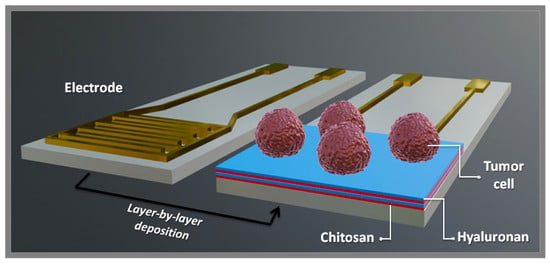
Polysaccharide Multilayer Films in Sensors for Detecting Prostate Tumor Cells Based on Hyaluronan-CD44 Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Preparation
2.3. Electrode Modification
2.4. Film Characterization
2.5. Detection of Tumor Cells
3. Results and Discussion
Physicochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Palmer, J.W. Polysaccharide of Vitreous Humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Heinegard, D.; Oldberg, A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989, 3, 2042–2051. [Google Scholar] [CrossRef] [Green Version]
- Comper, W.D.; Laurent, T.C. Physiological function of connective tissue polysaccharides. Physiol. Rev. 1978, 58, 255–315. [Google Scholar] [CrossRef]
- Brecht, M.; Mayer, U.; Schlosser, E.; Prehm, P. Increased hyaluronate synthesis is required for fibroblast detachment and mitosis. Biochem. J. 1986, 239, 445–450. [Google Scholar] [CrossRef]
- Turley, E.A. The role of a cell-associated hyaluronan-binding protein in fibroblast behaviour. Ciba Found. Symp. 1989, 143, 121–133. [Google Scholar]
- Underhill, C.B. The interaction of hyaluronate with the cell surface: The hyaluronate receptor and the core protein. Ciba Found. Symp. 1989, 143, 87–99. [Google Scholar]
- Underhill, C. CD44: The hyaluronan receptor. J. Cell Sci. 1992, 103, 293–298. [Google Scholar]
- Führmann, T.; Tam, R.Y.; Ballarin, B.; Coles, B.; Donaghue, I.E.; van der Kooy, D.; Nagy, A.; Tator, C.H.; Morshead, C.M.; Shoichet, M.S. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 2016, 83, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Swiston, A.J.; Cheng, C.; Um, S.H.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Surface functionalization of living cells with multilayer patches. Nanoletters 2008, 8, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Swiston, A.J.; Gilbert, J.B.; Irvine, D.J.; Cohen, R.E.; Rubner, M.F. Freely suspended cellular “backpacks” lead to cell aggregate self-assembly. Biomacromolecules 2010, 11, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.B.M.R.; Taketa, T.B.; Bataglioli, R.A.; Pimentel, S.B.; Santos, D.M.; Fiamingo, A.; Costa, C.A.R.; Campana-Filho, S.P.; Carvalho, H.F.; Beppu, M.M. Tailored chitosan/hyaluronan coatings for tumor cell adhesion: Effects of topography, charge density and surface composition. Appl. Surf. Sci. 2019, 486, 508–518. [Google Scholar] [CrossRef]
- Neto, J.B.M.R.; Neto, R.J.G.; Bataglioli, R.A.; Taketa, T.B.; Pimentel, S.B.; Baratti, M.O.; Costa, C.A.R.; Carvalho, H.F.; Beppu, M.M. Engineering the surface of prostate tumor cells and hyaluronan/chitosan multilayer films to modulate cell-substrate adhesion properties. Int. J. Biol. Macromol. 2020, 158, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Stott, S.L.; Richard, L.; Nagrath, S.; Min, Y.; Miyamoto, D.T.; Ulkus, L.; Inserra, E.J.; Ulman, M.; Springer, S.; Nakamura, Z.; et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci. Transl. Med. 2010, 2. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Ju, C.; Li, Y.; Shang, Z.; Wu, Y.; Jia, Y.; Niu, Y. Detection of circulating tumor cells in prostate cancer based on carboxylated graphene oxide modified light addressable potentiometric sensor. Biosens. Bioelectron. 2015, 66, 24–31. [Google Scholar] [CrossRef]
- Ivanov, I.; Stojcic, J.; Stanimirovic, A.; Sargent, E.; Nam, R.K.; Kelley, S.O. Chip-based nanostructured sensors enable accurate identification and classification of circulating tumor cells in prostate cancer patient blood samples. Anal. Chem. 2013, 85, 398–403. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Fletcher, J.E.; Stafford, C.G.; Daniels, P.B.; Bacarese-Hamilton, T. Use of an optical biosensor to measure prostate-specific antigen in whole blood. Sensors Actuators B Chem. 1995, 29, 79–83. [Google Scholar] [CrossRef]
- Jolly, P.; Tamboli, V.; Harniman, R.L.; Estrela, P.; Allender, C.J.; Bowen, J.L. Aptamer-MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen. Biosens. Bioelectron. 2016, 75, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graça, J.S.; Miyazaki, C.M.; Shimizu, F.M.; Volpati, D.; Mejía-Salazar, J.R.; Oliveira, O.N.; Ferreira, M. On the importance of controlling film architecture in detecting prostate specific antigen. Appl. Surf. Sci. 2018, 434, 1175–1182. [Google Scholar] [CrossRef]
- Bussemakers, M.J.G.; van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.M.; Schalken, J.A.; Debruyne, F.M.J.; Ru, N.; Isaacs, W.B. DD3:A New Prostate-specific Gene, Highly Overexpressed in Prostate Cancer. Cancer Res. 1999, 59, 5975–5979. [Google Scholar] [PubMed]
- Sioss, J.A.; Bhiladvala, R.B.; Pan, W.; Li, M.; Patrick, S.; Xin, P.; Dean, S.L.; Keating, C.D.; Mayer, T.S.; Clawson, G.A. Nanoresonator chip-based RNA sensor strategy for detection of circulating tumor cells: Response using PCA3 as a prostate cancer marker, Nanomedicine Nanotechnology. Biol. Med. 2012, 8, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.C.; Soares, A.C.; Rodrigues, V.C.; Melendez, M.E.; Santos, A.C.; Faria, E.F.; Reis, R.M.; Carvalho, A.L.; Oliveira, O.N. Detection of the Prostate Cancer Biomarker PCA3 with Electrochemical and Impedance-Based Biosensors. ACS Appl. Mater. Interfaces 2019, 11, 46645–46650. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Ariga, K.; Yamauchi, Y.; Rydzek, G.; Ji, Q.; Yonamine, Y.; Wu, K.C.-W.; Hill, J.P. Layer-by-layer nanoarchitectonics: Invention, innovation, and evolution. Chem. Lett. 2014, 43, 36–68. [Google Scholar] [CrossRef]
- Soares, A.C.; Soares, J.C.; Shimizu, F.M.; Rodrigues, V.d.; Awan, I.T.; Melendez, M.E.; Piazzetta, M.H.O.; Gobbi, A.L.; Reis, R.M.; Fregnani, J.H.T.G. A simple architecture with self-assembled monolayers to build immunosensors for detecting the pancreatic cancer biomarker CA19-9. Analyst 2018, 143, 3302–3308. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.B. Film coating theory and practice. J. Pharm. Sci. 1966, 55, 81–89. [Google Scholar] [CrossRef]
- Paulovich, F.V.; Moraes, M.L.; Maki, R.M.; Ferreira, M.; Oliveira Jr, O.N.; de Oliveira, M.C.F. Information visualization techniques for sensing and biosensing. Analyst 2011, 136, 1344–1350. [Google Scholar] [CrossRef]
- Paulovich, F.V.; Maki, R.M.; de Oliveira, M.C.F.; Colhone, M.C.; Santos, F.R.; Migliaccio, V.; Ciancaglini, P.; Perez, K.R.; Stabeli, R.G.; Perinoto, Â.C. Using multidimensional projection techniques for reaching a high distinguishing ability in biosensing. Anal. Bioanal. Chem. 2011, 400, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejada, E.; Minghim, R.; Nonato, L.G. On Improved Projection Techniques to Support Visual Exploration of Multi-Dimensional Data Sets. Inf. Vis. 2003, 2, 218–231. [Google Scholar] [CrossRef]
- Minghim, R.; Paulovich, F.V.; de Andrade Lopes, A. Content-based text mapping using multi-dimensional projections for exploration of document collections. Vis. Data Anal. 2006. [Google Scholar] [CrossRef]
- Almodóvar, J.; Place, L.W.; Gogolski, J.; Erickson, K.; Kipper, M.J. Layer-by-layer assembly of polysaccharide-based polyelectrolyte multilayers: A spectroscopic study of hydrophilicity, composition, and ion pairing. Biomacromolecules 2011, 12, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Hill, J.P.; Ji, Q. Layer-by-layer assembly as a versatile bottom-up nanofabrication technique for exploratory research and realistic application. Phys. Chem. Chem. Phys. 2007, 9, 2319–2340. [Google Scholar] [CrossRef] [PubMed]
- Colthup, N.B. LH-Daly and SE Wiberley. Introduction to Infrared and Raman Spectroscopy; Academic Press: Cambridge, MA, USA, 1964. [Google Scholar]
- Wieckowski, A.; Korzeniewski, C.; Braunschweig, B. Vibrational Spectroscopy at Electrified Interfaces; Wiley Online Library: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Taketa, T.B.; Beppu, M.M. Layer-by-Layer Thin Films of Alginate/Chitosan and Hyaluronic Acid/Chitosan with Tunable Thickness and Surface Roughness. Mater. Sci. Forum. 2014, 783–786, 1226–1231. [Google Scholar] [CrossRef]
- Vasconcellos, F.C.; Swiston, A.J.; Beppu, M.M.; Cohen, R.E.; Rubner, M.F. Bioactive Polyelectrolyte Multilayers: Hyaluronic Acid Mediated B Lymphocyte Adhesion. Biomacromolecules 2010, 11, 2407–2414. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Caseli, L.; Oliveira, O.N., Jr. Chitosan in nanostructured thin films. Biomacromolecules 2010, 11, 1897–1908. [Google Scholar] [CrossRef]
- Yoshida, K.; Hasebe, Y.; Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer deposited nano-and micro-assemblies for insulin delivery: A review. Mater. Sci. Eng. C 2014, 34, 384–392. [Google Scholar] [CrossRef]
- Hedayatipour, A.; Aslanzadeh, S.; McFarlane, N. CMOS based whole cell impedance sensing: Challenges and future outlook. Biosens. Bioelectron. 2019, 143, 111600. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Cheng, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Amancio, D.R.; Nunes, M.G.V.; Oliveira Jr, O.N.; Pardo, T.A.S.; Antiqueira, L.; Costa, L.D.F. Using metrics from complex networks to evaluate machine translation. Phys. A Stat. Mech. Appl. 2011, 390, 131–142. [Google Scholar] [CrossRef]
- Shimizu, F.M.; Pasqualeti, A.M.; Todão, F.R.; de Oliveira, J.F.; Vieira, L.C.; Gonçalves, S.P.; da Silva, G.H.; Cardoso, M.B.; Gobbi, A.L.; Martinez, D.S.; et al. Monitoring the surface chemistry of functionalized nanomaterials with a microfluidic electronic tongue. ACS Sens. 2018, 3, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Daikuzono, C.M.; Shimizu, F.M.; Manzoli, A.; Riul, A., Jr.; Piazzetta, M.H.; Gobbi, A.L.; Correa, D.S.; Paulovich, F.V.; Oliveira, O.N., Jr. Information visualization and feature selection methods applied to detect gliadin in gluten-containing foodstuff with a microfluidic electronic tongue. ACS Appl. Mater. Interfaces 2017, 9, 19646–19652. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha Neto, J.B.M.; Soares, A.C.; Bataglioli, R.A.; Carr, O.; Costa, C.A.R.; Oliveira, O.N., Jr.; Beppu, M.M.; Carvalho, H.F. Polysaccharide Multilayer Films in Sensors for Detecting Prostate Tumor Cells Based on Hyaluronan-CD44 Interactions. Cells 2020, 9, 1563. https://doi.org/10.3390/cells9061563
Rocha Neto JBM, Soares AC, Bataglioli RA, Carr O, Costa CAR, Oliveira ON Jr., Beppu MM, Carvalho HF. Polysaccharide Multilayer Films in Sensors for Detecting Prostate Tumor Cells Based on Hyaluronan-CD44 Interactions. Cells. 2020; 9(6):1563. https://doi.org/10.3390/cells9061563
Chicago/Turabian StyleRocha Neto, João Batista Maia, Andrey Coatrini Soares, Rogério Aparecido Bataglioli, Olívia Carr, Carlos Alberto Rodrigues Costa, Osvaldo N. Oliveira, Jr., Marisa Masumi Beppu, and Hernandes F. Carvalho. 2020. "Polysaccharide Multilayer Films in Sensors for Detecting Prostate Tumor Cells Based on Hyaluronan-CD44 Interactions" Cells 9, no. 6: 1563. https://doi.org/10.3390/cells9061563