Application of Salivary Biomarkers in the Diagnosis of Fibromyalgia
Abstract
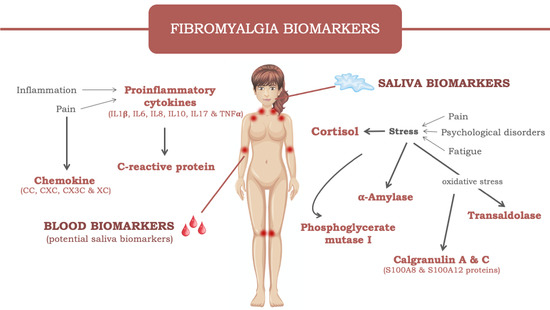
:1. Introduction
2. Fibromyalgia
3. Salivary biomarkers in FM
3.1. Cortisol
3.2. Enzymes
3.2.1. α-Amylase
3.2.2. Transaldolase
3.2.3. Phosphoglycerate mutase 1
3.3. Calgranulin
3.4. Other Biomolecules
4. Potential Salivary Biomarkers in FM
4.1. C-reactive Protein
4.2. Cytokines
4.2.1. IL-1 β
4.2.2. IL-6
4.2.3. IL-8
4.2.4. IL-10
4.2.5. IL-17
4.2.6. TNF-α
4.2.7. Chemokines
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, S.B.; Maixner, D.W.; Fillingim, R.B.; Slade, G.; Gracely, R.H.; Ambrose, K.; Zaykin, D.V.; Hyde, C.; John, S.; Tan, K.; et al. Large Candidate Gene Association Study Reveals Genetic Risk Factors and Therapeutic Targets for Fibromyalgia. Arthritis Rheum. 2012, 64, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchers, A.T.; Gershwin, M.E. Fibromyalgia: A Critical and Comprehensive Review. Clin. Rev. Allergy. Immunol. 2015, 49, 100–151. [Google Scholar] [CrossRef] [PubMed]
- McLean, S.A.; Williams, D.A.; Harris, R.E.; Kop, W.J.; Groner, K.H.; Ambrose, K.; Lyden, A.K.; Gracely, R.H.; Crofford, L.J.; Geisser, M.E.; et al. Momentary Relationship between Cortisol Secretion and Symptoms in Patients with Fibromyalgia. Arthritis Rheum. 2005, 52, 3660–3669. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, V.; Era, B.; Cacace, E.; Molin, L.; Corda, M.; Fais, A.; Utzeri, S. A Preliminary Study on Serum Proteomics in Fibromyalgia Syndrome. Clin. Chem. Lab. Med. (CCLM) 2014, 52, e207–e210. [Google Scholar] [CrossRef] [Green Version]
- Mendieta, D.; De la Cruz-Aguilera, D.L.; Barrera-Villalpando, M.I.; Becerril-Villanueva, E.; Arreola, R.; Hernández-Ferreira, E.; Pérez-Tapia, S.M.; Pérez-Sánchez, G.; Garcés-Alvarez, M.E.; Aguirre-Cruz, L.; et al. IL-8 and IL-6 Primarily Mediate the Inflammatory Response in Fibromyalgia Patients. J. Neuroimmunol. 2016, 290, 22–25. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Wong, D.T. Saliva: An Emerging Biofluid for Early Detection of Diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar]
- Zhang, C.-Z.; Cheng, X.-Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the Diagnosis of Diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; Manzano-Moreno, F.J.; Ruiz, C.; Illescas-Montes, R. Salivary Biomarkers and Their Application in the Diagnosis and Monitoring of the Most Common Oral Pathologies. Int. J. Mol. Sci. 2020, 21, 5173. [Google Scholar] [CrossRef]
- Laroche, F. Fibromyalgia. Rev. Prat. 2019, 69, 649–651. [Google Scholar]
- Marques, A.P.; Santo, A.; Santo, A.d.S.d.E.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of Fibromyalgia: Literature Review Update. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Sluka, K.A.; Clauw, D.J. Neurobiology of Fibromyalgia and Chronic Widespread Pain. Neuroscience 2016, 338, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Benlidayi, I.C. Role of Inflammation in the Pathogenesis and Treatment of Fibromyalgia. Rheumatol. Int. 2019, 39, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.E.; Chalder, T.; Sharpe, M.; White, P.D. Heterogeneity in Chronic Fatigue Syndrome—Empirically Defined Subgroups from the PACE Trial. Psychol. Med. 2017, 47, 1454–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, A.; Schweiger, V.; Del Balzo, G.; Sartori, G.; Parolini, M.; Chinellato, E.; Sarzi-Puttini, P.; Polati, E. Epidemiological, Pharmacological and Sociodemographic Characterisation of a Population of 386 Fibromyalgic Patients Referred to a Tertiary Pain Centre in Italy. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S116), 27–38. [Google Scholar]
- Fietta, P.; Fietta, P.; Manganelli, P. Fibromyalgia and Psychiatric Disorders. Acta Biomed. 2007, 78, 88–95. [Google Scholar]
- Loganathan, M.; Ladani, A.; Lippmann, S. Fibromyalgia, Sjogren’s & Depression: Linked? Postgrad. Med. 2020, 132, 575–580. [Google Scholar] [CrossRef]
- Wilson, A.; Hickie, I.; Hadzi-Pavlovic, D.; Wakefield, D.; Parker, G.; Straus, S.E.; Dale, J.; McCluskey, D.; Hinds, G.; Brickman, A.; et al. What Is Chronic Fatigue Syndrome? Heterogeneity within an International Multicentre Study. Aust. N. Z. J. Psychiatry 2001, 35, 520–527. [Google Scholar] [CrossRef]
- Collin, S.M.; Nikolaus, S.; Heron, J.; Knoop, H.; White, P.D.; Crawley, E. Chronic Fatigue Syndrome (CFS) Symptom-Based Phenotypes in Two Clinical Cohorts of Adult Patients in the UK and The Netherlands. J. Psychosom. Res. 2016, 81, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Cash, E.; Salmon, P.; Weissbecker, I.; Rebholz, W.N.; Bayley-Veloso, R.; Zimmaro, L.A.; Floyd, A.; Dedert, E.; Sephton, S.E. Mindfulness Meditation Alleviates Fibromyalgia Symptoms in Women: Results of a Randomized Clinical Trial. Ann. Behav. Med. 2015, 49, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Littlejohn, G.; Guymer, E. Neurogenic Inflammation in Fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Tejero, J.A.; Martínez-Lara, E.; Rus, A.; Camacho, M.V.; Del Moral, M.L.; Siles, E. Insight into the Biological Pathways Underlying Fibromyalgia by a Proteomic Approach. J. Proteom. 2018, 186, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Oh, H.J.; Lee, Y.J.; Song, Y.W. Prevalence and Clinical Impact of Fibromyalgia in Patients with Primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2016, 34, S9–S13. [Google Scholar] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR Revised Recommendations for the Management of Fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Talotta, R.; Bazzichi, L.; Franco, M.D.; Casale, R.; Batticciotto, A.; Gerardi, M.C.; Sarzi-Puttini, P. One Year in Review 2017: Fibromyalgia. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S105), 6–12. [Google Scholar]
- Wolfe, F. Editorial: The Status of Fibromyalgia Criteria. Arthritis Rheumatol. 2015, 67, 330–333. [Google Scholar] [CrossRef]
- Mas, A.J.; Carmona, L.; Valverde, M.; Ribas, B. EPISER Study Group Prevalence and Impact of Fibromyalgia on Function and Quality of Life in Individuals from the General Population: Results from a Nationwide Study in Spain. Clin. Exp. Rheumatol. 2008, 26, 519–526. [Google Scholar]
- Cabo-Meseguer, A.; Cerdá-Olmedo, G.; Trillo-Mata, J.L. Fibromyalgia: Prevalence, Epidemiologic Profiles and Economic Costs. Med. Clin. (Barc) 2017, 149, 441–448. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.C.; Moochhala, S. Mini-Review Article—Current Opinion on Salivary Biomarkers as a Measurement for Stress and Fatigue. Open Biomark. J. 2013, 6, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Catley, D.; Kaell, A.T.; Kirschbaum, C.; Stone, A.A. A Naturalistic Evaluation of Cortisol Secretion in Persons with Fibromyalgia and Rheumatoid Arthritis. Arthritis Care Res. 2000, 13, 51–61. [Google Scholar] [CrossRef]
- Fischer, S.; Doerr, J.M.; Strahler, J.; Mewes, R.; Thieme, K.; Nater, U.M. Stress Exacerbates Pain in the Everyday Lives of Women with Fibromyalgia Syndrome--The Role of Cortisol and Alpha-Amylase. Psychoneuroendocrinology 2016, 63, 68–77. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.R.; Gonçalves, L.C.V.; Borghi, F.; da Silva, L.G.R.V.; Gomes, A.E.; Trevisan, G.; de Souza, A.L.; Grassi-Kassisse, D.M.; de Oliveira Crege, D.R.X. Massage Therapy in Cortisol Circadian Rhythm, Pain Intensity, Perceived Stress Index and Quality of Life of Fibromyalgia Syndrome Patients. Complementary Ther. Clin. Pract. 2018, 30, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.J.; Miranda, M.B.; Loures, L.F.; Mainieri, A.G.; Mármora, C.H.C. A Systematic Review of Psychoneuroimmunology-Based Interventions. Psychol. Health Med. 2018, 23, 635–652. [Google Scholar] [CrossRef]
- Wingenfeld, K.; Nutzinger, D.; Kauth, J.; Hellhammer, D.H.; Lautenbacher, S. Salivary Cortisol Release and Hypothalamic Pituitary Adrenal Axis Feedback Sensitivity in Fibromyalgia Is Associated with Depression but Not with Pain. J. Pain 2010, 11, 1195–1202. [Google Scholar] [CrossRef]
- Coppens, E.; Kempke, S.; Van Wambeke, P.; Claes, S.; Morlion, B.; Luyten, P.; Van Oudenhove, L. Cortisol and Subjective Stress Responses to Acute Psychosocial Stress in Fibromyalgia Patients and Control Participants. Psychosom. Med. 2018, 80, 317–326. [Google Scholar] [CrossRef]
- Tak, L.M.; Cleare, A.J.; Ormel, J.; Manoharan, A.; Kok, I.C.; Wessely, S.; Rosmalen, J.G.M. Meta-Analysis and Meta-Regression of Hypothalamic-Pituitary-Adrenal Axis Activity in Functional Somatic Disorders. Biol. Psychol. 2011, 87, 183–194. [Google Scholar] [CrossRef]
- Riva, R.; Mork, P.J.; Westgaard, R.H.; Rø, M.; Lundberg, U. Fibromyalgia Syndrome Is Associated with Hypocortisolism. Int.J. Behav. Med. 2010, 17, 223–233. [Google Scholar] [CrossRef]
- Wingenfeld, K.; Heim, C.; Schmidt, I.; Wagner, D.; Meinlschmidt, G.; Hellhammer, D.H. HPA Axis Reactivity and Lymphocyte Glucocorticoid Sensitivity in Fibromyalgia Syndrome and Chronic Pelvic Pain. Psychosom. Med. 2008, 70, 65–72. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Peñacoba-Puente, C.; Cigarán-Méndez, M.; Díaz-Rodríguez, L.; Rubio-Ruiz, B.; Arroyo-Morales, M. Has Catechol-O-Methyltransferase Genotype (Val158Met) an Influence on Endocrine, Sympathetic Nervous and Humoral Immune Systems in Women with Fibromyalgia Syndrome? Clin. J. Pain 2014, 30, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pintor, F.M.; Escolano, A.F.; Rodríguez, J.R.; Ardid, J.M.C.; Gourlot, A.R. Niveles de α-Amilasa seriada salival en mujeres con fibromialgia. Cuad. Med. Psicosomática Psiquiatr. Enlace 2014, 110, 18–24. [Google Scholar]
- Sánchez, P.T.; Sánchez, J.M.; Lamo, M.d.; Peiró, G. Psicobioquimica (Estrés, Ansiedad y Depresión) En Fibromialgia. Cuad. Monográficos Psicobioquimica 2014, 3, 55–68. [Google Scholar]
- Skoluda, N.; Linnemann, A.; Nater, U.M. The Role of Week(End)-Day and Awakening Time on Cortisol and Alpha-Amylase Awakening Responses. Stress 2016, 19, 333–338. [Google Scholar] [CrossRef]
- Ahmadi-Motamayel, F.; Shahriari, S.; Goodarzi, M.T.; Moghimbeigi, A.; Jazaeri, M.; Babaei, P. The Relationship between the Level of Salivary Alpha Amylase Activity and Pain Severity in Patients with Symptomatic Irreversible Pulpitis. Restor. Dent. Endod. 2013, 38, 141–145. [Google Scholar] [CrossRef]
- Vahedi, M.; Mazdeh, M.; Hajilooi, M.; Farhadian, M.; Barakian, Y.; Sadr, P. The Relationship Between Salivary Alpha Amylase Activity and Score of McGill Pain Questionnaire in Patients with Tension Type Headache. Basic Clin. Neurosci. 2018, 9, 59–64. [Google Scholar] [CrossRef]
- Bazzichi, L.; Ciregia, F.; Giusti, L.; Baldini, C.; Giannaccini, G.; Giacomelli, C.; Sernissi, F.; Bombardieri, S.; Lucacchini, A. Detection of Potential Markers of Primary Fibromyalgia Syndrome in Human Saliva. Proteom. Clin. Appl. 2009, 3, 1296–1304. [Google Scholar] [CrossRef]
- Ciregia, F.; Giacomelli, C.; Giusti, L.; Boldrini, C.; Piga, I.; Pepe, P.; Consensi, A.; Gori, S.; Lucacchini, A.; Mazzoni, M.R.; et al. Putative Salivary Biomarkers Useful to Differentiate Patients with Fibromyalgia. J. Proteom. 2019, 190, 44–54. [Google Scholar] [CrossRef]
- Bazzichi, L.; Da Valle, Y.; Rossi, A.; Giacomelli, C.; Sernissi, F.; Giannaccini, G.; Betti, L.; Ciregia, F.; Giusti, L.; Scarpellini, P.; et al. A Multidisciplinary Approach to Study the Effects of Balneotherapy and Mud-Bath Therapy Treatments on Fibromyalgia. Clin. Exp. Rheumatol. 2013, 31, S111–S120. [Google Scholar]
- Giacomelli, C.; Bazzichi, L.; Ciregia, C.; Baldini, Y.; Da Valle, F.; Sernissi, F.; Rossi, A.; Bombardieri, S.; Lucacchini, A. MALDI-TOF and SELDI-TOF Analysis: “Tandem” Techniques to Identify Potential Biomarker in Fibromyalgia. Reumatismo 2011, 63, 166–170. [Google Scholar] [CrossRef]
- Angelousi, A.; Margioris, A.N.; Tsatsanis, C. ACTH action on the adrenals. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dungan, K., Grossman, A., Hershman, J.M., Hofland, H.J., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Thau, L.; Gandhi, J.; Sharma, S. Physiology, cortisol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lisurek, M.; Bernhardt, R. Modulation of Aldosterone and Cortisol Synthesis on the Molecular Level. Mol. Cell. Endocrinol. 2004, 215, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids-Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary Cortisol as a Biomarker in Stress Research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.M. Cortisol and Immunity. Med. Hypotheses 1991, 34, 198–208. [Google Scholar] [CrossRef]
- Khani, S.; Tayek, J.A. Cortisol Increases Gluconeogenesis in Humans: Its Role in the Metabolic Syndrome. Clin. Sci. (Lond) 2001, 101, 739–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, M.; Donelli, D. Effects of Balneotherapy and Spa Therapy on Levels of Cortisol as a Stress Biomarker: A Systematic Review. Int. J. Biometeorol. 2018, 62, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.D.; Bloom, M.S.; Frye, C.A.; Walf, A.A.; Parsons, P.J.; Steuerwald, A.J.; Ilonze, C.; Fujimoto, V.Y. Urine Cortisol Concentration as a Biomarker of Stress Is Unrelated to IVF Outcomes in Women and Men. J. Assist. Reprod. Genet. 2014, 31, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.D.; Hickman, R.; Laudenslager, M.L. Hair Cortisol Analysis: A Promising Biomarker of HPA Activation in Older Adults. Gerontologist 2015, 55 (Suppl. S1), S140–S145. [Google Scholar] [CrossRef] [Green Version]
- Weibel, L. [Methodological guidelines for the use of salivary cortisol as biological marker of stress]. Presse Med. 2003, 32, 845–851. [Google Scholar]
- Riva, R.; Mork, P.J.; Westgaard, R.H.; Lundberg, U. Comparison of the Cortisol Awakening Response in Women with Shoulder and Neck Pain and Women with Fibromyalgia. Psychoneuroendocrinology 2012, 37, 299–306. [Google Scholar] [CrossRef]
- Romano, G.F.; Tomassi, S.; Russell, A.; Mondelli, V.; Pariante, C.M. Fibromyalgia and Chronic Fatigue: The Underlying Biology and Related Theoretical Issues. Adv. Psychosom. Med. 2015, 34, 61–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nater, U.M.; Rohleder, N. Salivary Alpha-Amylase as a Non-Invasive Biomarker for the Sympathetic Nervous System: Current State of Research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.; Kirschbaum, C.; Fydrich, T.; Ströhle, A. Is Salivary Alpha-Amylase an Indicator of Autonomic Nervous System Dysregulations in Mental Disorders?—A Review of Preliminary Findings and the Interactions with Cortisol. Psychoneuroendocrinology 2013, 38, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Capranica, L.; Condello, G.; Tornello, F.; Iona, T.; Chiodo, S.; Valenzano, A.; De Rosas, M.; Messina, G.; Tessitore, A.; Cibelli, G. Salivary Alpha-Amylase, Salivary Cortisol, and Anxiety during a Youth Taekwondo Championship: An Observational Study. Medicine (Baltimore) 2017, 96, e7272. [Google Scholar] [CrossRef]
- Inagaki, T.K.; Eisenberger, N.I. Giving Support to Others Reduces Sympathetic Nervous System-Related Responses to Stress. Psychophysiology 2016, 53, 427–435. [Google Scholar] [CrossRef] [Green Version]
- van Stegeren, A.; Rohleder, N.; Everaerd, W.; Wolf, O.T. Salivary Alpha Amylase as Marker for Adrenergic Activity during Stress: Effect of Betablockade. Psychoneuroendocrinology 2006, 31, 137–141. [Google Scholar] [CrossRef]
- Doerr, J.M.; Fischer, S.; Nater, U.M.; Strahler, J. Influence of Stress Systems and Physical Activity on Different Dimensions of Fatigue in Female Fibromyalgia Patients. J. Psychosom. Res. 2017, 93, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Linnemann, A.; Kappert, M.B.; Fischer, S.; Doerr, J.M.; Strahler, J.; Nater, U.M. The Effects of Music Listening on Pain and Stress in the Daily Life of Patients with Fibromyalgia Syndrome. Front. Hum. Neurosci. 2015, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Samland, A.K.; Sprenger, G.A. Transaldolase: From Biochemistry to Human Disease. Int. J. Biochem. Cell Biol. 2009, 41, 1482–1494. [Google Scholar] [CrossRef]
- Gutiérrez, P.B.; Dafa, L.M. Glucose-6-Phosphate Dehydrogenase Deficiency: A Case Report and Review. Pediatría Atención Primaria 2015, 17, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Quintanar-Escorza, M.; Calderon, V. La Capacidad Antioxidante Total. Bases y Aplicaciones. Rev. Educ. Bioquímica 2009, 28, 89–101. [Google Scholar]
- Gvozdjáková, A.; Kucharská, J.; Sykora, L.; Singh, R. Balneotherapy and Coenzyme Q10 in Clinical and Experimental Medicine. Front. Biosci. 2014. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M. Lehninger´s Principles of Biochemistry: International Edition, 4th ed.; WH Freeman and Company: New York, NY, USA, 2005; pp. 521–525. [Google Scholar]
- Morán, G.A.G.; Mejía, Ó.R.; Vela, S.H.; Cardona, A.G.; Grimaldi, D.C.; Baez, S.A.; Muñoz, J.G. Biomedicina (Biología, Patobiología, Bioclínica y Fármaco-Terapéutica) de la Familia de las Proteínas S100 en la Especie Humana. Rev. Univ. Ind. Santander. Salud 2006, 38, 128–152. [Google Scholar]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Moroz, O.V.; Dodson, G.G.; Wilson, K.S.; Lukanidin, E.; Bronstein, I.B. Multiple Structural States of S100A12: A Key to Its Functional Diversity. Microsc. Res. Tech. 2003, 60, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Donato, R. Functional Roles of S100 Proteins, Calcium-Binding Proteins of the EF-Hand Type. Biochim. Biophys. Acta 1999, 1450, 191–231. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tao, T.; Raftery, M.J.; Youssef, P.; Girolamo, N.D.; Geczy, C.L. Proinflammatory Properties of the Human S100 Protein S100A12. J. Leukoc. Biol. 2001, 69, 986–994. [Google Scholar]
- Cole, A.M.; Kim, Y.H.; Tahk, S.; Hong, T.; Weis, P.; Waring, A.J.; Ganz, T. Calcitermin, a Novel Antimicrobial Peptide Isolated from Human Airway Secretions. FEBS Lett. 2001, 504, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Satoh, K.; Matoba, T.; Suzuki, J.; O’Dell, M.R.; Nigro, P.; Cui, Z.; Mohan, A.; Pan, S.; Li, L.; Jin, Z.-G.; et al. Cyclophilin A Mediates Vascular Remodeling by Promoting Inflammation and Vascular Smooth Muscle Cell Proliferation. Circulation 2008, 117, 3088–3098. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, J.; Jin, Z.-G.; Meoli, D.F.; Matoba, T.; Berk, B.C. Cyclophilin A Is Secreted by a Vesicular Pathway in Vascular Smooth Muscle Cells. Circ. Res. 2006, 98, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in Cell Biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.Z. The Proteasome: Subtypes and Involvement in Central Tolerance. Rev. Cuba. Investig. Biomed. 2019, 38, 227–249. [Google Scholar]
- Nielsen, M.J.; Petersen, S.V.; Jacobsen, C.; Oxvig, C.; Rees, D.; Møller, H.J.; Moestrup, S.K. Haptoglobin-Related Protein Is a High-Affinity Hemoglobin-Binding Plasma Protein. Blood 2006, 108, 2846–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Xiao, Y.; Haynes, W.L.; Michalek, J.E.; Russell, I.J. Elevated Serum High-Sensitivity C-Reactive Protein Levels in Fibromyalgia Syndrome Patients Correlate with Body Mass Index, Interleukin-6, Interleukin-8, Erythrocyte Sedimentation Rate. Rheumatol. Int. 2013, 33, 1259–1264. [Google Scholar] [CrossRef]
- Feinberg, T.; Sambamoorthi, U.; Lilly, C.; Innes, K.K. Potential Mediators between Fibromyalgia and C-Reactive Protein: Results from a Large U.S. Community Survey. BMC Musculoskelet. Disord. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Kingsbury, C.; Karelis, A.D.; Hains-Monfette, G.; Bernard, P. Association between Daily Level of Objective Physical Activity and C-Reactive Protein in a Representative National Sample of Adults with Self-Reported Diagnosed Arthritis or Fibromyalgia. Rheumatol. Int. 2020, 40, 1463–1471. [Google Scholar] [CrossRef]
- Imamura, M.; Targino, R.A.; Hsing, W.T.; Imamura, S.; Azevedo, R.S.; Boas, L.S.V.; Tozetto-Mendoza, T.R.; Alfieri, F.M.; Filippo, T.R.; Battistella, L.R. Concentration of Cytokines in Patients with Osteoarthritis of the Knee and Fibromyalgia. Clin. Interv. Aging 2014, 9, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Wallace, D.J.; Linker-Israeli, M.; Hallegua, D.; Silverman, S.; Silver, D.; Weisman, M.H. Cytokines Play an Aetiopathogenetic Role in Fibromyalgia: A Hypothesis and Pilot Study. Rheumatology (Oxford) 2001, 40, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of Central Inflammation in Fibromyalgia-Increased Cerebrospinal Fluid Interleukin-8 Levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Kutu, F.C.; Özdolap, Ş.; Sarikaya, S. Pro-Inflammatory Cytokines and Oxidized Low-Density-Lipoprotein in Patients With Fibromyalgia. Arch. Rheumatol. 2019, 34, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salemi, S.; Rethage, J.; Wollina, U.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Detection of Interleukin 1beta (IL-1beta), IL-6, and Tumor Necrosis Factor-Alpha in Skin of Patients with Fibromyalgia. J. Rheumatol. 2003, 30, 146–150. [Google Scholar] [PubMed]
- Christidis, N.; Ghafouri, B.; Larsson, A.; Palstam, A.; Mannerkorpi, K.; Bileviciute-Ljungar, I.; Löfgren, M.; Bjersing, J.; Kosek, E.; Gerdle, B.; et al. Comparison of the Levels of Pro-Inflammatory Cytokines Released in the Vastus Lateralis Muscle of Patients with Fibromyalgia and Healthy Controls during Contractions of the Quadriceps Muscle—A Microdialysis Study. PLoS ONE 2015, 10, e0143856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uçeyler, N.; Häuser, W.; Sommer, C. Systematic Review with Meta-Analysis: Cytokines in Fibromyalgia Syndrome. BMC Musculoskelet. Disord. 2011, 12, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilioni, I.; Russell, I.J.; Stewart, J.M.; Gleason, R.M.; Theoharides, T.C. Neuropeptides CRH, SP, HK-1, and Inflammatory Cytokines IL-6 and TNF Are Increased in Serum of Patients with Fibromyalgia Syndrome, Implicating Mast Cells. J. Pharmacol. Exp. Ther. 2016, 356, 664–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolcino, M.; Tinazzi, E.; Puccetti, A.; Lunardi, C. Gene Expression Profiling in Fibromyalgia Indicates an Autoimmune Origin of the Disease and Opens New Avenues for Targeted Therapy. J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef]
- Malhotra, D.; Saxena, A.K.; Dar, S.A.; Kumar, V.; Nasare, N.; Tripathi, A.K.; Banerjee, B.D. Evaluation of Cytokine Levels in Fibromyalgia Syndrome Patients and Its Relationship to the Severity of Chronic Pain†. J. Musculoskelet. Pain 2012, 20, 164–169. [Google Scholar] [CrossRef]
- Geiss, A.; Rohleder, N.; Anton, F. Evidence for an Association between an Enhanced Reactivity of Interleukin-6 Levels and Reduced Glucocorticoid Sensitivity in Patients with Fibromyalgia. Psychoneuroendocrinology 2012, 37, 671–684. [Google Scholar] [CrossRef]
- Bazzichi, L.; Rossi, A.; Massimetti, G.; Giannaccini, G.; Giuliano, T.; De Feo, F.; Ciapparelli, A.; Dell’Osso, L.; Bombardieri, S. Cytokine Patterns in Fibromyalgia and Their Correlation with Clinical Manifestations. Clin. Exp. Rheumatol. 2007, 25, 225–230. [Google Scholar]
- Wang, H.; Buchner, M.; Moser, M.T.; Daniel, V.; Schiltenwolf, M. The Role of IL-8 in Patients with Fibromyalgia: A Prospective Longitudinal Study of 6 Months. Clin. J. Pain 2009, 25, 1–4. [Google Scholar] [CrossRef]
- Ang, D.C.; Moore, M.N.; Hilligoss, J.; Tabbey, R. MCP-1 and IL-8 as Pain Biomarkers in Fibromyalgia: A Pilot Study. Pain Med. 2011, 12, 1154–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Weber, A.; Schiltenwolf, M.; Amelung, D. Attachment style and cytokine levels in patients with fibromyalgia. A prospective longitudinal study. Schmerz 2014, 28, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Pernambuco, A.P.; Schetino, L.P.L.; Alvim, C.C.; Murad, C.M.; Viana, R.S.; Carvalho, L.S.C.; Reis, D.Á. Increased Levels of IL-17A in Patients with Fibromyalgia. Clin. Exp. Rheumatol. 2013, 31, S60–S63. [Google Scholar] [PubMed]
- Furer, V.; Hazan, E.; Mor, A.; Segal, M.; Katav, A.; Aloush, V.; Elkayam, O.; George, J.; Ablin, J.N. Elevated Levels of Eotaxin-2 in Serum of Fibromyalgia Patients. Pain Res. Manag. 2018, 2018. [Google Scholar] [CrossRef]
- Volanakis, J.E. Human C-Reactive Protein: Expression, Structure, and Function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Invest. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Rus, A.; Molina, F.; Gassó, M.; Camacho, M.V.; Peinado, M.Á.; Moral, M.L.d. Nitric Oxide, Inflammation, Lipid Profile, and Cortisol in Normal- and Overweight Women with Fibromyalgia. Biol. Res. Nurs. 2015. [Google Scholar] [CrossRef]
- Rodriguez-Pintó, I.; Agmon-Levin, N.; Howard, A.; Shoenfeld, Y. Fibromyalgia and Cytokines. Immunol. Lett. 2014, 161, 200–203. [Google Scholar] [CrossRef]
- Peck, M.M.; Maram, R.; Mohamed, A.; Crespo, D.O.; Kaur, G.; Ashraf, I.; Malik, B.H. The Influence of Pro-Inflammatory Cytokines and Genetic Variants in the Development of Fibromyalgia: A Traditional Review. Cureus 2020, 12, e10276. [Google Scholar] [CrossRef]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain Regulation by Non-Neuronal Cells and Inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Sims, J.E.; Smith, D.E. The IL-1 Family: Regulators of Immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Cê, P.S.; Barreiro, B.B.; Silva, R.B.; Oliveira, R.B.; Heitz, C.; Campos, M.M. Salivary Levels of Interleukin-1β in Temporomandibular Disorders and Fibromyalgia. J. Oral Facial Pain Headache 2018, 32, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trikha, M.; Corringham, R.; Klein, B.; Rossi, J.-F. Targeted Anti-Interleukin-6 Monoclonal Antibody Therapy for Cancer: A Review of the Rationale and Clinical Evidence. Clin. Cancer Res. 2003, 9, 4653–4665. [Google Scholar] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Liu, Z.; Liu, Z.-H.; Chen, S.-P.; Li, M.; Shahveranov, A.; Ye, D.-W.; Tian, Y.-K. Interleukin-6: An Emerging Regulator of Pathological Pain. J. Neuroinflamm. 2016, 13, 141. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential Involvement of Interleukin-8 (IL-8) in Acute Inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on Erythrocytes, Platelets and Clot Viscoelasticity. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Mumm, J.B.; Emmerich, J.; Zhang, X.; Chan, I.; Wu, L.; Mauze, S.; Blaisdell, S.; Basham, B.; Dai, J.; Grein, J.; et al. IL-10 Elicits IFNγ-Dependent Tumor Immune Surveillance. Cancer Cell 2011, 20, 781–796. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, M.; O’Garra, A. The Regulation of IL-10 Production by Immune Cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Abusleme, L.; Moutsopoulos, N.M. IL-17: Overview and Role in Oral Immunity and Microbiome. Oral Dis. 2017, 23, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, R.C.-M.; Mak, A. The Role of Interleukin (IL)-17 in Anxiety and Depression of Patients with Rheumatoid Arthritis. Int. J. Rheum. Dis. 2012, 15, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Lao, L.; Saito, R.; Li, A.; Bäckman, C.M.; Berman, B.M.; Ren, K.; Wei, P.-K.; Zhang, R.-X. Spinal Interleukin-17 Promotes Thermal Hyperalgesia and NMDA NR1 Phosphorylation in an Inflammatory Pain Rat Model. Pain 2013, 154, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baral, P.; Udit, S.; Chiu, I.M. Pain and Immunity: Implications for Host Defence. Nat. Rev. Immunol. 2019, 19, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Chemokines and Cytokines in Neuroinflammation Leading to Neuropathic Pain. Curr. Opin. Pharmacol. 2012, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- García, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered Profile of Chemokines in Fibromyalgia Patients. Ann. Clin. Biochem. 2014, 51, 576–581. [Google Scholar] [CrossRef] [PubMed]
| Biomarker | Salivary Levels in Patients | Findings |
|---|---|---|
| Cortisol | Increased levels [31,32] | Association between high levels of cortisol in early stages of the pathology [31,32] that correspond to peaks in pain, stress [33,34,35], and depression [36]. |
| Decreased levels [37,38] | Low cortisol levels are associated with the duration of the disease, and may be the cause of chronic adaptation to stress in fibromyalgia patients [37,39,40]. | |
| α-Amylase | Increased levels [33,41,42,43] | Fluctuations in amylase levels were observed depending on the timing of the sampling [42,44]. These changes may have been related to pain and stress in patients with fibromyalgia [33,45,46]. |
| Transaldolasa | Increased levels [47,48] | Overexpression of transaldolase in FM-affected women that may have been due to decreased oxidative tissue damage [47]. Increased expression of transaldolase by two-dimensional electrophoresis was not useful to differentiate between FM and migraine [48]. |
| Unaltered levels [49] | Absence of changes in transaldolase expression in response to thermal treatments [49]. | |
| Phosphoglycerate mutase 1 | Increased levels [47,48] | Overexpression of PGM1 determined by ELISA, Western blot, and total optical density [47]. This increase in PGM1 expression can also be detected in patients with migraine [48]. |
| Decreased levels [49] | FGM1 was significantly reduced in FM subjects after receiving mud-balneotherapy [49]. | |
| Calgranulina | Increased levels [47,50] | Increased levels of calgranulin A and C in fibromyalgia patients [47], which may be explained by their protective role against oxidative stress [50]. |
| Biomarker | Fluid | Biomarker Levels in Fibromyalgia Patients | Findings |
|---|---|---|---|
| CRP | Serum | Increased levels [88,89] | CRP has been found to provide information on the inflammatory status of patients with FM [88,89,90]. |
| IL-1β | Serum | Increased levels [87,91,92] | Higher serum levels were found in patients with fibromyalgia. No differences after adjustment of age as a covariate [91]. IL-1β, released by thalamic mast cells, contributes to inflammation and pain and can also stimulate thalamic nociceptive neurons [87]. |
| Unaltered levels [93,94] | No differences in serum levels between healthy and fibromyalgia patients [93,94]. | ||
| Skin biopsies | Increased levels [95] | There was presence of IL-1β in skin biopsies of fibromyalgia patients compared to healthy controls, which may be associated with an inflammatory component in the induction of pain [95]. | |
| Muscle samples | Unaltered levels [96] | No differences between cytokine concentrations in healthy control compared to patients with fibromyalgia [96]. | |
| Cerebrospinal fluid | Unaltered levels [93] | No differences of IL-1β were found [93]. | |
| IL-6 | Serum | Increased levels [5,87,91,97,98,99,100,101] | Higher serum levels were found in patients with fibromyalgia, which was significantly associated with fatigue and pain ratings [5,100,101]. No differences after adjustment of age as a covariate [91]. Higher levels of IL-6 in peripheral blood of fibromyalgia patients were related to hyperalgesia [92]. IL-6 released by thalamic mast cells contributed to inflammation and pain and can also stimulate thalamic nociceptive neurons [87]. This elevation also contributes to the symptoms of fibromyalgia [98,99]. |
| Skin biopsies | Increased levels [95] | There was presence of IL-6 in skin biopsies of fibromyalgia patients compared to healthy controls, which may be associated with an inflammatory component in the induction of pain [95]. | |
| Muscle samples | Unaltered levels [96] | No differences between cytokine concentrations in healthy control compared to patients with fibromyalgia [96]. | |
| IL-8 | Serum | Increased levels [5,91,92,102,103,104] | Higher serum levels were found in patients with fibromyalgia. No differences after adjustment of age as a covariate [91]. Fatigue and depression were associated with high levels of IL-8 with the promotion of sympathetic pain [5,92,104]. This increase also suggests the presence of anti-inflammatory response and a link with clinical symptoms [102]. Wang el al. did not correlate this cytokine in relation to pain intensity [103]. |
| Unaltered levels [94] | Severity of pain was positively correlated with IL-8 [94]. | ||
| Cerebrospinal fluid | Increased levels [93] | Higher levels of IL-8 in serum and cerebrospinal fluid of fibromyalgia patients [93]. | |
| Muscle samples | Unaltered levels [96] | No differences between cytokine concentrations in healthy control compared to patients with fibromyalgia [96]. | |
| IL-10 | Serum | Increased levels [91,92,100,102,105] | Higher serum levels were found in patients with fibromyalgia. No differences after adjustment of age as a covariate [91]. This market was also positively correlated with various pain scores [100]. This increase also suggests the presence of anti-inflammatory response and a link with clinical symptoms [102]. |
| IL-17 | Serum | Increased levels [106] | Fibromyalgia patients showed elevated levels of IL-17a as well as positive correlations with levels of IL-2, IL-4, IL-10, TNF, and IFN-γ [106]. |
| Intracellular levels of mononuclear blood cells | Increased levels [99] | Higher intracellular levels of IL-17 in peripheral blood mononuclear cells in fibromyalgia patients [99]. | |
| TNF- α | Serum | Increased levels [87,91,92,98,99,102,105] | Higher serum levels were found in patients with fibromyalgia. No differences after adjustment of age as a covariate [91]. TNF-α released by thalamic mast cells contributes to inflammation and pain and can also stimulate thalamic nociceptive neurons [87]. This elevation also contributes to the symptoms of fibromyalgia [98]. This increase also suggests the presence of anti-inflammatory response and a link with clinical symptoms [99,102]. |
| Unaltered levels [94] | Severity of pain was positively correlated to TNF-α [94]. | ||
| Skin biopsies | Increased levels [95] | There was presence of TNF-α in skin biopsies of fibromyalgia patients compared to healthy controls, which may be associated with an inflammatory component in the induction of pain [95]. | |
| Muscle samples | Unaltered levels [96] | No differences between cytokine concentrations in healthy control compared to patients with fibromyalgia [96]. | |
| Eoxatin-2 | Serum | Increased levels [107] | Significantly increased circulating levels of eotaxin-2 in serum of fibromyalgia patients compared with healthy controls [107]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illescas-Montes, R.; Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; De Luna-Bertos, E.; Ruiz, C.; Ramos-Torrecillas, J. Application of Salivary Biomarkers in the Diagnosis of Fibromyalgia. Diagnostics 2021, 11, 63. https://doi.org/10.3390/diagnostics11010063
Illescas-Montes R, Costela-Ruiz VJ, Melguizo-Rodríguez L, De Luna-Bertos E, Ruiz C, Ramos-Torrecillas J. Application of Salivary Biomarkers in the Diagnosis of Fibromyalgia. Diagnostics. 2021; 11(1):63. https://doi.org/10.3390/diagnostics11010063
Chicago/Turabian StyleIllescas-Montes, Rebeca, Víctor J. Costela-Ruiz, Lucía Melguizo-Rodríguez, Elvira De Luna-Bertos, Concepción Ruiz, and Javier Ramos-Torrecillas. 2021. "Application of Salivary Biomarkers in the Diagnosis of Fibromyalgia" Diagnostics 11, no. 1: 63. https://doi.org/10.3390/diagnostics11010063