In Vitro Investigation of Corrosion Control of Magnesium with Degradable Polycaprolactone Coatings for Cardiovascular Grafts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation for the Mg Corrosion Experiment
2.2. Polymeric Coating for the Mg Corrosion Experiment
2.3. Characterization of the Coating
2.4. Magnesium Corrosion Experiment
2.5. PCL Fiber Degradation
2.6. Characterization of the Fiber Mats
2.7. Statistical Analysis
3. Results
3.1. Characterization of the Coating
3.2. Magnesium Corrosion Experiment
3.3. Fiber Degradation Experiment
4. Discussion
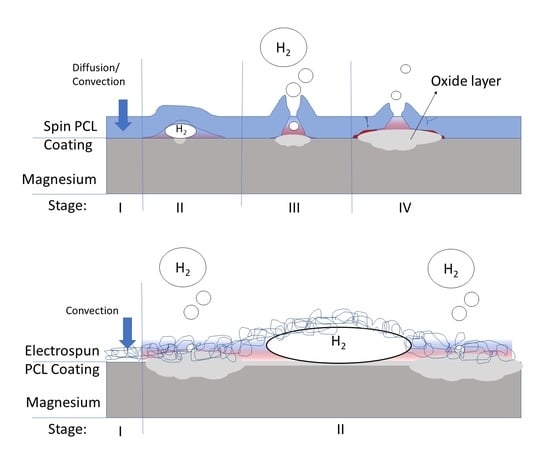
4.1. Corrosion Protection of Magnesium by the Dense Coating
4.2. Corrosion Protection of Magnesium by Fibrous Coating
4.3. The Effects of Magnesium Corrosion on the Degradation of PCL Coatings
5. Conclusions
- Spin coating can decrease the initial strong corrosion reaction;
- A dense coating has a prolonged decelerating effect on corrosion because of the diffusion barrier and autoinhibition of the corrosion process;
- Fiber coatings do not provide efficient protection against corrosive attacks in the conducted experimental conditions;
- The degradation of the polymer is accelerated in the presence of magnesium corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Zhang, X.; Niu, J.; Shi, Y.; Zhu, Z.; Dai, D.; Chen, C.; Pei, J.; Yuan, G.; Zhang, R. Biosafety and efficacy evaluation of a biodegradable magnesium-based drug-eluting stent in porcine coronary artery. Sci. Rep. 2021, 11, 7330. [Google Scholar] [CrossRef] [PubMed]
- Toušek, P.; Lazarák, T.; Varvařovský, I.; Nováčková, M.; Neuberg, M.; Kočka, V. Comparison of a Bioresorbable, Magnesium-Based Sirolimus-Eluting Stent with a Permanent, Everolimus-Eluting Metallic Stent for Treating Patients with Acute Coronary Syndrome: The PRAGUE-22 Study. Cardiovasc. Drugs Ther. 2022, 36, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Rukshin, V.; Santos, R.; Gheorghiu, M.; Shah, P.K.; Kar, S.; Padmanabhan, S.; Azarbal, B.; Tsang, V.T.; Makkar, R.; Samuels, B.; et al. A prospective, nonrandomized, open-labeled pilot study investigating the use of magnesium in patients undergoing nonacute percutaneous coronary intervention with stent implantation. J. Cardiovasc. Pharmacol. Ther. 2003, 8, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Bauer, M.; Biskup, C.; Haverich, A.; Hassel, T. Engineering of biodegradable magnesium alloy scaffolds to stabilize biological myocardial grafts. Biomed. Tech. 2017, 62, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Brandes, G.; Tudorache, I.; Cebotari, S.; Hilfiker, A.; Meyer, T.; Biskup, C.; Bauer, M.; Waldmann, K.-H.; Bach, F.-W.; et al. In vivo degradation of magnesium alloy LA63 scaffolds for temporary stabilization of biological myocardial grafts in a swine model. Biomed. Tech. 2013, 58, 407–416. [Google Scholar] [CrossRef]
- Schilling, T.; Meyer, T.; Brandes, G.; Hartung, D.; Tudorache, I.; Nolte, I.; Wacker, F.; Hilfiker, A.; Hoeffler, K.; Haverich, A.; et al. Left Ventricular Wall Reconstruction with Autologous Vascularized Gastric Graft in a Porcine Pilot Model. Eur. Surg. Res. 2022.
- Dor, V.; Saab, M.; Coste, P.; Kornaszewska, M.; Montiglio, F. Left ventricular aneurysm: A new surgical approach. Thorac. Cardiovasc. Surg. 1989, 37, 11–19. [Google Scholar] [CrossRef]
- Tudorache, I.; Kostin, S.; Meyer, T.; Teebken, O.; Bara, C.; Hilfiker, A.; Haverich, A.; Cebotari, S. Viable vascularized autologous patch for transmural myocardial reconstruction. Eur. J. Cardiothorac. Surg. 2009, 36, 306–311; discussion 311. [Google Scholar] [CrossRef]
- Willumeit, R.; Fischer, J.; Feyerabend, F.; Hort, N.; Bismayer, U.; Heidrich, S.; Mihailova, B. Chemical surface alteration of biodegradable magnesium exposed to corrosion media. Acta Biomater. 2011, 7, 2704–2715. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Han, E.H. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application. Mater. Lett. 2008, 62, 3276–3279. [Google Scholar] [CrossRef]
- Imwinkelried, T.; Beck, S.; Iizuka, T.; Schaller, B. Effect of a plasmaelectrolytic coating on the strength retention of in vivo and in vitro degraded magnesium implants. Acta Biomater. 2013, 9, 8643–8649. [Google Scholar] [CrossRef]
- Lin, X.; Tan, L.; Wang, Q.; Zhang, G.; Zhang, B.; Yang, K. In vivo degradation and tissue compatibility of ZK60 magnesium alloy with micro-arc oxidation coating in a transcortical model. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3881–3888. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Barakat, N.A.; Lim, J.K. Influence of electrospinning and dip-coating techniques on the degradation and cytocompatibility of Mg-based alloy. Colloids Surf. A Physicochem. Eng. Asp. 2013, 420, 37–45. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Interaction between a high purity magnesium surface and PCL and PLA coatings during dynamic degradation. Biomed. Mater. 2011, 6, 25005. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A biodegradable polymer-based coating to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yamamoto, A. Characteristics and cytocompatibility of biodegradable polymer film on magnesium by spin coating. Colloids Surf. B Biointerfaces 2012, 93, 67–74. [Google Scholar] [CrossRef]
- Repanas, A.; Glasmacher, B. Dipyridamole embedded in Polycaprolactone fibers prepared by coaxial electrospinning as a novel drug delivery system. J. Drug Deliv. Sci. Technol. 2015, 29, 132–142. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.-J.; Dean, D.R.; Jun, H.-W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef] [Green Version]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [CrossRef]
- Bode, M.; Mueller, M.; Zernetsch, H.; Glasmacher, B. Electrospun vascular grafts with anti-kinking properties. Curr. Dir. Biomed. Eng. 2015, 1, 459. [Google Scholar] [CrossRef]
- Suresh, S.; Gryshkov, O.; Glasmacher, B. Impact of setup orientation on blend electrospinning of poly-ε-caprolactone-gelatin scaffolds for vascular tissue engineering. Int. J. Artif. Organs 2018, 41, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Degner, J.; Singer, F.; Cordero, L.; Boccaccini, A.R.; Virtanen, S. Electrochemical investigations of magnesium in DMEM with biodegradable polycaprolactone coating as corrosion barrier. Appl. Surf. Sci. 2013, 282, 264–270. [Google Scholar] [CrossRef]
- Knigge, S.R.; Glasmacher, B. Comparison between three in vitro methods to measure magnesium degradation and their suitability for predicting in vivo degradation. Int. J. Artif. Organs 2018, 41, 772–778. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.-M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. A 2003, 65, 188–195. [Google Scholar] [CrossRef]
- Nidadavolu, E.P.S.; Feyerabend, F.; Ebel, T.; Willumeit-Römer, R.; Dahms, M. On the Determination of Magnesium Degradation Rates under Physiological Conditions. Materials 2016, 9, 627. [Google Scholar] [CrossRef] [Green Version]
- DIN EN ISO 10993-1:2017-04; Biologische Beurteilung von Medizinprodukten—Teil 1: Beurteilung und Prüfungen im Rahmen eines Risikomanagementsystems. Beuth Verlag GmbH: Berlin, Germany, 2017.
- Poh, P.S.P.; Hege, C.; Chhaya, M.P.; Balmayor, E.R.; Foehr, P.; Burgkart, R.H.; Schantz, J.-T.; Schiller, S.M.; Schilling, A.F.; Hutmacher, D.W. Evaluation of polycaprolactone—poly-D,L-lactide copolymer as biomaterial for breast tissue engineering. Polym. Int. 2017, 66, 77–84. [Google Scholar] [CrossRef]
- Fricke, D.; Becker, A.; Jütte, L.; Bode, M.; de Cassan, D.; Wollweber, M.; Glasmacher, B.; Roth, B. Mueller Matrix Measurement of Electrospun Fiber Scaffolds for Tissue Engineering. Polymers 2019, 11, 2062. [Google Scholar] [CrossRef] [Green Version]
- Wesełucha-Birczyńska, A.; Swiętek, M.; Sołtysiak, E.; Galiński, P.; Płachta, Ł.; Piekara, K.; Błażewicz, M. Raman spectroscopy and the material study of nanocomposite membranes from poly(ε-caprolactone) with biocompatibility testing in osteoblast-like cells. Analyst 2015, 140, 2311–2320. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Cassan, D.; Becker, A.; Glasmacher, B.; Roger, Y.; Hoffmann, A.; Gengenbach, T.R.; Easton, C.D.; Hänsch, R.; Menzel, H. Blending chitosan-g-poly(caprolactone) with poly(caprolactone) by electrospinning to produce functional fiber mats for tissue engineering applications. J. Appl. Polym. Sci. 2020, 137, 48650. [Google Scholar] [CrossRef]
- Burugapalli, K.; Pandit, A. Characterization of tissue response and in vivo degradation of cholecyst-derived extracellular matrix. Biomacromolecules 2007, 8, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.A.; Sellke, F.W.; Bianchi, C.; Khan, T.A.; Faro, R.; Zhang, J.-P.; Cohn, W.E. Endogenous myocardial angiogenesis and revascularization using a gastric submucosal patch. Ann. Thorac. Surg. 2003, 75, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Kochupura, P.V.; Cohen, I.S.; Doronin, S.V.; Saltman, A.E.; Gilbert, T.W.; Kelly, D.J.; Ignotz, R.A.; Gaudette, G.R. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006, 15 (Suppl. 1), S29–S40. [Google Scholar] [CrossRef]
- Chang, Y.; Chen, S.-C.; Wei, H.-J.; Wu, T.-J.; Liang, H.-C.; Lai, P.-H.; Yang, H.-H.; Sung, H.-W. Tissue regeneration observed in a porous acellular bovine pericardium used to repair a myocardial defect in the right ventricle of a rat model. J. Thorac. Cardiovasc. Surg. 2005, 130, 705–711. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—a critical review. J. Alloy. Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Ostrowski, N.; Lee, B.; Enick, N.; Carlson, B.; Kunjukunju, S.; Roy, A.; Kumta, P.N. Corrosion protection and improved cytocompatibility of biodegradable polymeric layer-by-layer coatings on AZ31 magnesium alloys. Acta Biomater. 2013, 9, 8704–8713. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—a review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Intranuovo, F.; Gristina, R.; Brun, F.; Mohammadi, S.; Ceccone, G.; Sardella, E.; Rossi, F.; Tromba, G.; Favia, P. Plasma Modification of PCL Porous Scaffolds Fabricated by Solvent-Casting/Particulate-Leaching for Tissue Engineering. Plasma Process. Polym. 2014, 11, 184–195. [Google Scholar] [CrossRef]
- Hatami, M.; Yeganeh, M.; Keyvani, A.; Saremi, M.; Naderi, R. Electrochemical behavior of polypyrrole-coated AZ31 alloy modified by fluoride anions. J. Solid State Electrochem. 2017, 21, 777–785. [Google Scholar] [CrossRef]
- Taylor, M.S.; Daniels, A.U.; Andriano, K.P.; Heller, J. Six bioabsorbable polymers: In vitro acute toxicity of accumulated degradation products. J. Appl. Biomater. 1994, 5, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kotula, A.P.; Snyder, C.R.; Migler, K.B. Determining conformational order and crystallinity in polycaprolactone via Raman spectroscopy. Polymer 2017, 117, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, K.; McVenes, R.; Anderson, J.M. Polyurethane elastomer biostability. J. Biomater. Appl. 1995, 9, 321–354. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.; Coury, A.; Urbanski, P. Autooxidative Degradation of Implanted Polyether Polyurethane Devices. J. Biomater. Appl. 1986, 1, 411–448. [Google Scholar] [CrossRef] [PubMed]
- Jawad, H.; Ali, N.N.; Lyon, A.; Chen, Q.Z.; Harding, S.E.; Boccaccini, A.R. Myocardial tissue engineering: A review. J. Tissue Eng. Regen. Med 2007, 1, 327–342. [Google Scholar] [CrossRef]
- Stevens, J.S.; de Luca, A.C.; Downes, S.; Terenghi, G.; Schroeder, S.L.M. Immobilisation of cell-binding peptides on poly-ε-caprolactone (PCL) films: A comparative XPS study of two chemical surface functionalisation methods. Surf. Interface Anal. 2014, 10–11, 673–678. [Google Scholar] [CrossRef]
- Boffito, M.; Di Meglio, F.; Mozetic, P.; Giannitelli, S.M.; Carmagnola, I.; Castaldo, C.; Nurzynska, D.; Sacco, A.M.; Miraglia, R.; Montagnani, S.; et al. Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS ONE 2018, 13, e0199896. [Google Scholar] [CrossRef]











| Ion | Concentration in mM | |||
|---|---|---|---|---|
| rSBF | mSBF | 0.1 M NaOH | H2O Dest. | |
| Na+ | 142.0 | 142.0 | 100 | - |
| K+ | 5.0 | 5.0 | - | - |
| Mg2+ | 1.5 | 1.5 | - | - |
| Ca2+ | 2.5 | 2.5 | - | - |
| Cl− | 103.0 | 103.0 | - | - |
| HCO2− | 27.0 | 10.0 | - | - |
| HPO42− | 1.0 | 1.0 | - | - |
| SO42− | 0.5 | 0.5 | - | - |
| pH | 7.4 | 7.4 | 13 | 7 |
| Experiment | Magnesium Corrosion | Electrospun Fiber Degradation | ||||
|---|---|---|---|---|---|---|
| Sample | MgUc | MgSc | MgFc | efPCL | ||
| Test fluid | rSBF | mSBF | in 0.1M NaOH (pH 13) | H2O dist. | ||
| Determination of mass loss | H2-measuring | Mass loss | ||||
| Coating characterization | - | Adhesion test | - | |||
| Coating/fiber morphology | - | SEM | SEM | |||
| Surface characterization | Optical | - | ||||
| Molecular structure of fiber/ coating | - | Raman spectroscopy | Raman spectroscopy and DSC | |||
| Parameter | Tg in °C | Tm in °C | ΔHm in J/g | xc in % |
|---|---|---|---|---|
| Dist. water | −61.2 | 60.8 | 80.2 | 58 |
| mSBF | −60.8 | 60.2 | 79.9 | 57.3 |
| NaOH solution | −61 | 61 | 84.7 | 60.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knigge, S.; Mueller, M.; Fricke, L.; Schilling, T.; Glasmacher, B. In Vitro Investigation of Corrosion Control of Magnesium with Degradable Polycaprolactone Coatings for Cardiovascular Grafts. Coatings 2023, 13, 94. https://doi.org/10.3390/coatings13010094
Knigge S, Mueller M, Fricke L, Schilling T, Glasmacher B. In Vitro Investigation of Corrosion Control of Magnesium with Degradable Polycaprolactone Coatings for Cardiovascular Grafts. Coatings. 2023; 13(1):94. https://doi.org/10.3390/coatings13010094
Chicago/Turabian StyleKnigge, Sara, Marc Mueller, Lara Fricke, Tobias Schilling, and Birgit Glasmacher. 2023. "In Vitro Investigation of Corrosion Control of Magnesium with Degradable Polycaprolactone Coatings for Cardiovascular Grafts" Coatings 13, no. 1: 94. https://doi.org/10.3390/coatings13010094