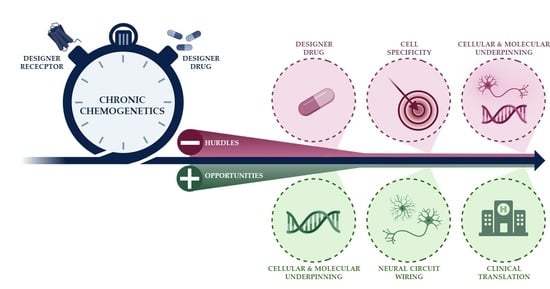
The DREADDful Hurdles and Opportunities of the Chronic Chemogenetic Toolbox
Abstract
:1. Introduction
2. DREADDful Hurdles in (Chronic) Chemogenetic Studies
2.1. The DREADD Actuator CNO
2.2. Cell Specificity
2.3. Lack of Fundamental Knowledge of Chronic DREADD Neuromodulation
2.3.1. Receptor Desensitization
2.3.2. Neuroadaptive Changes
3. DREADDful Opportunities
3.1. Cellular and Molecular Fingerprints of Neuroscience
3.2. Untangling Network Activation
3.3. Exploring the Road towards Clinical Translation
3.4. Neurotrophic Factors as an Interesting Example for a Future Therapeutic Direction
4. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, B.N.; Li, X.; Pausch, M.H.; Herlitze, S.; Roth, B.L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. USA 2007, 104, 5163–5168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, B.L. DREADDs for Neuroscientists. Neuron 2016, 89, 683–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørgensen, S.H.; Fitzpatrick, C.M.; Gether, U.; Woldbye, D.P.D.; Sørensen, A.T. Chemogenetic Modulation of G Protein-Coupled Receptor Signalling in Visual Attention Research. Basic Clin. Pharmacol. Toxicol. 2017, 121, 373–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogan, S.C.; Roth, B.L. Remote Control of Neuronal Signaling. Pharmacol. Rev. 2011, 63, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Kolesov, D.V.; Sokolinskaya, E.L.; Lukyanov, K.A.; Bogdanov, A.M. Molecular Tools for Targeted Control of Nerve Cell Electrical Activity. Part II. Acta Nat. 2021, 13, 17–32. [Google Scholar] [CrossRef]
- Atasoy, D.; Sternson, S.M. Chemogenetic tools for causal cellular and neuronal biology. Physiol. Rev. 2018, 98, 391–418. [Google Scholar] [CrossRef]
- Poth, K.M.; Texakalidis, P.; Boulis, N.M. Chemogenetics: Beyond Lesions and Electrodes. Neurosurgery 2021, 89, 185–195. [Google Scholar] [CrossRef]
- Alexander, G.M.; Rogan, S.C.; Abbas, A.I.; Armbruster, B.N.; Pei, Y.; Allen, J.A.; Nonneman, R.J.; Hartmann, J.; Moy, S.S.; Nicolelis, M.A.; et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009, 63, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Desloovere, J.; Boon, P.; Larsen, L.E.; Merckx, C.; Goossens, M.; van den Haute, C.; Baekelandt, V.; de Bundel, D.; Carrette, E.; Delbeke, J.; et al. Long-term chemogenetic suppression of spontaneous seizures in a mouse model for temporal lobe epilepsy. Epilepsia 2019, 60, 2314–2324. [Google Scholar] [CrossRef]
- Paretkar, T.; Dimitrov, E. Activation of enkephalinergic (Enk) interneurons in the central amygdala (CeA) buffers the behavioral effects of persistent pain. Neurobiol. Dis. 2019, 124, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Lau, J.; Ip, C.K.; Qi, Y.; Shi, Y.-C.; Zhang, L.; Tasan, R.; Sperk, G.; Herzog, H. Arcuate nucleus and lateral hypothalamic CART neurons in the mouse brain exert opposing effects on energy expenditure. eLife 2018, 7, e36494. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, D.A.A.; Browne, R.W.; Shaw, J.K.; Krishnan Radhakrishnan, S.; Khare, P.; España, R.A.; Clark, S.D. Clozapine N-Oxide Administration Produces Behavioral Effects in Long–Evans Rats: Implications for Designing DREADD Experiments. eNeuro 2016, 3, ENEURO.0219-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Bonaventura, J.; Lesniak, W.; Mathews, W.B.; Sysa-Shah, P.; Rodriguez, L.A.; Ellis, R.J.; Richie, C.T.; Harvey, B.K.; Dannals, R.F.; et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 2017, 357, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manvich, D.F.; Webster, K.A.; Foster, S.L.; Farrell, M.S.; Ritchie, J.C.; Porter, J.H.; Weinshenker, D. The DREADD agonist clozapine N-oxide (CNO) is reverse-metabolized to clozapine and produces clozapine-like interoceptive stimulus effects in rats and mice. Sci. Rep. 2018, 8, 3840. [Google Scholar] [CrossRef]
- Mahler, S.V.; Aston-Jones, G. CNO Evil? Considerations for the Use of DREADDs in Behavioral Neuroscience. Neuropsychopharmacology 2018, 43, 934–936. [Google Scholar] [CrossRef]
- Runegaard, A.H.; Sørensen, A.T.; Fitzpatrick, C.M.; Jørgensen, S.H.; Petersen, A.V.; Hansen, N.W.; Weikop, P.; Andreasen, J.T.; Mikkelsen, J.D.; Perrier, J.-F.; et al. Locomotor- and Reward-Enhancing Effects of Cocaine Are Differentially Regulated by Chemogenetic Stimulation of Gi-Signaling in Dopaminergic Neurons. eNeuro 2018, 5, ENEURO.0345-17.2018. [Google Scholar] [CrossRef]
- Grafe, L.A.; Eacret, D.; Dobkin, J.; Bhatnagar, S. Reduced Orexin System Function Contributes to Resilience to Repeated Social Stress. eNeuro 2018, 5, ENEURO.0273-17.2018. [Google Scholar] [CrossRef] [Green Version]
- Campbell, E.J.; Marchant, N.J. The use of chemogenetics in behavioural neuroscience: Receptor variants, targeting approaches and caveats. Br. J. Pharmacol. 2018, 175, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Tran, F.H.; Spears, S.L.; Ahn, K.J.; Eisch, A.J.; Yun, S. Does chronic systemic injection of the DREADD agonists clozapine-N-oxide or Compound 21 change behavior relevant to locomotion, exploration, anxiety, and depression in male non-DREADD-expressing mice? Neurosci. Lett. 2020, 739, 135432. [Google Scholar] [CrossRef]
- Nawreen, N.; Cotella, E.M.; Morano, R.; Mahbod, P.; Dalal, K.S.; Fitzgerald, M.; Martelle, S.; Packard, B.A.; Franco-Villanueva, A.; Moloney, R.D.; et al. Chemogenetic Inhibition of Infralimbic Prefrontal Cortex GABAergic Parvalbumin Interneurons Attenuates the Impact of Chronic Stress in Male Mice. eNeuro 2020, 7, ENEURO.0423-19.2020. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Umschweif, G.; Leung, J.; Sagi, Y.; Greengard, P. HCN2 Channels in Cholinergic Interneurons of Nucleus Accumbens Shell Regulate Depressive Behaviors. Neuron 2019, 101, 662.e5–672.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Morgan, D.A.; Cui, H.; Rahmouni, K. Activation of hypothalamic AgRP and POMC neurons evokes disparate sympathetic and cardiovascular responses. Am. J. Physiol. Circ. Physiol. 2020, 319, H1069–H1077. [Google Scholar] [CrossRef] [PubMed]
- Pozhidayeva, D.Y.; Farris, S.P.; Goeke, C.M.; Firsick, E.J.; Townsley, K.G.; Guizzetti, M.; Ozburn, A.R. Chronic Chemogenetic Stimulation of the Nucleus Accumbens Produces Lasting Reductions in Binge Drinking and Ameliorates Alcohol-Related Morphological and Transcriptional Changes. Brain Sci. 2020, 10, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, K.J.; Khajehali, E.; Bradley, S.J.; Navarrete, J.S.; Huang, X.P.; Slocum, S.; Jin, J.; Liu, J.; Xiong, Y.; Olsen, R.H.J.; et al. DREADD Agonist 21 Is an Effective Agonist for Muscarinic-Based DREADDs in Vitro and in Vivo. ACS Pharmacol. Transl. Sci. 2018, 1, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Weston, M.; Kaserer, T.; Wu, A.; Mouravlev, A.; Carpenter, J.C.; Snowball, A.; Knauss, S.; von Schimmelmann, M.; During, M.J.; Lignani, G.; et al. Olanzapine: A potent agonist at the hM4D(Gi) DREADD amenable to clinical translation of chemogenetics. Sci. Adv. 2019, 5, eaaw1567. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Choo, H.; Huang, X.-P.; Yang, X.; Stone, O.; Roth, B.L.; Jin, J. The First Structure–Activity Relationship Studies for Designer Receptors Exclusively Activated by Designer Drugs. ACS Chem. Neurosci. 2015, 6, 476–484. [Google Scholar] [CrossRef]
- Nagai, Y.; Miyakawa, N.; Takuwa, H.; Hori, Y.; Oyama, K.; Ji, B.; Takahashi, M.; Huang, X.-P.; Slocum, S.T.; DiBerto, J.F.; et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat. Neurosci. 2020, 23, 1157–1167. [Google Scholar] [CrossRef]
- Bonaventura, J.; Eldridge, M.A.G.; Hu, F.; Gomez, J.L.; Sanchez-Soto, M.; Abramyan, A.M.; Lam, S.; Boehm, M.A.; Ruiz, C.; Farrell, M.R.; et al. High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat. Commun. 2019, 10, 4627. [Google Scholar] [CrossRef] [Green Version]
- Berglund, K.; Fernandez, A.M.; Gutekunst, C.N.; Hochgeschwender, U.; Gross, R.E. Step-function luminopsins for bimodal prolonged neuromodulation. J. Neurosci. Res. 2020, 98, 422–436. [Google Scholar] [CrossRef]
- Lieb, A.; Weston, M.; Kullmann, D.M. Designer receptor technology for the treatment of epilepsy. eBioMedicine 2019, 43, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnus, C.J.; Lee, P.H.; Atasoy, D.; Su, H.H.; Looger, L.L.; Sternson, S.M. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 2011, 333, 1292–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternson, S.M.; Atasoy, D.; Betley, J.N.; Henry, F.E.; Xu, S. An emerging technology framework for the neurobiology of appetite. Cell Metab. 2016, 23, 234–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnus, C.J.; Lee, P.H.; Bonaventura, J.; Zemla, R.; Gomez, J.L.; Ramirez, M.H.; Hu, X.; Galvan, A.; Basu, J.; Michaelides, M.; et al. Ultrapotent chemogenetics for research and potential clinical applications. Science 2019, 364, eaav5282. [Google Scholar] [CrossRef] [PubMed]
- Raper, J.; Eldridge, M.A.G.; Sternson, S.M.; Shim, J.Y.; Fomani, G.P.; Richmond, B.J.; Wichmann, T.; Galvan, A. Characterization of ultrapotent chemogenetic ligands for research applications in non-human primates. bioRxiv 2022, 475241. [Google Scholar] [CrossRef]
- Poyraz, F.C.; Holzner, E.; Bailey, M.R.; Meszaros, J.; Kenney, L.; Kheirbek, M.A.; Balsam, P.D.; Kellendonk, C. Decreasing Striatopallidal Pathway Function Enhances Motivation by Energizing the Initiation of Goal-Directed Action. J. Neurosci. 2016, 36, 5988–6001. [Google Scholar] [CrossRef]
- Nation, H.L.; Nicoleau, M.; Kinsman, B.J.; Browning, K.N.; Stocker, S.D. DREADD-induced activation of subfornical organ neurons stimulates thirst and salt appetite. J. Neurophysiol. 2016, 115, 3123–3129. [Google Scholar] [CrossRef] [Green Version]
- Urban, D.J.; Zhu, H.; Marcinkiewcz, C.A.; Michaelides, M.; Oshibuchi, H.; Rhea, D.; Aryal, D.K.; Farrell, M.S.; Lowery-Gionta, E.; Olsen, R.H.J.; et al. Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons. Neuropsychopharmacology 2016, 41, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Schalbetter, S.M.; Mueller, F.S.; Scarborough, J.; Richetto, J.; Weber-Stadlbauer, U.; Meyer, U.; Notter, T. Oral application of clozapine-N-oxide using the micropipette-guided drug administration (MDA) method in mouse DREADD systems. Lab. Anim. 2021, 50, 69–75. [Google Scholar] [CrossRef]
- Keenan, W.T.; Fernandez, D.C.; Shumway, L.J.; Zhao, H.; Hattar, S. Eye-Drops for Activation of DREADDs. Front. Neural Circuits 2017, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Goossens, M.; Boon, P.; Wadman, W.; van den Haute, C.; Baekelandt, V.; Verstraete, A.G.; Vonck, K.; Larsen, L.E.; Sprengers, M.; Carrette, E.; et al. Long-term chemogenetic suppression of seizures in a multifocal rat model of temporal lobe epilepsy. Epilepsia 2021, 62, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-C.; Fu, S.; Liu, F.-Y.; Cui, S.; Yi, M.; Wan, Y. Hypersensitivity of Prelimbic Cortex Neurons Contributes to Aggravated Nociceptive Responses in Rats With Experience of Chronic Inflammatory Pain. Front. Mol. Neurosci. 2018, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Chen, R.; LaRocca, A.A.; Christiansen, M.G.; Senko, A.W.; Shi, C.H.; Chiang, P.H.; Varnavides, G.; Xue, J.; Zhou, Y.; et al. Remotely controlled chemomagnetic modulation of targeted neural circuits. Nat. Nanotechnol. 2019, 14, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Cassataro, D.; Sjulson, L. The Use of DREADDs (Designer Receptors Exclusively Activated by Designer Receptors) in Transgenic Mouse Behavioral Models. In Designer Receptors Exclusively Activated by Designer Drugs; Thiel, G., Ed.; Humana Press: New York, NY, USA, 2015; Volume 108, pp. 95–108. [Google Scholar] [CrossRef]
- Ozawa, A.; Arakawa, H. Chemogenetics drives paradigm change in the investigation of behavioral circuits and neural mechanisms underlying drug action. Behav. Brain Res. 2021, 406, 113234. [Google Scholar] [CrossRef]
- Sciolino, N.R.; Plummer, N.W.; Chen, Y.-W.; Alexander, G.M.; Robertson, S.D.; Dudek, S.M.; McElligott, Z.A.; Jensen, P. Recombinase-Dependent Mouse Lines for Chemogenetic Activation of Genetically Defined Cell Types. Cell Rep. 2016, 15, 2563–2573. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Aryal, D.K.; Olsen, R.H.J.; Urban, D.J.; Swearingen, A.; Forbes, S.; Roth, B.L.; Hochgeschwender, U. Cre-dependent DREADD (Designer Receptors Exclusively Activated by Designer Drugs) mice. Genesis 2016, 54, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Botterill, J.J.; Khlaifia, A.; Walters, B.J.; Brimble, M.A.; Scharfman, H.E.; Arruda-Carvalho, M. Off-Target Expression of Cre-Dependent Adeno-Associated Viruses in Wild-Type C57BL/6J Mice. eNeuro 2021, 8, ENEURO.0363-21.2021. [Google Scholar] [CrossRef]
- Page, C.E.; Shepard, R.; Heslin, K.; Coutellier, L. Prefrontal parvalbumin cells are sensitive to stress and mediate anxiety-related behaviors in female mice. Sci. Rep. 2019, 9, 19772. [Google Scholar] [CrossRef] [Green Version]
- Torre-Muruzabal, T.; Devoght, J.; van den Haute, C.; Brône, B.; van der Perren, A.; Baekelandt, V. Chronic nigral neuromodulation aggravates behavioral deficits and synaptic changes in an α-synuclein based rat model for Parkinson’s disease. Acta Neuropathol. Commun. 2019, 7, 160. [Google Scholar] [CrossRef] [Green Version]
- Soumier, A.; Sibille, E. Opposing Effects of Acute versus Chronic Blockade of Frontal Cortex Somatostatin-Positive Inhibitory Neurons on Behavioral Emotionality in Mice. Neuropsychopharmacology 2014, 39, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Binning, W.; Hogan-Cann, A.E.; Yae Sakae, D.; Maksoud, M.; Ostapchenko, V.; Al-Onaizi, M.; Matovic, S.; Lu, W.-Y.; Prado, M.A.M.; Inoue, W.; et al. Chronic hM3Dq signaling in microglia ameliorates neuroinflammation in male mice. Brain. Behav. Immun. 2020, 88, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Zhou, J.; Feng, Q.; Zhang, J.-E.; Lin, S.; Bao, J.; Wu, P.; Luo, M. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J. Neurosci. 2013, 33, 3624–3632. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Reynolds, R.P.; Petrof, I.; White, A.; Rivera, P.D.; Segev, A.; Gibson, A.D.; Suarez, M.; DeSalle, M.J.; Ito, N.; et al. Stimulation of entorhinal cortex–dentate gyrus circuitry is antidepressive. Nat. Med. 2018, 24, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Mistretta, O.; Ward, P.; English, A. Chemogenetic Enhancement of Axon Regeneration Following Peripheral Nerve Injury in the SLICK-A Mouse. Brain Sci. 2018, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Bączyk, M.; Alami, N.O.; Delestrée, N.; Martinot, C.; Tang, L.; Commisso, B.; Bayer, D.; Doisne, N.; Frankel, W.; Manuel, M.; et al. Synaptic restoration by cAMP/PKA drives activity-dependent neuroprotection to motoneurons in ALS. J. Exp. Med. 2020, 217, e20191734. [Google Scholar] [CrossRef]
- Bockaert, J.; Pin, J.P. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999, 18, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Seifert, R.; Wenzel-Seifert, K. Constitutive activity of G-protein-coupled receptors: Cause of disease and common property of wild-type receptors. Naunyn-Schmiedeberg Arch. Pharmacol. 2002, 366, 381–416. [Google Scholar] [CrossRef]
- Rajagopal, S.; Shenoy, S.K. GPCR desensitization: Acute and prolonged phases. Cell. Signal. 2018, 41, 9–16. [Google Scholar] [CrossRef]
- Mangmool, S.; Kurose, H. Gi/o Protein-Dependent and -Independent Actions of Pertussis Toxin (PTX). Toxins 2011, 3, 884–899. [Google Scholar] [CrossRef] [Green Version]
- Bharati, K.; Ganguly, N.K. Cholera toxin: A paradigm of a multifunctional protein. Indian J. Med. Res. 2011, 133, 179–187. [Google Scholar]
- Urban, D.J.; Roth, B.L. DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): Chemogenetic Tools with Therapeutic Utility. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Premont, R.T.; Bohn, L.M.; Lefkowitz, R.J.; Caron, M.G. Desensitization of G Protein–Coupled Receptors and Neuronal Functions. Annu. Rev. Neurosci. 2004, 27, 107–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, J.B.; Premont, R.T.; Daaka, Y. Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin. Cell Dev. Biol. 2016, 50, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöneberg, T. Tolerance and Desensitization. In Encyclopedia of Molecular Pharmacology; Offermans, S., Rosenthal, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1203–1207. [Google Scholar] [CrossRef]
- Lee, G.H.; Kim, S.S. Therapeutic Strategies for Neuropathic Pain: Potential Application of Pharmacosynthetics and Optogenetics. Mediators Inflamm. 2016, 2016, 5808215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, M.S.; Roth, B.L. Pharmacosynthetics: Reimagining the pharmacogenetic approach. Brain Res. 2013, 1511, 6–20. [Google Scholar] [CrossRef] [Green Version]
- Libbrecht, S.; van den Haute, C.; Welkenhuysen, M.; Braeken, D.; Haesler, S.; Baekelandt, V. Chronic chemogenetic stimulation of the anterior olfactory nucleus reduces newborn neuron survival in the adult mouse olfactory bulb. J. Neurochem. 2021, 158, 1186–1198. [Google Scholar] [CrossRef]
- Ewbank, S.N.; Campos, C.A.; Chen, J.Y.; Bowen, A.J.; Padilla, S.L.; Dempsey, J.L.; Cui, J.Y.; Palmiter, R.D. Chronic G q signaling in AgRP neurons does not cause obesity. Proc. Natl. Acad. Sci. USA 2020, 117, 20874–20880. [Google Scholar] [CrossRef]
- Xu, J.; Gao, P.; Wu, Y.; Yin, S.-Q.; Zhu, L.; Xu, S.-H.; Tang, D.; Cheung, C.-W.; Jiao, Y.-F.; Yu, W.-F.; et al. G protein-coupled estrogen receptor in the rostral ventromedial medulla contributes to the chronification of postoperative pain. CNS Neurosci. Ther. 2021, 27, 1313–1326. [Google Scholar] [CrossRef]
- Saloman, J.L.; Scheff, N.N.; Snyder, L.M.; Ross, S.E.; Davis, B.M.; Gold, M.S. Gi-DREADD Expression in Peripheral Nerves Produces Ligand-Dependent Analgesia, as well as Ligand-Independent Functional Changes in Sensory Neurons. J. Neurosci. 2016, 36, 10769–10781. [Google Scholar] [CrossRef] [Green Version]
- Varin, C.; Bonnavion, P. Pharmacosynthetic Deconstruction of Sleep-Wake Circuits in the Brain. In Sleep-Wake Neurobiology and Pharmacology; Landolt, H.P., Dijk, D.J., Eds.; Springer: Cham, Switzerland, 2018; pp. 153–206. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.; Rozman, P.A.; Valero, M.; Doyle, W.K.; Buzsáki, G. Mechanisms and plasticity of chemogenically induced interneuronal suppression of principal cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2014157118. [Google Scholar] [CrossRef]
- Jong, Y.-J.I.; Harmon, S.K.; O’Malley, K.L. Intracellular GPCRs Play Key Roles in Synaptic Plasticity. ACS Chem. Neurosci. 2018, 9, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Atwood, B.K.; Lovinger, D.M.; Mathur, B.N. Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends Neurosci. 2014, 37, 663–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, C.; Wong, Y. Role of G Protein-Coupled Receptors in the Regulation of Structural Plasticity and Cognitive Function. Molecules 2017, 22, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, J.F.; Jackson, M.F.; Beazely, M.A. G protein-coupled receptors control NMDARs and metaplasticity in the hippocampus. Biochim. Biophys. Acta Biomembr. 2007, 1768, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salesse, C.; Charest, J.; Doucet-Beaupré, H.; Castonguay, A.-M.; Labrecque, S.; de Koninck, P.; Lévesque, M. Opposite Control of Excitatory and Inhibitory Synapse Formation by Slitrk2 and Slitrk5 on Dopamine Neurons Modulates Hyperactivity Behavior. Cell Rep. 2020, 30, 2374.e5–2386.e5. [Google Scholar] [CrossRef] [Green Version]
- Xie, A.X.; Lee, J.J.; McCarthy, K.D. Ganglionic GFAP+ glial Gq-GPCR signaling enhances heart functions in vivo. JCI Insight 2017, 2, e90565. [Google Scholar] [CrossRef]
- Ying, Y.; Wang, J.-Z. Illuminating Neural Circuits in Alzheimer’s Disease. Neurosci. Bull. 2021, 37, 1203–1217. [Google Scholar] [CrossRef]
- Hallock, H.L.; Quillian, H.M.; Maynard, K.R.; Mai, Y.; Chen, H.-Y.; Hamersky, G.R.; Shin, J.H.; Maher, B.J.; Jaffe, A.E.; Martinowich, K. Molecularly Defined Hippocampal Inputs Regulate Population Dynamics in the Prelimbic Cortex to Suppress Context Fear Memory Retrieval. Biol. Psychiatry 2020, 88, 554–565. [Google Scholar] [CrossRef]
- Sun, D.; Milibari, L.; Pan, J.-X.; Ren, X.; Yao, L.-L.; Zhao, Y.; Shen, C.; Chen, W.-B.; Tang, F.-L.; Lee, D.; et al. Critical Roles of Embryonic Born Dorsal Dentate Granule Neurons for Activity-Dependent Increases in BDNF, Adult Hippocampal Neurogenesis, and Antianxiety-like Behaviors. Biol. Psychiatry 2021, 89, 600–614. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Chen, H.; Zhou, H.; Liu, Z.; Liu, Z.; Zhou, Y.; Zhou, X.; Yu, L.; Jiang, H. Sympathetic Nervous System Mediates Cardiac Remodeling After Myocardial Infarction in a Circadian Disruption Model. Front. Cardiovasc. Med. 2021, 8, 668387. [Google Scholar] [CrossRef]
- Dumrongprechachan, V.; Salisbury, R.B.; Soto, G.; Kumar, M.; MacDonald, M.L.; Kozorovitskiy, Y. Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum. Nat. Commun. 2021, 12, 4855. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.S.; Munji, R.N.; Chan, T.C.; Quirk, C.R.; Weiner, G.A.; Weger, B.D.; Rossi, M.J.; Elmsaouri, S.; Malfavon, M.; Deng, A.; et al. Neuronal Activity Regulates Blood-Brain Barrier Efflux Transport through Endothelial Circadian Genes. Neuron 2020, 108, 937.e7–952.e7. [Google Scholar] [CrossRef] [PubMed]
- Nagai, J.; Rajbhandari, A.K.; Gangwani, M.R.; Hachisuka, A.; Coppola, G.; Masmanidis, S.C.; Fanselow, M.S.; Khakh, B.S. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 2019, 177, 1280.e20–1292.e20. [Google Scholar] [CrossRef]
- Philtjens, S.; Turnbull, M.T.; Thedy, B.P.; Moon, Y.; Kim, J. Single-cell resolution analysis of the crosstalk between chemogenically activated astrocytes and microglia. bioRxiv 2021, 064881. [Google Scholar] [CrossRef]
- Yu, S.; Munzberg, H. Testing Effects of Chronic Chemogenetic Neuronal Stimulation on Energy Balance by Indirect Calorimetry. Bio-Protocol 2018, 8, e2811. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Nagai, J.; Marti-Solano, M.; Soto, J.S.; Coppola, G.; Babu, M.M.; Khakh, B.S. Context-Specific Striatal Astrocyte Molecular Responses Are Phenotypically Exploitable. Neuron 2020, 108, 1146–1162.e10. [Google Scholar] [CrossRef] [PubMed]
- Fellin, T. Communication between neurons and astrocytes: Relevance to the modulation of synaptic and network activity. J. Neurochem. 2009, 108, 533–544. [Google Scholar] [CrossRef]
- Covelo, A.; Araque, A. Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife 2018, 7, e32237. [Google Scholar] [CrossRef]
- Hasel, P.; Dando, O.; Jiwaji, Z.; Baxter, P.; Todd, A.C.; Heron, S.; Márkus, N.M.; McQueen, J.; Hampton, D.W.; Torvell, M.; et al. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat. Commun. 2017, 8, 15132. [Google Scholar] [CrossRef]
- Dani, J.W.; Chernjavsky, A.; Smith, S.J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 1992, 8, 429–440. [Google Scholar] [CrossRef]
- Jeon, M.; Moon, G.J.; Kim, S.; Choi, M.; Oh, Y.; Kim, D.W.; Kim, H.; Lee, K.J.; Choe, Y.; Ha, C.M.; et al. Neurotrophic interactions between neurons and astrocytes following AAV1-Rheb(S16H) transduction in the hippocampus in vivo. Br. J. Pharmacol. 2020, 177, 668–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakovcevic, D.; Harder, D.R. Role of Astrocytes in Matching Blood Flow to Neuronal Activity. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 79, pp. 75–97. [Google Scholar] [CrossRef]
- Park, S.; Koppes, R.A.; Froriep, U.P.; Jia, X.; Achyuta, A.K.H.; McLaughlin, B.L.; Anikeeva, P. Optogenetic control of nerve growth. Sci. Rep. 2015, 5, 9669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umpierre, A.D.; Bystrom, L.L.; Ying, Y.; Liu, Y.U.; Worrell, G.; Wu, L.-J. Microglial calcium signaling is attuned to neuronal activity in awake mice. eLife 2020, 9, e56502. [Google Scholar] [CrossRef] [PubMed]
- Salmina, A.B.; Gorina, Y.V.; Erofeev, A.I.; Balaban, P.M.; Bezprozvanny, I.B.; Vlasova, O.L. Optogenetic and chemogenetic modulation of astroglial secretory phenotype. Rev. Neurosci. 2021, 32, 459–479. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Heuvel, F.O.; Tar, L.; Hagenston, A.M.; Palmer, A.; Linkus, B.; Ludolph, A.C.; Huber-Lang, M.; Boeckers, T.; Bading, H.; et al. Parvalbumin Interneurons Shape Neuronal Vulnerability in Blunt TBI. Cereb. Cortex 2019, 29, 2701–2715. [Google Scholar] [CrossRef]
- Geeraerts, E.; Claes, M.; Dekeyster, E.; Salinas-Navarro, M.; de Groef, L.; van den Haute, C.; Scheyltjens, I.; Baekelandt, V.; Arckens, L.; Moons, L. Optogenetic Stimulation of the Superior Colliculus Confers Retinal Neuroprotection in a Mouse Glaucoma Model. J. Neurosci. 2019, 39, 2313–2325. [Google Scholar] [CrossRef] [Green Version]
- Varadarajan, S.G.; Dhande, O.S.; Le, P.; Huberman, A.D. Probing the role of retinorecipient target cells in visual circuit regeneration. In Proceedings of the 2021 Neuroscience Meeting Planner, Chicago, IL, USA, 8–11 November 2021. [Google Scholar]
- Hennes, M.; Lombaert, N.; Wahis, J.; van den Haute, C.; Holt, M.G.; Arckens, L. Astrocytes shape the plastic response of adult cortical neurons to vision loss. Glia 2020, 68, 2102–2118. [Google Scholar] [CrossRef]
- Gonzalez, D.; Satriotomo, I.; Miki, T.; Lee, K.-Y.; Yokoyama, T.; Touge, T.; Matsumoto, Y.; Li, H.-P.; Kuriyama, S.; Takeuchi, Y. Changes of parvalbumin immunoreactive neurons and GFAP immunoreactive astrocytes in the rat lateral geniculate nucleus following monocular enucleation. Neurosci. Lett. 2006, 395, 149–154. [Google Scholar] [CrossRef]
- Cornett, A.; Sucic, J.F.; Hillsburg, D.; Cyr, L.; Johnson, C.; Polanco, A.; Figuereo, J.; Cabine, K.; Russo, N.; Sturtevant, A.; et al. Altered glial gene expression, density, and architecture in the visual cortex upon retinal degeneration. Brain Res. 2011, 1422, 46–56. [Google Scholar] [CrossRef]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef]
- Wang, F.; Qin, Z.; Lu, H.; He, S.; Luo, J.; Jin, C.; Song, X. Clinical translation of gene medicine. J. Gene Med. 2019, 21, e3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Bruggeman, K.F.; Franks, S.; Gautam, V.; Hodgetts, S.I.; Harvey, A.R.; Williams, R.J.; Nisbet, D.R. Is Viral Vector Gene Delivery More Effective Using Biomaterials? Adv. Healthc. Mater. 2021, 10, 2001238. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Ramos, M.; Gallego, I.; Villate-Beitia, I.; Zarate, J.; Maldonado, I.; Puras, G.; Pedraz, J.L. How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? Int. J. Mol. Sci. 2021, 22, 7545. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Jang, M.J.; Yoo, B.B.; Greenbaum, A.; Ravi, N.; Wu, W.L.; Sánchez-Guardado, L.; Lois, C.; Mazmanian, S.K.; Deverman, B.E.; et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017, 20, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Szablowski, J.O.; Lee-Gosselin, A.; Lue, B.; Malounda, D.; Shapiro, M.G. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng. 2018, 2, 475–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, M.A.; Bonaventura, J.; Gomez, J.L.; Solís, O.; Stein, E.A.; Bradberry, C.W.; Michaelides, M. Translational PET applications for brain circuit mapping with transgenic neuromodulation tools. Pharmacol. Biochem. Behav. 2021, 204, 173147. [Google Scholar] [CrossRef]
- Alcantara, C.C.; García-Salazar, L.F.; Silva-Couto, M.A.; Santos, G.L.; Reisman, D.S.; Russo, T.L. Post-stroke BDNF Concentration Changes Following Physical Exercise: A Systematic Review. Front. Neurol. 2018, 9, 637. [Google Scholar] [CrossRef]
- Berchtold, N.C.; Chinn, G.; Chou, M.; Kesslak, J.P.; Cotman, C.W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 2005, 133, 853–861. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary Exercise Induces a BDNF-Mediated Mechanism That Promotes Neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef] [Green Version]
- Sabatier, M.J.; Redmon, N.; Schwartz, G.; English, A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008, 211, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Willand, M.P.; Nguyen, M.-A.; Borschel, G.H.; Gordon, T. Electrical Stimulation to Promote Peripheral Nerve Regeneration. Neurorehabil. Neural Repair 2016, 30, 490–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mui, A.M.; Yang, V.; Aung, M.H.; Fu, J.; Adekunle, A.N.; Prall, B.C.; Sidhu, C.S.; Park, H.N.; Boatright, J.H.; Iuvone, P.M.; et al. Daily visual stimulation in the critical period enhances multiple aspects of vision through BDNF-mediated pathways in the mouse retina. PLoS ONE 2018, 13, e0192435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Majed, A.A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief Electrical Stimulation Promotes the Speed and Accuracy of Motor Axonal Regeneration. J. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef] [PubMed]
- Koppes, A.N.; Nordberg, A.L.; Paolillo, G.; Goodsell, N.; Darwish, H.; Zhang, L.; Thompson, D.M. Electrical Stimulation of Schwann Cells Promotes Sustained Increases in Neurite Outgrowth. Tissue Eng. Part A 2013, 20, 494–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geremia, N.M.; Gordon, T.; Brushart, T.M.; Al-Majed, A.A.; Verge, V.M.K. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp. Neurol. 2007, 205, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Yamamoto, N. Neuronal activity patterns regulate BDNF expression in cortical neurons via synaptic connections and calcium signaling. bioRxiv 2021, 433239. [Google Scholar] [CrossRef]
- Deng, W.; Wu, G.; Min, L.; Feng, Z.; Chen, H.; Tan, M.; Sui, J.; Liu, H.; Hou, J. Optogenetic Neuronal Stimulation Promotes Functional Recovery After Spinal Cord Injury. Front. Neurosci. 2021, 15, 640255. [Google Scholar] [CrossRef]
- Cheng, M.Y.; Wang, E.H.; Woodson, W.J.; Wang, S.; Sun, G.; Lee, A.G.; Arac, A.; Fenno, L.E.; Deisseroth, K.; Steinberg, G.K. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc. Natl. Acad. Sci. USA 2014, 111, 12913–12918. [Google Scholar] [CrossRef] [Green Version]
- Chavoshinezhad, S.; Zibaii, M.I.; Seyed Nazari, M.H.; Ronaghi, A.; Asgari Taei, A.; Ghorbani, A.; Pandamooz, S.; Salehi, M.S.; Valian, N.; Motamedi, F.; et al. Optogenetic stimulation of entorhinal cortex reveals the implication of insulin signaling in adult rat’s hippocampal neurogenesis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110344. [Google Scholar] [CrossRef]
- Lim, J.-H.A.; Stafford, B.K.; Nguyen, P.L.; Lien, B.V.; Wang, C.; Zukor, K.; He, Z.; Huberman, A.D. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci. 2016, 19, 1073–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yang, C.; Zhang, L.; Gao, X.; Wang, X.; Liu, W.; Wang, Y.; Jiang, S.; Wong, Y.H.; Zhang, Y.; et al. Promoting axon regeneration in the adult CNS by modulation of the melanopsin/GPCR signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 1937–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blázquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; García-Rincón, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galve-Roperh, I.; et al. The CB1 cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- West, A.E.; Pruunsild, P.; Timmusk, T. Neurotrophins: Transcription and Translation. In Neurotrophic Factors; Lewin, G., Carter, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 67–100. [Google Scholar] [CrossRef]
- Corredor, R.G.; Goldberg, J.L. Electrical activity enhances neuronal survival and regeneration. J. Neural Eng. 2009, 6, 055001. [Google Scholar] [CrossRef]
- Kolarow, R.; Kuhlmann, C.R.W.; Munsch, T.; Zehendner, C.; Brigadski, T.; Luhmann, H.J.; Lessmann, V. BDNF-induced nitric oxide signals in cultured rat hippocampal neurons: Time course, mechanism of generation, and effect on neurotrophin secretion. Front. Cell. Neurosci. 2014, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Hogan, M.K.; Hamilton, G.F.; Horner, P.J. Neural Stimulation and Molecular Mechanisms of Plasticity and Regeneration: A Review. Front. Cell. Neurosci. 2020, 14, 271. [Google Scholar] [CrossRef]
- Finkbeiner, S. Calcium regulation of the brain-derived neurotrophic factor gene. Cell. Mol. Life Sci. 2000, 57, 394–401. [Google Scholar] [CrossRef]
- Xia, S.-H.; Hu, S.-W.; Ge, D.-G.; Liu, D.; Wang, D.; Zhang, S.; Zhang, Q.; Yuan, L.; Li, Y.-Q.; Yang, J.-X.; et al. Chronic Pain Impairs Memory Formation via Disruption of Neurogenesis Mediated by Mesohippocampal Brain-Derived Neurotrophic Factor Signaling. Biol. Psychiatry 2020, 88, 597–610. [Google Scholar] [CrossRef]
- Xiu, J.; Han, R.; Liu, Z.; Li, J.; Liu, S.; Shen, Y.; Ding, Y.-Q.; Xu, Q. Hijacking Dorsal Raphe to Improve Metabolism and Depression-like Behaviors via BDNF Gene Transfer in Mice. Diabetes 2021, 70, 1780–1793. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Claes, M.; de Groef, L.; Moons, L. Target-derived neurotrophic factor deprivation puts retinal ganglion cells on death row: Cold hard evidence and caveats. Int. J. Mol. Sci. 2019, 20, 4314. [Google Scholar] [CrossRef] [Green Version]

| Authors | Targeted Area | Chemogenetic Platform | Ligand Concentration | Ligand Administration | Chronic Administration Scheme | Diverse Effects on … | |
|---|---|---|---|---|---|---|---|
| Zhan et al., 2013 [53] | Murine hypothalamus (arcuate nucleus) | hM3Dq | CNO | 1 mg/kg | i.p. injection(s) | every 5 h for 3 consecutive days | Animal feeding behavior |
| Nawreen et al., 2020 [21] | Murine prefrontal cortex | hM4Di | CNO | 1 mg/kg | i.p. injection(s) | Twice daily for 2 weeks | Stress coping strategies |
| Jiang et al., 2020 [23] | Murine hypothalamus (arcuate nucleus) | hM3Dq | CNO | 1 mg/kg (i.p.) 2 mg/kg (pump) | i.p. injection (acute) osmotic micropump (s.c., chronic) | 2 weeks | Blood pressure |
| Torre-Muruzabal et al., 2019 [50] | Rat substantia nigra | hM3Dq | CNO | 1 mg/kg | i.p. injection(s) | 3 weeks of daily injections (5 days/week) | Motor deficits |
| Soumier and Sibille, 2014 [51] | Murine prefrontal cortex | hM4Di | CNO | 0.5 mg/kg | i.p. injection(s) | Twice daily for 2 weeks | Behavioral emotionality |
| Binning et al., 2020 [52] | Murine microglia | hM3Dq | CNO | 1 mg/kg | i.p. injection(s) | 4 days of daily injections | Pro-inflammatory cytokine expression |
| Poyraz et al., 2016 [36] | Murine striatum | hM4Di | CNO | 0.25 mg/kg | drinking water | 2 weeks | Motivation |
| Goossens et al., 2016 [41] | Rat hippocampus | hM4Di | Clozapine, olanzapine | 0.4 mg/kg/day | Osmotic minipump (s.c.) | 1 week | Seizure suppression |
| Nation et al. [37] | Murine subfornical organ | hM3Dq | CNO | 3 mg/kg/day | drinking water | 3 days | Salt appetite |
| Cheng et al., 2019 [22] | Murine nucleus accumbens | rM3D | CNO | 1 mg/kg | i.p. injection(s) | 2 weeks of daily injections | Social avoidance |
| Yun et al., 2018 [54] | Murine entorhinal cortex circuitry | hM3Dq | CNO | 2 mg/kg | i.p. injection(s) | 4 weeks of daily injections | Antidepressive-like effects |
| Jaiswal et al., 2018 [55] | Murine spinal cord | hM3Dq | CNO | 1 mg/kg | i.p. injection(s) | 2 weeks of injections (5 days/week) | Axonal regeneration |
| Urban et al., 2016 [38] | Murine dorsal raphe nucleus | hM3Dq | CNO | 2 (acute) or 5 (chronic) mg/kg | i.p. injection (acute) drinking water (chronic) | 3 weeks | Antidepressive-like effects |
| Bązyk et al., 2020 [56] | Murine spinal cord | rM3D or PSAM | CNO or PSEM308 | 5 mg/kg | s.c. injection(s) | 1 week of daily injections | Synaptic impairment |
| Authors | Targeted Area | Chemogenetic Platform | Goal | Molecular Signature | |
|---|---|---|---|---|---|
| Pozhidayeva et al., 2020 [24] | Murine nucleus accumbens | hM3Dq, hM4Di | Chronic, daily CNO injections: 1 mg/kg for 4 weeks | Study binge-like drinking behavior | Transcriptome of neurons |
| Hallock et al., 2020 [81] | Murine medial prefrontal cortex | hM3Dq | Single injection of 5 mg/kg CNO | Study the link of the hippocampal-prelimbic circuitry on context-fear memory retrieval | Transcriptome of neurons |
| Sun et al., 2021 [82] | Murine dorsal dentate gyrus | hM3Dq | Single injection of 2 mg/kg CNO | Study the suppression of antianxiety-like behavior and neurogenesis | Transcriptome of neurons |
| Nagai et al., 2019 [86]. | Murine striatum | hM4Di | Single injection of 1 mg/kg CNO | Study the roles of neuron-astrocyte interactions in the striatum | Transcriptome of astrocytes |
| Philtjens et al., 2021 [87] | Murine hippocampus and cortex | hM3Dq | Chronic CNO administration via drinking water: 5 mg/kg/day for 8 weeks | Study the effect of chronic activation of astrocytes and the microglial crosstalk | Single-cell transcriptome of astrocytes and microglia |
| Wang et al., 2021 [83] | Rat superior cervical ganglion | hM4Di | Chronic, daily CNO injections: 3.3 mg/kg for 30 days | Study circadian disruption and remodeling after myocardial infarction | Transcriptome of neurons |
| Yu et al., 2020 [89] | Murine striatum | rM3Ds, hM3Dq, hM4Di | Single injection or 1 injection every other day for 5–6 weeks of 1 mg/kg CNO | Study the astrocytic response in the striatum to different experimental perturbations and their role in Huntington’s Disease | Transcriptome of astrocytes |
| Dumrongprechachan et al., 2021 [84] | Murine striatum | hM3Dq | Single injection of 3 mg/kg CNO | Study the proteomic landscape of the striatum | Proteome of neurons |
| Pulido et al., 2020 [85] | Murine cortex/hippocampus | hM3Dq, hM4Di | Single injection of 0.5 mg/kg (hM3Dq) or 1 mg/kg (hM4Di) CNO | Study how neuronal activity regulations endothelial cells in the brain | Transcriptome of endothelial cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claes, M.; De Groef, L.; Moons, L. The DREADDful Hurdles and Opportunities of the Chronic Chemogenetic Toolbox. Cells 2022, 11, 1110. https://doi.org/10.3390/cells11071110
Claes M, De Groef L, Moons L. The DREADDful Hurdles and Opportunities of the Chronic Chemogenetic Toolbox. Cells. 2022; 11(7):1110. https://doi.org/10.3390/cells11071110
Chicago/Turabian StyleClaes, Marie, Lies De Groef, and Lieve Moons. 2022. "The DREADDful Hurdles and Opportunities of the Chronic Chemogenetic Toolbox" Cells 11, no. 7: 1110. https://doi.org/10.3390/cells11071110