Zein-Functionalized MCM-41 Silica Nanoparticles with Enzyme-Responsive for Controlled Release in Antibacterial Activity
Abstract
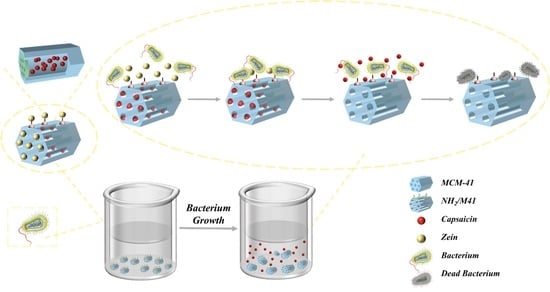
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparing Antimicrobial Supports: Preparation of NH2-MCM-41 (NH2/M41)
2.3. Preparation of Capsaicin-NH2-MCM-41 (CAP@ NH2/M41)
2.4. Preparation of Zein-Capsaicin-NH2-MCM-41 (CAP@ Zein/M41)
2.5. Characterization of CAP@ Zein/M41
2.5.1. Scanning Electron Microscope (SEM)
2.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.3. Thermogravimetric Analysis (TGA)
2.5.4. Particle Size and Zeta Potential
2.5.5. Nitrogen Adsorption/Desorption
2.6. Measurement of Capsaicin Release by High-Performance Liquid Chromatography (HPLC)
2.7. Antibacterial Performance
2.7.1. Strains and Growth Conditions
2.7.2. Bactericidal Activity of Capsaicin
2.7.3. Bactericidal Activity of CAP@ Zein/M41
2.7.4. Morphological Changes in E. coli and S. aureus
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of CAP@ Zein/M41
3.1.1. Scanning Electron Microscope (SEM)
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.1.3. Thermogravimetric Analysis (TGA)
3.1.4. Zeta Potential
3.1.5. Nitrogen Adsorption/Desorption
3.2. Sustained Release Test
3.3. Antibacterial Performance
3.4. Antibacterial Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Saricaoglu, F.T.; Avena-Bustillos, R.J.; Bridges, D.F.; Takeoka, G.R.; Wu, V.; Chiou, B.S.; Wood, D.F.; Mchugh, T.H. Preparation of fish skin gelatin-based nanofibers incorporating cinnamaldehyde by solution blow spinning 4. Int. J. Mol. Sci. 2019, 19, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriotis, E.G.; Papi, R.M.; Paraskevopoulou, A.; Achilias, D.S. Synthesis of D-Limonene loaded polymeric nanoparticles with enhanced antimicrobial properties for potential application in food packaging. Nanomaterials 2021, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Huang, T.Y.; Mao, Y.Z.; Chen, Y.N.; Shi, F.; Peng, R.X.; Chen, J.X.; Yuan, L.; Bai, C.Y.; Chen, L.; et al. Study on the viable but non-culturable (VBNC) state formation of staphylococcus aureus and its control in food system. Front. Microbiol. 2020, 11, 599739. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Huang, M.Y.; Zeng, X.M.; Peng, B.; Xu, X.L.; Zhou, G.H. Resistance profiles of salmonella isolates exposed to stresses and the expression of small non-coding RNAs. Front. Microbiol. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paden, H.; Ilic, S.; Hatsu, I.; Kane, K.; Lustberg, M.; Grenade, C.; Bhatt, A.; Pardo, D.D.; Beery, A. Assessment of food safety knowledge and behaviors of cancer patients receiving treatment. J. Acad. Nutr. Diet. 2020, 120, A46. [Google Scholar] [CrossRef]
- Onyebuchi, C.; Kavaz, D. Chitosan and N, N, N-Trimethyl chitosan nanoparticle encapsulation Of ocimum gratissimum essential oil: Optimised synthesis, in vitro release And bioactivity. Int. J. Nanomed. 2019, 14, 7707–7727. [Google Scholar] [CrossRef] [Green Version]
- Lien, K.W.; Yang, M.X.; Ling, M.P. Microbial risk assessment of escherichia coli O157:H7 in beef imported from the United States of America to Taiwan. Microorganisms 2020, 8, 676. [Google Scholar] [CrossRef]
- Jaiswal, S. Salmonella, Food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar]
- Qian, X.; Yanagi, K.; Kane, A.V.; Alden, N.; Lei, M.; Snydman, D.R.; Vickers, R.J.; Lee, K.; Thorpe, C.M. Ridinilazole, a narrow spectrum antibiotic for treatment of clostridioides difficile infection, enhances preservation of microbiota-dependent bile acids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 319, G227–G237. [Google Scholar] [CrossRef]
- Carlson, T.J.; Gonzales-Luna, A.J.; Garey, K.W. Recent developments in antimicrobial therapy for gastrointestinal infections. Curr. Opin. Gastroenterol. 2021, 37, 30–36. [Google Scholar] [CrossRef]
- Schlattmann, A.; Lützau, K.; Kaspar, U.; Becker, K. The porcine nasal microbiota with particular attention to livestock-associated methicillin-resistant staphylococcus aureus in Germany—A culturomic approach. Microorganisms 2020, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.; Abu-Dieyeh, M.H. Novel Prosopis juliflora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Sci. Rep. 2021, 11, 7871. [Google Scholar] [CrossRef] [PubMed]
- Abu Ali, O.A.; El-Naggar, M.E.; Abdel-Aziz, M.S.; Saleh, D.I.; Abu-Saied, M.A.; El-Sayed, W.A. Facile synthesis of natural anise-based nanoemulsions and their antimicrobial activity. Polymers 2021, 13, 2009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Luo, Y.; Wang, M.X.; Chen, F.; Liu, J.K.; Meng, K.; Zhao, H.J. Double-layered microcapsules significantly improve the long-term effectiveness of essential oil. Polymers 2020, 12, 1651. [Google Scholar] [CrossRef]
- Wiatrak, K.; Morawiec, T.; Roj, R.; Kownacki, P.; Nitecka-Buchta, A.; Niedzielski, D.; Wychowanski, P.; Machorowska-Pieniazek, A.; Cholewka, A.; Baldi, D.; et al. Evaluation of Effectiveness of a Toothpaste Containing tea tree oil and ethanolic extract of propolis on the improvement of oral health in patients using removable partial dentures. Molecules 2021, 26, 4071. [Google Scholar] [CrossRef]
- Valkova, V.; Duranova, H.; Galovicova, L.; Vukovic, N.L.; Vukic, M.; Kacaniova, M. In vitro antimicrobial activity of lavender, mint, and rosemary essential oils and the effect of their vapours on growth of penicillium spp. in a bread model system. Molecules 2021, 26, 3859. [Google Scholar] [CrossRef]
- Muta, T.; Parikh, A.; Kathawala, K.; Haidari, H.; Song, Y.M.; Thomas, J.; Garg, S. Quality-by-Design approach for the development of nano-sized tea tree oil formulation-impregnated biocompatible gel with antimicrobial properties. Pharmaceutics 2020, 12, 1091. [Google Scholar] [CrossRef]
- Partheniadis, I.; Zarafidou, E.; Litinas, K.E.; Nikolakakis, I. Enteric Release Essential Oil Prepared by co-spray drying methacrylate/polysaccharides-influence of starch type. Pharmaceutics 2020, 12, 571. [Google Scholar] [CrossRef]
- Yong, G.R.; Gebru, Y.A.; Kim, D.W.; Kim, D.H.; Han, H.A.; Kim, Y.H.; Kim, M.K. Chemical composition and antioxidant activity of steam-distilled essential oil and glycosidically bound volatiles from maclura tricuspidata fruit. Foods 2019, 8, 659. [Google Scholar] [CrossRef] [Green Version]
- Kuzma, M.; Fodor, K.; Almasi, A.; Mozsik, G.; Past, T.; Perjesi, P. Toxicokinetic study of a gastroprotective dose of capsaicin by HPLC-FLD method. Molecules 2019, 24, 2848. [Google Scholar] [CrossRef]
- Kursunluoglu, G.; Taskiran, D.; Kayali, H.A. The investigation of the antitumor agent toxicity and capsaicin effect on the electron transport chain enzymes, catalase activities and lipid peroxidation levels in lung, heart and brain tissues of rats. Molecules 2018, 23, 3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, S.; Saurina, J.; Puignou, L.; Nunez, O. Targeted UHPLC-HRMS (Orbitrap) polyphenolic and capsaicinoid profiling for the chemometric characterization and classification of paprika with protected designation of origin (PDO) attributes. Molecules 2020, 25, 1623. [Google Scholar] [CrossRef] [Green Version]
- Iturrioz-Rodriguez, N.; Correa-Duarte, M.A.; Valiente, R.; Fanarraga, M.L. Engineering sub-cellular targeting strategies to enhance safe cytosolic silica particle dissolution in cells. Pharmaceutics 2020, 12, 487. [Google Scholar] [CrossRef]
- Jin, L.; Huang, Q.J.; Zeng, H.Y.; Du, J.Z.; Xu, S.; Chen, C.R. Hydrotalcite-gated hollow mesoporous silica delivery system for controlled drug release. Microporous Mesoporous Mater. 2019, 274, 304–312. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jin, L.; Wang, C.; Sheng, J.; Song, Y.S. Thymol-functionalized hollow mesoporous silica spheres nanoparticles: Preparation, characterization and bactericidal activity. Bull. Mater. Sci. 2021, 44, 126. [Google Scholar] [CrossRef]
- Raja, R.K.; Nguyen-Tri, P.; Balasubramani, G.; Alagarsamy, A.; Hazir, S.; Ladhari, S.; Saidi, A.; Pugazhendhi, A.; Samy, A.A. SARS-CoV-2 and its new variants: A comprehensive review on nanotechnological application insights into potential approaches. Appl. Nanosci. 2021. [Google Scholar] [CrossRef]
- Lesniewska, B.; Arciszewska, Z.; Wawrzynczak, A.; Jarmolinska, S.; Nowak, I.; Godlewska-Zylkiewicz, B. Method development for determination of trace amounts of palladium in environmental water samples by ICP-MS/MS after pre-concentration on thiol-functionalized MCM-41 materials. Talanta 2020, 217, 121004. [Google Scholar] [CrossRef]
- Ozcelik, N.; Yurtcan, A.B. Drug loading with supercritical carbon dioxide deposition on different silica derivatives: Carvedilol study. J. Drug Deliv. Sci. Technol. 2019, 53, 101213. [Google Scholar] [CrossRef]
- Jin, L.; Teng, J.; Hu, L.H.; Lan, X.T.; Xu, Y.; Sheng, J.; Song, Y.S.; Wang, M.F. Pepper fragrant essential oil (PFEO) and functionalized MCM-41 nanoparticles: Formation, characterization, and bactericidal activity. J. Sci. Food Agric. 2019, 99, 5168–5175. [Google Scholar] [CrossRef]
- Yang, B.Y.; Dong, Y.X.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Edo, G.; Llinas, M.C.; Borros, S.; Sanchez-Garcia, D. Isothiocyanate-Functionalized mesoporous silica nanoparticles as building blocks for the design of nanovehicles with optimized drug release profile. Nanomaterials 2019, 9, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Pahal, V.; Gupta, A.; Vadhan, R.; Chandra, H.; Dubey, R.C. Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays. Sci. Rep. 2020, 10, 20409. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of horseradish (Armoracia rusticana L.) juice using spray-drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.J.; Weng, R.Y.; Jiang, W.W.; Sun, H.M.; Xia, J.T.; Liu, Y.T.; Sheng, J.; Song, Y.S. In-situ preparation of novel sedimentary rock-like Fe3O4 by rice-husk mesoporous silica as templates for effective remove As(III) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 105866. [Google Scholar] [CrossRef]
- Hou, J.J.; Xia, J.T.; Weng, R.Y.; Liu, Y.T.; Li, L.Y.; Liu, K.Y.; Sheng, J.; Song, Y.S. Mesoporous silicon extracted from rice husk for remediation of different sorts of dyestuffs from simulated textile effluent: Kinetic, isotherm, and mechanism study. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, H.J.; Shen, Z.C.; Zhu, G.W.; Hao, L.; Chen, H.Y.; Xu, H.; Zhou, X.H. Long-lasting anti-bacterial activity and bacteriostatic mechanism of tea tree oil adsorbed on the amino-functionalized mesoporous silica-coated by PAA. Colloids Surf. B Biointerfaces 2020, 188, 110784. [Google Scholar] [CrossRef]
- Sanchez-Arreguin, A.; Carriles, R.; Ochoa-Alejo, N.; Lopez, M.G.; Sanchez-Segura, L. Generation of BSA-capsaicin nanoparticles and their hormesis effect on the rhodotorula mucilaginosa yeast. Molecules 2019, 24, 2800. [Google Scholar] [CrossRef] [Green Version]
- Chamignon, C.; Gueneau, V.; Medina, S.; Deschamps, J.; Gil-Izquierdo, A.; Briandet, R.; Mousset, P.Y.; Langella, P.; Lafay, S.; Bermudez-Humaran, L.G. Evaluation of the probiotic properties and the capacity to form biofilms of variouslactobacillusstrains. Microorganisms 2020, 8, 1053. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garnaes, J.; Mijakovic, I. A sustainable approach for the green synthesis of silver nanoparticles fromsolibacillus isronensissp. and their application in biofilm inhibition. Molecules 2020, 25, 2783. [Google Scholar] [CrossRef]
- Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmalek, T.; Kapron, B.; Plech, T.; Szymanowska, D.; Karazniewicz-Lada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel delivery system containingcalendulae floslyophilized extract with chitosan as a supporting strategy for wound healing applications. Pharmaceutics 2020, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, L.; Nardello-Rataj, V. How to improve the chemical disinfection of contaminated surfaces by viruses, bacteria and fungus? Eur. J. Pharm. Sci. 2020, 155, 105559. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Gai, Q.Y.; Jiao, J.; Wang, W.; Zu, Y.G.; Fu, Y.J. Antibacterial activity of fructus forsythia essential oil and the application of EO-loaded nanoparticles to food-borne pathogens. Foods 2016, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Ribes, S.; Ruiz-Rico, M.; Perez-Esteve, E.; Fuentes, A.; Talens, P.; Martinez-Manez, R.; Barat, J.M. Eugenol and thymol immobilised on mesoporous silica-based material as an innovative antifungal system: Application in strawberry jam. Food Control 2017, 81, 181–188. [Google Scholar] [CrossRef]
- Shi, F.; Wu, J.C.; Zhao, Y.; Zhao, B.; Kong, X.T. Controllable assembly and application of janus smart nanosheets for oil displacement. Front. Chem. 2020, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suttiruengwong, S.; Pivsa-Art, S.; Chareonpanich, M. Hydrophilic and hydrophobic mesoporous silica derived from rice husk ash as a potential drug carrier. Materials 2018, 11, 1142. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.D.; He, P.L.; Yu, H.D.; Gu, L.; Ni, B.; Wang, D.; Wang, X. Single molecule-mediated assembly of polyoxometalate single-cluster rings and their three-dimensional superstructures. Sci. Adv. 2019, 5, eaax1081. [Google Scholar] [CrossRef] [Green Version]
- Tong, Z.W.; Gul, H.; Awais, M.; Saddick, S.; Khan, F.S.; Gulfraz, M.; Afzal, U.; Nazir, K.; Malik, M.Y.; Khan, S.U.; et al. Determination of in vivo biological activities of Dodonaea viscosa flowers against CCL4 toxicity in albino mice with bioactive compound detection. Sci. Rep. 2021, 11, 13336. [Google Scholar] [CrossRef]
- Akhmetova, A.; Lanno, G.M.; Kogermann, K.; Malmsten, M.; Rades, T.; Heinz, A. Highly elastic and water stable zein microfibers as a potential drug delivery system for wound healing. Pharmaceutics 2020, 12, 458. [Google Scholar] [CrossRef]
- Boateng-Marfo, Y.; Dong, Y.C.; Ng, W.K.; Lin, H.S. Artemether-loaded zein nanoparticles: An innovative intravenous dosage form for the management of severe malaria. Int. J. Mol. Sci. 2021, 22, 1141. [Google Scholar] [CrossRef]
- Esposito, D.; Dal Poggetto, G.; Demont, A.; Kraut, N.; Miro, A.; Ungaro, F.; Laurienzo, P.; Quaglia, F. Zein Beta-cyclodextrin micropowders for iron bisglycinate delivery. Pharmaceutics 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Chen, M.X.; Chu, S.Q.; Lin, Y.; Liang, H.W.; Zhang, J.; Yu, S.H. Switching Co/N/C catalysts for heterogeneous catalysis and electrocatalysis by controllable pyrolysis of cobalt porphyrin. Iscience 2019, 15, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uemura, T.; Kaseda, T.; Sasaki, Y.; Inukai, M.; Toriyama, T.; Takahara, A.; Jinnai, H.; Kitagawa, S. Mixing of immiscible polymers using nanoporous coordination templates. Nat. Commun. 2015, 6, 7473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotjanasuworapong, K.; Lerdwijitjarud, W.; Sirivat, A. Synthesis and Characterization of Fe0.8Mn0.2Fe2O4 Ferrite Nanoparticle with high saturation magnetization via the surfactant assisted co-precipitation. Nanomaterials 2021, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Juarez, L.A.; Anon, E.; Gimenez, C.; Sancenon, F.; Martinez-Manez, R.; Costero, A.M.; Gavina, P.; Parra, M.; Bernardos, A. Self-immolative linkers as caps for the design of gated silica mesoporous supports. Chem.—Eur. J. 2016, 22, 14126–14130. [Google Scholar] [CrossRef]
- Aznar, E.; Villalonga, R.; Gimenez, C.; Sancenon, F.; Marcos, M.D.; Martinez-Manez, R.; Diez, P.; Pingarron, J.M.; Amoros, P. Glucose-triggered release using enzyme-gated mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 6391–6393. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Y.; He, Y.; Zhang, B.K.; Zhao, L.; Tian, S.M.; Wang, Q.M.; Chen, S.A.; Li, Z.K.; Liang, S.; et al. Intestinal-targeted nanotubes-in-microgels composite carriers for capsaicin delivery and their effect for alleviation of Salmonella induced enteritis. Biomaterials 2022, 287, 121613. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Guari-Borras, G.; Ruiz-Rico, M.; Morella-Aucejo, A.; Aznar, E.; Barat, J.M.; Martinez-Manez, R.; Marcos, M.D.; Bernardos, A. Towards the enhancement of essential oil components’ antimicrobial activity using new zein protein-gated mesoporous silica microdevices. Int. J. Mol. Sci. 2021, 22, 3795. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.S.; Chen, L.; Wu, Y.; Liu, L.L.; Addicoat, M.; Irle, S.; Dong, Y.P.; Jiang, D.L. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci. Rep. 2016, 6, 32944. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Zhang, J.H.; Wang, J.; Li, Q.Y.; Xu, C.W.; Lu, X.H. Three-dimensional ordered mesoporous Co3O4 enhanced by Pd for oxygen evolution reaction. Sci. Rep. 2017, 7, 41542. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, A.; Popova, M.; Goshev, I.; Mihály, J. Effect of amine functionalization of spherical MCM-41 and SBA-15 on controlled drug release. J. Solid State Chem. 2011, 184, 1201–1207. [Google Scholar] [CrossRef]
- Li, B.Y.; Yang, X.J.; Xia, L.L.; Majeed, M.I.; Tan, B. Hollow microporous organic capsules. Sci. Rep. 2013, 3, 2128. [Google Scholar] [CrossRef] [Green Version]
- Pawar, R.C.; Kang, S.; Park, J.H.; Kim, J.H.; Ahn, S.; Lee, C.S. Room-temperature synthesis of nanoporous 1D microrods of graphitic carbon nitride (g-C3N4) with highly enhanced photocatalytic activity and stability. Sci. Rep. 2016, 6, 31147. [Google Scholar] [CrossRef] [Green Version]
- Polo, L.; Gomez-Cerezo, N.; Garcia-Fernandez, A.; Aznar, E.; Vivancos, J.L.; Arcos, D.; Vallet-Regi, M.; Martinez-Manez, R. Mesoporous bioactive glasses equipped with stimuli-responsive molecular gates for controlled delivery of levofloxacin against bacteria. Chem.—Eur. J. 2018, 24, 18944–18951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhakta, H.C.; Lin, J.M.; Grover, W.H. Measuring dissolution profiles of single controlled-release drug pellets. Sci. Rep. 2020, 10, 19734. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.T.; Wang, Z.R.; Li, Z.H.; Gu, L.S. A two-phase and long-lasting multi-antibacterial coating enables titanium biomaterials to prevent implants-related infections. Mater. Today Bio 2022, 15, 100330. [Google Scholar] [CrossRef] [PubMed]
- Wenting, L.; Siying, L.; Shiti, S.; Yuqing, Z.; Sameen, D.E.; Li, H.; Yaowen, L. Investigation of ultrasonic treatment on physicochemical, structural and morphological properties of sodium alginate/AgNPs/apple polyphenol films and its preservation effect on strawberry. Polymers 2020, 12, 2096. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Perez-Esteve, E.; de la Torre, C.; Jimenez-Belenguer, A.I.; Quiles, A.; Marcos, M.D.; Martinez-Manez, R.; Barat, J.M. Improving the antimicrobial power of low-effective antimicrobial molecules through nanotechnology. J. Food Sci. 2018, 83, 2140–2147. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Moreno, Y.; Barat, J.M. In vitro antimicrobial activity of immobilised essential oil components against Helicobacter pylori. World J. Microbiol. Biotechnol. 2020, 36, 3. [Google Scholar] [CrossRef]
- Pena-Gomez, N.; Ruiz-Rico, M.; Fernandez-Segovia, I.; Barat, J.M. Study of apple juice preservation by filtration through silica microparticles functionalised with essential oil components. Food Control 2019, 106, 106749. [Google Scholar] [CrossRef]
- Zhou, S.W.; Du, X.Z.; Cui, F.B.; Zhang, X.F. Multi-responsive and logic controlled release of dnagated mesoporous silica vehicles functionalized with intercalators for multiple delivery. Small 2014, 10, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, W.R.; Wang, S.Y.; Zhang, X.; Zhao, Y.B.; Cao, L.Q.; Sun, L. Construction of multifunctional porous silica nanocarriers for pH/enzyme-responsive drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 485–491. [Google Scholar] [CrossRef] [PubMed]











| Nanoparticles | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| MCM-41 | 636.68 | 0.7121 | 4.2736 |
| NH2/M41 | 368.26 | 0.3931 | 4.4693 |
| Zein/M41 | 290.32 | 0.3898 | 5.3706 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Lu, Y.; Sheng, J.; Song, Y. Zein-Functionalized MCM-41 Silica Nanoparticles with Enzyme-Responsive for Controlled Release in Antibacterial Activity. Coatings 2023, 13, 57. https://doi.org/10.3390/coatings13010057
Sun H, Lu Y, Sheng J, Song Y. Zein-Functionalized MCM-41 Silica Nanoparticles with Enzyme-Responsive for Controlled Release in Antibacterial Activity. Coatings. 2023; 13(1):57. https://doi.org/10.3390/coatings13010057
Chicago/Turabian StyleSun, Huimin, Yuyang Lu, Jie Sheng, and Yishan Song. 2023. "Zein-Functionalized MCM-41 Silica Nanoparticles with Enzyme-Responsive for Controlled Release in Antibacterial Activity" Coatings 13, no. 1: 57. https://doi.org/10.3390/coatings13010057