlein, O.; Robbins, C.S.; Monaco, C.; Park, I.; McNamara, C.A.; et al. Meta-Analysis of Leukocyte Diversity in Atherosclerotic Mouse Aortas. Circ. Res. 2020, 127, 402–426. [Google Scholar] [CrossRef]
- Paulsson, G.; Zhou, X.; Tornquist, E.; Hansson, G.K. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arter. Thromb. Vasc. Biol. 2000, 20, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Qian, S.; Gong, Y.; Ren, J.; Zhao, L.; Wang, D.; Wang, X.; Zhang, Y.; Wang, Z.; Zhang, Q. Deep sequencing of the T cell receptor beta repertoire reveals signature patterns and clonal drift in atherosclerotic plaques and patients. Oncotarget 2017, 8, 99312–99322. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, R.; Brokopp, C.E.; Grives, A.; Courtier, A.; Jaguszewski, M.; Pasqual, N.; Vlaskou Badra, E.; Lewandowski, A.; Gaemperli, O.; Hoerstrup, S.P.; et al. Clonal restriction and predominance of regulatory T cells in coronary thrombi of patients with acute coronary syndromes. Eur. Heart J. 2015, 36, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Oksenberg, J.R.; Stavri, G.T.; Jeong, M.C.; Garovoy, N.; Salisbury, J.R.; Erusalimsky, J.D. Analysis of the T-cell receptor repertoire in human atherosclerosis. Cardiovasc Res. 1997, 36, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Kilic, A.; Mandal, K. Heat Shock Proteins: Pathogenic Role in Atherosclerosis and Potential Therapeutic Implications. Autoimmune Dis. 2012, 2012, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, C. Tolerization against atherosclerosis using heat shock protein 60. Cell Stress Chaperones 2015. [Google Scholar] [CrossRef] [Green Version]
- Wick, G.; Jakic, B.; Buszko, M.; Wick, M.C.; Grundtman, C. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 516–529. [Google Scholar] [CrossRef]
- Zhu, J.; Quyyumi, A.A.; Rott, D.; Csako, G.; Wu, H.; Halcox, J.; Epstein, S.E. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: Evidence for an autoimmune component of atherogenesis. Circulation 2001, 103, 1071–1075. [Google Scholar] [CrossRef] [Green Version]
- Kervinen, H.; Huittinen, T.; Vaarala, O.; Leinonen, M.; Saikku, P.; Manninen, V.; Manttari, M. Antibodies to human heat shock protein 60, hypertension and dyslipidemia. A study of joint effects on coronary risk. Atherosclerosis 2003, 169, 339–344. [Google Scholar] [CrossRef]
- Xu, Q.; Dietrich, H.; Steiner, H.J.; Gown, A.M.; Schoel, B.; Mikuz, G.; Kaufmann, S.H.; Wick, G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arter. Thromb. 1992, 12, 789–799. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Shoenfeld, Y.; Afek, A.; Gilburd, B.; Keren, P.; Shaish, A.; Kopolovic, J.; Wick, G.; Harats, D. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arter. Thromb. Vasc. Biol. 1999, 19, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xiong, Q.; Hu, X.; Sun, Y.; Tan, X.; Zhang, H.; Lu, Y.; Liu, J. A novel atherogenic epitope from Mycobacterium tuberculosis heat shock protein 65 enhances atherosclerosis in rabbit and LDL receptor-deficient mice. Heart Vessel. 2012, 27, 411–418. [Google Scholar] [CrossRef]
- Afek, A.; George, J.; Gilburd, B.; Rauova, L.; Goldberg, I.; Kopolovic, J.; Harats, D.; Shoenfeld, Y. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J Autoimmun 2000, 14, 115–121. [Google Scholar] [CrossRef]
- George, J.; Afek, A.; Gilburd, B.; Shoenfeld, Y.; Harats, D. Cellular and humoral immune responses to heat shock protein 65 are both involved in promoting fatty-streak formation in LDL-receptor deficient mice. J. Am. Coll. Cardiol. 2001, 38, 900–905. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.; Feng, J.; Zhang, Y.; Sun, Y.; Lu, Y.; Li, T.; Zhang, X.; Cao, R.; Jin, L.; Wu, J. Promotion of atherosclerosis in high cholesterol diet-fed rabbits by immunization with the P277 peptide. Immunol. Lett. 2016, 170, 80–87. [Google Scholar] [CrossRef]
- Klingenberg, R.; Ketelhuth, D.F.; Strodthoff, D.; Gregori, S.; Hansson, G.K. Subcutaneous immunization with heat shock protein-65 reduces atherosclerosis in Apoe(-)/(-) mice. Immunobiology 2012, 217, 540–547. [Google Scholar] [CrossRef]
- Long, J.; Lin, J.; Yang, X.; Yuan, D.; Wu, J.; Li, T.; Cao, R.; Liu, J. Nasal immunization with different forms of heat shock protein-65 reduced high-cholesterol-diet-driven rabbit atherosclerosis. Int. Immunopharmacol. 2012, 13, 82–87. [Google Scholar] [CrossRef]
- Van Puijvelde, G.H.; van Es, T.; van Wanrooij, E.J.; Habets, K.L.; de Vos, P.; van der Zee, R.; van Eden, W.; van Berkel, T.J.; Kuiper, J. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arter. Thromb. Vasc. Biol. 2007, 27, 2677–2683. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Tang, H.; Wang, X.; Zeng, Q.; Liu, Y.; Zhao, X.I.; Yu, K.; Shi, H.; Zhu, R.; Mao, X. Intranasal immunization with heat shock protein 60 induces CD4(+) CD25(+) GARP(+) and type 1 regulatory T cells and inhibits early atherosclerosis. Clin. Exp. Immunol. 2016, 183, 452–468. [Google Scholar] [CrossRef] [Green Version]
- Perschinka, H.; Mayr, M.; Millonig, G.; Mayerl, C.; van der Zee, R.; Morrison, S.G.; Morrison, R.P.; Xu, Q.; Wick, G. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arter. Thromb. Vasc. Biol. 2003, 23, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.J.; Horkko, S.; Dewan, A.; Chang, M.K.; Kieu, E.P.; Goodyear, C.S.; Shaw, P.X.; Palinski, W.; Witztum, J.L.; Silverman, G.J. Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003, 9, 736–743. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Matsuura, E.; Ichikawa, K.; Fujisaku, A.; Mukai, M.; Kobayashi, S.; Koike, T. Antibodies to beta 2-glycoprotein I and clinical manifestations in patients with systemic lupus erythematosus. Arthritis Rheum. 1996, 39, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, D.A.; Krilis, S.A. Beta 2-glycoprotein I. Lupus 1994, 3, 207–212. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Harats, D.; Gilburd, B.; Afek, A.; Levy, Y.; Schneiderman, J.; Barshack, I.; Kopolovic, J.; Shoenfeld, Y. Immunolocalization of beta2-glycoprotein I (apolipoprotein H) to human atherosclerotic plaques: Potential implications for lesion progression. Circulation 1999, 99, 2227–2230. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Afek, A.; Gilburd, B.; Blank, M.; Levy, Y.; Aron-Maor, A.; Levkovitz, H.; Shaish, A.; Goldberg, I.; Kopolovic, J.; et al. Induction of early atherosclerosis in LDL-receptor-deficient mice immunized with beta2-glycoprotein I. Circulation 1998, 98, 1108–1115. [Google Scholar] [CrossRef] [Green Version]
- Dunoyer-Geindre, S.; Kwak, B.R.; Pelli, G.; Roth, I.; Satta, N.; Fish, R.J.; Reber, G.; Mach, F.; Kruithof, E.K.; de Moerloose, P. Immunization of LDL receptor-deficient mice with beta2-glycoprotein 1 or human serum albumin induces a more inflammatory phenotype in atherosclerotic plaques. Thromb. Haemost. 2007, 97, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Afek, A.; George, J.; Shoenfeld, Y.; Gilburd, B.; Levy, Y.; Shaish, A.; Keren, P.; Janackovic, Z.; Goldberg, I.; Kopolovic, J.; et al. Enhancement of atherosclerosis in beta-2-glycoprotein I-immunized apolipoprotein E-deficient mice. Pathobiology 1999, 67, 19–25. [Google Scholar] [CrossRef]
- De Haro, J.; Esparza, L.; Bleda, S.; Varela, C.; Sanchez, C.; Acin, F. Attenuation of early atherosclerotic lesions by immunotolerance with beta2 glycoprotein I and the immunomodulatory effectors interleukin 2 and 10 in a murine model. J. Vasc. Surg. 2015, 62, 1625–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, J.; Harats, D.; Gilburd, B.; Afek, A.; Shaish, A.; Kopolovic, J.; Shoenfeld, Y. Adoptive transfer of beta(2)-glycoprotein I-reactive lymphocytes enhances early atherosclerosis in LDL receptor-deficient mice. Circulation 2000, 102, 1822–1827. [Google Scholar] [CrossRef] [Green Version]
- Sage, A.P.; Tsiantoulas, D.; Binder, C.J.; Mallat, Z. The role of B cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 180–196. [Google Scholar] [CrossRef]
- Hebsur, S.; Vakil, E.; Oetgen, W.J.; Kumar, P.N.; Lazarous, D.F. Influenza and coronary artery disease: Exploring a clinical association with myocardial infarction and analyzing the utility of vaccination in prevention of myocardial infarction. Rev. Cardiovasc Med. 2014, 15, 168–175. [Google Scholar] [PubMed]
- Lin, H.C.; Chien, C.W.; Ho, J.D. Herpes zoster ophthalmicus and the risk of stroke: A population-based follow-up study. Neurology 2010, 74, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.M.; Taubert, K.; Benin, A.L.; Brown, D.W.; Mensah, G.A.; Baddour, L.M.; Dunbar, S.; Krumholz, H.M.; American Heart, A.; American College of, C.; et al. Influenza vaccination as secondary prevention for cardiovascular disease: A science advisory from the American Heart Association/American College of Cardiology. J. Am. Coll. Cardiol. 2006, 48, 1498–1502. [Google Scholar] [CrossRef] [Green Version]
- Udell, J.A.; Zawi, R.; Bhatt, D.L.; Keshtkar-Jahromi, M.; Gaughran, F.; Phrommintikul, A.; Ciszewski, A.; Vakili, H.; Hoffman, E.B.; Farkouh, M.E.; et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: A meta-analysis. JAMA 2013, 310, 1711–1720. [Google Scholar] [CrossRef]
- Siriwardena, A.N.; Asghar, Z.; Coupland, C.C. Influenza and pneumococcal vaccination and risk of stroke or transient ischaemic attack-matched case control study. Vaccine 2014, 32, 1354–1361. [Google Scholar] [CrossRef]
- Macintyre, C.R.; Heywood, A.E.; Kovoor, P.; Ridda, I.; Seale, H.; Tan, T.; Gao, Z.; Katelaris, A.L.; Siu, H.W.; Lo, V.; et al. Ischaemic heart disease, influenza and influenza vaccination: A prospective case control study. Heart 2013, 99, 1843–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Famularo, G.; Trinchieri, V.; Santini, G.; De Simone, C. Infections, atherosclerosis, and coronary heart disease. Ann. Ital. Med. Int. 2000, 15, 144–155. [Google Scholar] [PubMed]
- Hemmat, N.; Ebadi, A.; Badalzadeh, R.; Memar, M.Y.; Baghi, H.B. Viral infection and atherosclerosis. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.E.; Campbell, L.A. Pathogens and atherosclerosis: Update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb. Haemost 2011, 106, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Wyde, P.R.; Litovsky, S.; Vela, D.; Ali, M.; Casscells, S.W.; Madjid, M. Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis 2010, 208, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Glenn, W.K.; Tran, D.D.; Ngan, C.C.; Duflou, J.A.; Whitaker, N.J. Identification of Human Papilloma Viruses in Atheromatous Coronary Artery Disease. Front Cardiovasc Med. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M.; Wyde, P.; Litovsky, S.; Madjid, M.; Akhtar, A.; Naguib, S.; Siadaty, M.S.; Sanati, S.; Casscells, W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 2003, 107, 762–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendren, N.S.; Drazner, M.H.; Bozkurt, B.; Cooper, L.T., Jr. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation 2020, 141, 1903–1914. [Google Scholar] [CrossRef]
- Nelson, R.W.; Beisang, D.; Tubo, N.J.; Dileepan, T.; Wiesner, D.L.; Nielsen, K.; Wuthrich, M.; Klein, B.S.; Kotov, D.I.; Spanier, J.A.; et al. T cell receptor cross-reactivity between similar foreign and self peptides influences naive cell population size and autoimmunity. Immunity 2015, 42, 95–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suthers, B.; Hansbro, P.; Thambar, S.; McEvoy, M.; Peel, R.; Attia, J. Pneumococcal vaccination may induce anti-oxidized low-density lipoprotein antibodies that have potentially protective effects against cardiovascular disease. Vaccine 2012, 30, 3983–3985. [Google Scholar] [CrossRef] [PubMed]
- Worzella, S.L.; Hayney, M.S. Inflammatory chronic diseases: Preventable by vaccines? J. Am. Pharm. Assoc. 2014, 54, 446–448. [Google Scholar] [CrossRef]
- Vila-Corcoles, A.; Ochoa-Gondar, O.; Rodriguez-Blanco, T.; de Diego, C.; Satue, E.; Group, E.S. Ineffectiveness of pneumococcal vaccination in cardiovascular prevention: The CAPAMIS study. JAMA Intern. Med. 2013, 173, 1918–1920. [Google Scholar] [CrossRef]
- Shiri-Sverdlov, R.; Dos Reis, I.M.; Oligschlaeger, Y.; Hendrikx, T.; Meesters, D.M.; Vanclooster, A.; Vanhoutvin, N.; Koek, G.H.; Westerterp, M.; Binder, C.J.; et al. The Influence of a Conjugated Pneumococcal Vaccination on Plasma Antibody Levels against Oxidized Low-Density Lipoprotein in Metabolic Disease Patients: A Single-Arm Pilot Clinical Trial. Antioxidants 2021, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A. Dendritic Cells in Atherosclerosis: Evidence in Mice and Humans. Arter. Thromb. Vasc. Biol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacRitchie, N.; Grassia, G.; Noonan, J.; Cole, J.E.; Hughes, C.E.; Schroeder, J.; Benson, R.A.; Cochain, C.; Zernecke, A.; Guzik, T.J.; et al. The aorta can act as a site of naive CD4+ T-cell priming. Cardiovasc Res. 2020, 116, 306–316. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Hennecke, J.; Wiley, D.C. T cell receptor-MHC interactions up close. Cell 2001, 104, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Zheng, L.; Lin, J.; Zhang, B.; Zhu, Y.; Li, N.; Xie, S.; Wang, Y.; Gao, N.; Huang, Z. Structural basis of assembly of the human T cell receptor-CD3 complex. Nature 2019, 573, 546–552. [Google Scholar] [CrossRef]
- Saito, T.; Yokosuka, T. Immunological synapse and microclusters: The site for recognition and activation of T cells. Curr. Opin. Immunol. 2006, 18, 305–313. [Google Scholar] [CrossRef]
- Frauwirth, K.A.; Thompson, C.B. Regulation of T lymphocyte metabolism. J. Immunol. 2004, 172, 4661–4665. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Vandenborre, K.; Van Gool, S.W.; Kasran, A.; Ceuppens, J.L.; Boogaerts, M.A.; Vandenberghe, P. Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology 1999, 98, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Zirlik, A.; Lutgens, E. An inflammatory link in atherosclerosis and obesity. Co-stimulatory molecules. Hamostaseologie 2015, 35, 272–278. [Google Scholar] [CrossRef]
- Ley, K.; Gerdes, N.; Winkels, H. ATVB Distinguished Scientist Award: How Costimulatory and Coinhibitory Pathways Shape Atherosclerosis. Arter. Thromb. Vasc. Biol. 2017, 37, 764–777. [Google Scholar] [CrossRef] [Green Version]
- Lievens, D.; Habets, K.L.; Robertson, A.K.; Laouar, Y.; Winkels, H.; Rademakers, T.; Beckers, L.; Wijnands, E.; Boon, L.; Mosaheb, M.; et al. Abrogated transforming growth factor beta receptor II (TGFbetaRII) signalling in dendritic cells promotes immune reactivity of T cells resulting in enhanced atherosclerosis. Eur. Heart J. 2013, 34, 3717–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermansson, A.; Johansson, D.K.; Ketelhuth, D.F.; Andersson, J.; Zhou, X.; Hansson, G.K. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation 2011, 123, 1083–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Cheong, C.; Dandamudi, D.B.; Park, C.G.; Rodriguez, A.; Mehandru, S.; Velinzon, K.; Jung, I.H.; Yoo, J.Y.; Oh, G.T.; et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity 2011, 35, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, M.; Thorp, E.; Hansson, G.K.; Tabas, I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs. J. Clin. Invest. 2013, 123, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Westerterp, M.; Gautier, E.L.; Ganda, A.; Molusky, M.M.; Wang, W.; Fotakis, P.; Wang, N.; Randolph, G.J.; D’Agati, V.D.; Yvan-Charvet, L.; et al. Cholesterol Accumulation in Dendritic Cells Links the Inflammasome to Acquired Immunity. Cell Metab. 2017, 25, 1294–1304 e1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clement, M.; Haddad, Y.; Raffort, J.; Lareyre, F.; Newland, S.A.; Master, L.; Harrison, J.; Ozsvar-Kozma, M.; Bruneval, P.; Binder, C.J.; et al. Deletion of IRF8 (Interferon Regulatory Factor 8)-Dependent Dendritic Cells Abrogates Proatherogenic Adaptive Immunity. Circ. Res. 2018, 122, 813–820. [Google Scholar] [CrossRef]
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T cell differentiation. Cell. Mol. Immunol. 2019, 16, 634–643. [Google Scholar] [CrossRef]
- Ley, K. The second touch hypothesis: T cell activation, homing and polarization. F1000Research 2014, 3, 37. [Google Scholar] [CrossRef]
- Koltsova, E.K.; Ley, K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011, 32, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Dustin, M.L. A dynamic view of the immunological synapse. Semin Immunol 2005, 17, 400–410. [Google Scholar] [CrossRef]
- Huppa, J.B.; Davis, M.M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 2003, 3, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.D.; Davis, M.M. MHC-peptide tetramers to visualize antigen-specific T cells. Curr. Protoc. Immunol. 2003, 53, 17.3.1–17.3.33. [Google Scholar] [CrossRef] [PubMed]
- Newell, E.W.; Davis, M.M. Beyond model antigens: High-dimensional methods for the analysis of antigen-specific T cells. Nat. Biotechnol. 2014, 32, 149–157. [Google Scholar] [CrossRef]
- Altman, J.D.; Moss, P.A.; Goulder, P.J.; Barouch, D.H.; McHeyzer-Williams, M.G.; Bell, J.I.; McMichael, A.J.; Davis, M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996. 274: 94-96. J. Immunol. 2011, 187, 7–9. [Google Scholar] [PubMed]
- Reiss, S.; Baxter, A.E.; Cirelli, K.M.; Dan, J.M.; Morou, A.; Daigneault, A.; Brassard, N.; Silvestri, G.; Routy, J.P.; Havenar-Daughton, C.; et al. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS ONE 2017, 12, e0186998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosono, M.; de Boer, O.J.; van der Wal, A.C.; van der Loos, C.M.; Teeling, P.; Piek, J.J.; Ueda, M.; Becker, A.E. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis 2003, 168, 73–80. [Google Scholar] [CrossRef]
- Poulton, T.A.; Gallagher, A.; Potts, R.C.; Beck, J.S. Changes in activation markers and cell membrane receptors on human peripheral blood T lymphocytes during cell cycle progression after PHA stimulation. Immunology 1988, 64, 419–425. [Google Scholar]
- Croft, M.; So, T.; Duan, W.; Soroosh, P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol. Rev. 2009, 229, 173–191. [Google Scholar] [CrossRef] [Green Version]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Frentsch, M.; Arbach, O.; Kirchhoff, D.; Moewes, B.; Worm, M.; Rothe, M.; Scheffold, A.; Thiel, A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 2005, 11, 1118–1124. [Google Scholar] [CrossRef]
- Roy, M.; Waldschmidt, T.; Aruffo, A.; Ledbetter, J.A.; Noelle, R.J. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 1993, 151, 2497–2510. [Google Scholar] [PubMed]
- Michel, N.A.; Zirlik, A.; Wolf, D. CD40L and Its Receptors in Atherothrombosis-An Update. Front Cardiovasc Med. 2017, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Yellin, M.J.; Sippel, K.; Inghirami, G.; Covey, L.R.; Lee, J.J.; Sinning, J.; Clark, E.A.; Chess, L.; Lederman, S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J. Immunol. 1994, 152, 598–608. [Google Scholar]
- Han, A.; Glanville, J.; Hansmann, L.; Davis, M.M. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol. 2014, 32, 684–692. [Google Scholar] [CrossRef]
- Abdelaal, H.M.; Cartwright, E.K.; Skinner, P.J. Detection of Antigen-Specific T Cells Using In Situ MHC Tetramer Staining. Int. J. Mol. Sci. 2019, 20, 5165. [Google Scholar] [CrossRef] [Green Version]
- Authors/Task Force, M.; Guidelines, E.S.C.C.f.P.; Societies, E.S.C.N.C. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [Green Version]
- Gero, S.; Gergely, J.; Jakab, L.; Szekely, J.; Virag, S.; Farkas, K.; Czuppon, A. Inhibition of cholesterol atherosclerosis by immunisation with beta-lipoprotein. Lancet 1959, 2, 6–7. [Google Scholar] [CrossRef]
- Freigang, S.; Horkko, S.; Miller, E.; Witztum, J.L.; Palinski, W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arter. Thromb. Vasc. Biol. 1998, 18, 1972–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palinski, W.; Miller, E.; Witztum, J.L. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl. Acad. Sci. USA 1995, 92, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Ameli, S.; Hultgardh-Nilsson, A.; Regnstrom, J.; Calara, F.; Yano, J.; Cercek, B.; Shah, P.K.; Nilsson, J. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arter. Thromb. Vasc. Biol. 1996, 16, 1074–1079. [Google Scholar] [CrossRef]
- George, J.; Afek, A.; Gilburd, B.; Levkovitz, H.; Shaish, A.; Goldberg, I.; Kopolovic, Y.; Wick, G.; Shoenfeld, Y.; Harats, D. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis 1998, 138, 147–152. [Google Scholar] [CrossRef]
- Zhou, X.; Caligiuri, G.; Hamsten, A.; Lefvert, A.K.; Hansson, G.K. LDL Immunization Induces T-Cell-Dependent Antibody Formation and Protection Against Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Chyu, K.Y.; Reyes, O.S.; Zhao, X.; Yano, J.; Dimayuga, P.; Nilsson, J.; Cercek, B.; Shah, P.K. Timing affects the efficacy of LDL immunization on atherosclerotic lesions in apo E (−/−) mice. Atherosclerosis 2004, 176, 27–35. [Google Scholar] [CrossRef]
- Zhou, X.; Robertson, A.K.; Rudling, M.; Parini, P.; Hansson, G.K. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ. Res. 2005, 96, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Wang, X.; Ji, Q.; Mao, X.; Tang, H.; Yi, G.; Meng, K.; Yang, X.; Zeng, Q. CD4+LAP + and CD4 +CD25 +Foxp3 + regulatory T cells induced by nasal oxidized low-density lipoprotein suppress effector T cells response and attenuate atherosclerosis in ApoE−/− mice. J. Clin. Immunol. 2012, 32, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Saigusa, R.; Ley, K. Vaccination against atherosclerosis. Curr. Opin. Immunol. 2019, 59, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Nettersheim, F.S.; De Vore, L.; Winkels, H. Vaccination in Atherosclerosis. Cells 2020, 9, 2560. [Google Scholar] [CrossRef]
- Davidson, N.O.; Shelness, G.S. APOLIPOPROTEIN B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 2000, 20, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, R.; Lebens, M.; Hermansson, A.; Fredrikson, G.N.; Strodthoff, D.; Rudling, M.; Ketelhuth, D.F.; Gerdes, N.; Holmgren, J.; Nilsson, J.; et al. Intranasal immunization with an apolipoprotein B-100 fusion protein induces antigen-specific regulatory T cells and reduces atherosclerosis. Arter. Thromb. Vasc. Biol. 2010, 30, 946–952. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Tse, K.; McArdle, S.; Gerhardt, T.; Miller, J.; Mikulski, Z.; Sidney, J.; Sette, A.; Wolf, D.; Ley, K. Atheroprotective vaccination with MHC-II-restricted ApoB peptides induces peritoneal IL-10-producing CD4 T cells. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H781–H790. [Google Scholar] [CrossRef]
- Kobiyama, K.; Vassallo, M.; Mitzi, J.; Winkels, H.; Pei, H.; Kimura, T.; Miller, J.; Wolf, D.; Ley, K. A clinically applicable adjuvant for an atherosclerosis vaccine in mice. Eur. J. Immunol. 2018, 48, 1580–1587. [Google Scholar] [CrossRef]
- Fredrikson, G.N.; Hedblad, B.; Berglund, G.; Alm, R.; Ares, M.; Cercek, B.; Chyu, K.Y.; Shah, P.K.; Nilsson, J. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arter. Thromb. Vasc. Biol. 2003, 23, 872–878. [Google Scholar] [CrossRef]
- Tse, K.Y.-B.; Gonen, A.; Sidney, J.; Ouyang, H.; Witztum, J.L.; Sette, A.; Tse, H.Y.; Ley, K. Atheroprotective vaccination with MHC-II restricted peptides from ApoB-100. Front. Immunol. 2013, 4, 493. [Google Scholar] [CrossRef] [PubMed]
- Gistera, A.; Hermansson, A.; Strodthoff, D.; Klement, M.L.; Hedin, U.; Fredrikson, G.N.; Nilsson, J.; Hansson, G.K.; Ketelhuth, D.F. Vaccination against T-cell epitopes of native ApoB100 reduces vascular inflammation and disease in a humanized mouse model of atherosclerosis. J. Intern. Med. 2017, 281, 383–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, M.K.; Tse, K.Y.; Zhao, X.; Welch, K.; Eitzman, D.T.; Thipparthi, R.R.; Montgomery, P.C.; Thummel, R.; Tse, H.Y. T-Cells Specific for a Self-Peptide of ApoB-100 Exacerbate Aortic Atheroma in Murine Atherosclerosis. Front Immunol. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Tse, K.; Tse, H.; Sidney, J.; Sette, A.; Ley, K. T cells in atherosclerosis. Int. Immunol. 2013, 25, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Wigren, M.; Bengtsson, D.; Duner, P.; Olofsson, K.; Bjorkbacka, H.; Bengtsson, E.; Fredrikson, G.N.; Nilsson, J. Atheroprotective effects of Alum are associated with capture of oxidized LDL antigens and activation of regulatory T cells. Circ. Res. 2009, 104, e62–e70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbin, O.; Ait-Oufella, H.; Yu, W.; Fredrikson, G.N.; Aubier, B.; Perez, N.; Barateau, V.; Nilsson, J.; Tedgui, A.; Mallat, Z. Regulatory T-cell response to apolipoprotein B100-derived peptides reduces the development and progression of atherosclerosis in mice. Arter. Thromb. Vasc. Biol. 2012, 32, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Wigren, M.; Rattik, S.; Yao Mattisson, I.; Tomas, L.; Gronberg, C.; Soderberg, I.; Alm, R.; Sundius, L.; Ljungcrantz, I.; Bjorkbacka, H.; et al. Lack of Ability to Present Antigens on Major Histocompatibility Complex Class II Molecules Aggravates Atherosclerosis in ApoE(−/−) Mice. Circulation 2019, 139, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Ley, K. 2015 Russell Ross Memorial Lecture in Vascular Biology: Protective Autoimmunity in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2016, 36, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Burnet, F.M. Immunological recognition of self. Science 1961, 133, 307–311. [Google Scholar] [CrossRef]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Metzger, T.C.; Anderson, M.S. Control of central and peripheral tolerance by Aire. Immunol. Rev. 2011, 241, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Juang, J.; Ebert, P.J.; Feng, D.; Garcia, K.C.; Krogsgaard, M.; Davis, M.M. Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J. Exp. Med. 2010, 207, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Jiang, N.; Ebert, P.J.; Kidd, B.A.; Muller, S.; Lund, P.J.; Juang, J.; Adachi, K.; Tse, T.; Birnbaum, M.E.; et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity 2015, 42, 929–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Villar, M.; Baecher-Allan, C.M.; Hafler, D.A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat. Med. 2011, 17, 673–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurence, A.; Amarnath, S.; Mariotti, J.; Kim, Y.C.; Foley, J.; Eckhaus, M.; O’Shea, J.J.; Fowler, D.H. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity 2012, 37, 209–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClymont, S.A.; Putnam, A.L.; Lee, M.R.; Esensten, J.H.; Liu, W.; Hulme, M.A.; Hoffmuller, U.; Baron, U.; Olek, S.; Bluestone, J.A.; et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 2011, 186, 3918–3926. [Google Scholar] [CrossRef]
- Pugliese, A. Autoreactive T cells in type 1 diabetes. J. Clin. Invest. 2017, 127, 2881–2891. [Google Scholar] [CrossRef]
- Libby, P.; Lichtman, A.H.; Hansson, G.K. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity 2013, 38, 1092–1104. [Google Scholar] [CrossRef] [Green Version]
- Reiner, Z.; Laufs, U.; Cosentino, F.; Landmesser, U. The year in cardiology 2018: Prevention. Eur. Heart J. 2019, 40, 336–344. [Google Scholar] [CrossRef]
- Aday, A.W.; Ridker, P.M. Targeting Residual Inflammatory Risk: A Shifting Paradigm for Atherosclerotic Disease. Front Cardiovasc Med. 2019, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [Green Version]
- Peikert, A.; Kaier, K.; Merz, J.; Manhart, L.; Schafer, I.; Hilgendorf, I.; Hehn, P.; Wolf, D.; Willecke, F.; Sheng, X.; et al. Residual inflammatory risk in coronary heart disease: Incidence of elevated high-sensitive CRP in a real-world cohort. Clin. Res. Cardiol. 2020, 109, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frostegard, J. Atherosclerosis in patients with autoimmune disorders. Arter. Thromb. Vasc. Biol. 2005, 25, 1776–1785. [Google Scholar] [CrossRef] [PubMed]


| Lineage | TF | Effector Cytokines | Role in Mouse Atherosclerosis | Regulation in Human Atherosclerosis |
|---|---|---|---|---|
| TH1 | T-bet | INF-γ, IL-2, IL-3, IL-6, TNF-α, lymphotoxin | Pro-atherogenic [37,39,40,41,42,43,44] | TH1 dominance in atherosclerotic lesions [35,36], higher IFN-γ plasma levels in patients with CAD [127], higher IL-6 and TNF-α plasma levels in patients with MI [128]. |
| TH2 | GATA3 | IL-4, IL-5, IL-10, IL-13 | Pro-atherogenic [79,82] Atheroprotective [80] No effect [81] | Lower TH2 cell numbers and decreased IL-4 secretion by CD4+ in patients with MI [83], lower IL-5 plasma levels in patients with subclinical atherosclerosis [84] |
| TH9 | FoxO1, BATF, IRF4 | IL-9 | Pro-atherogenic [107] | Higher IL-9 plasma levels in patients with atherosclerosis and ACS [105] Unchanged TH9 numbers in patients with ACS [106] |
| TH17 | RORγT | IL-17A, IL-17-F, IL-21, IL-22 | Pro-atherogenic [64,65,66,67] Atheroprotective [70,71,72,73,74] No effect [76] | Higher IL-17 plasma levels in patients with unstable angina or MI [68,69], lower IL-17 plasma levels in patients with MI [75], unchanged IL-17 plasma levels patients in patients with CAD [77] |
| TH22 | AHR | IL-22 | Pro-atherogenic [109] | Higher TH22 cell counts and IL-22 plasma level in patient with an ACS [106,110] |
| Treg | FoxP3, CD25 | IL-10, TGF-β | Atheroprotective [54,55,56] | Lower Treg numbers in blood from patients with MI [57] and ACS [68,69], low Treg numbers predict MI [58], higher Treg numbers in blood of patients with CAD [49] |
| TFH | Bcl6 | IL-21 | Pro-atherogenic [50,87,88] | Higher TFH count in patients with advanced atherosclerosis [90] |
| CD4+ CTL | TNF-α, INF-γ, perforin, granzyme A, B | Not present in mice | Higher numbers in blood from patients with ACS [95] and with end-stage renal disease and atherosclerosis [129], enrichment in unstable atherosclerotic lesions [97,98,99] | |
| NK T cells | Multiple, including perforin and granzymes | Controversial [113] | Accumulation of NKT cells in rupture-prone atherosclerotic plaques [112]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchini, T.; Hansen, S.; Wolf, D. ApoB-Specific CD4+ T Cells in Mouse and Human Atherosclerosis. Cells 2021, 10, 446. https://doi.org/10.3390/cells10020446
Marchini T, Hansen S, Wolf D. ApoB-Specific CD4+ T Cells in Mouse and Human Atherosclerosis. Cells. 2021; 10(2):446. https://doi.org/10.3390/cells10020446
Chicago/Turabian StyleMarchini, Timoteo, Sophie Hansen, and Dennis Wolf. 2021. "ApoB-Specific CD4+ T Cells in Mouse and Human Atherosclerosis" Cells 10, no. 2: 446. https://doi.org/10.3390/cells10020446