Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques
Abstract
:1. Introduction
2. Essential Concepts of Gas Hydrate Formation
2.1. Gas Hydrate Formation
2.2. Gas Hydrate Structures
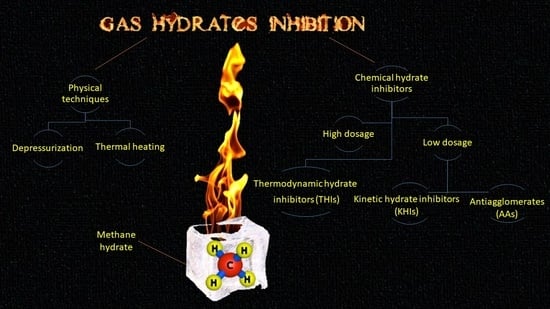
3. Gas Hydrate Inhibition
3.1. Heating
3.2. Depressurization
3.3. Thermodynamic Inhibitors (THIs)
3.3.1. Alcohols as THIs
3.3.2. Electrolytes as THIs
3.4. Low Dosage Hydrate Inhibitors (LDHIs)
3.4.1. Early History of KHIs
3.4.2. Kinetic Hydrate Inhibitors (KHIs)
- I.
- Polymeric compounds as KHIs
- II.
- Poly(vinyl pyrrolidone) (PVP) as KHI
- III.
- Poly (vinyl caprolactam) (PVCap) as a KHI.
- IV.
- Vinyl pyrrolidone/vinyl caprolactam copolymer as a KHI
- V.
- Poly (N-vinyl piperidone) as a KHI
- VI.
- VP/VCap/dimethylaminoethyl methacrylate terpolymer as a KHI
3.4.3. Anti-Agglomerates (AAs)
3.5. Dual Function Gas Hydrate Inhibitors
3.5.1. Ionic Liquids
- I.
- Ionic liquids as THIs
- II.
- Ionic liquids as KHIs
3.5.2. Amino Acids
- I.
- Amino acids as THIs
- II.
- Amino acids as KHIs
3.5.3. Nanoparticles (NPs)
- I.
- Nano particles as THIs
- II.
- Nano particles as KHIs
4. Drawbacks of Using THIs and KHIs, along with Ways to Minimize Their Negative Impacts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Okereke, N.U.; Edet, P.E.; Baba, Y.D.; Izuwa, N.C.; Kanshio, S.; Nwogu, N.; Afolabi, F.A.; Nwanwe, O. An assessment of hydrates inhibition in deepwater production systems using low-dosage hydrate inhibitor and monoethylene glycol. J. Pet. Explor. Prod. Technol. 2020, 10, 1169–1182. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, A.; Nashed, O.; Lal, B.; Foo, K.S. Experimental and modeling studies on enhancing the thermodynamic hydrate inhibition performance of monoethylene glycol via synergistic green material. Sci. Rep. 2021, 11, 2396. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Sohn, Y.h.; Chang, D.; Seo, Y.; Kang, S.-P. Prevention of methane hydrate re-formation in transport pipeline using thermodynamic and kinetic hydrate inhibitors. J. Pet. Sci. Eng. 2017, 154, 114–125. [Google Scholar] [CrossRef]
- Kvamme, B.; Kuznetsova, T.; Bauman, J.M.; Sjöblom, S.; Avinash Kulkarni, A. Hydrate Formation during Transport of Natural Gas Containing Water and Impurities. J. Chem. Eng. Data 2016, 61, 936–949. [Google Scholar] [CrossRef]
- Schicks, J.M. Gas Hydrates: Formation, Structures, and Properties. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Wilkes, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–15. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Galib, M.; Nguyen, A.V. Critical Review on Gas Hydrate Formation at Solid Surfaces and in Confined Spaces—Why and How Does Interfacial Regime Matter? Energy Fuels 2020, 34, 6751–6760. [Google Scholar] [CrossRef]
- Venet, S.; Guerton, F.; Desmedt, A.; Broseta, D. Insights into the porous structure of surfactant-promoted gas hydrate. Chem. Eng. Sci. 2022, 248, 117193. [Google Scholar] [CrossRef]
- Dhamu, V.; Thakre, N.; Jana, A.K. Structure-H hydrate of mixed gases: Phase equilibrium modeling and experimental validation. J. Mol. Liq. 2021, 343, 117605. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Vasheghani Farahani, M.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, Q.; Chen, Z.; Li, X.; Zhang, Y.; Xu, C.; Yan, K. Review of methods and applications for promoting gas hydrate formation process. J. Nat. Gas Sci. Eng. 2022, 101, 104528. [Google Scholar] [CrossRef]
- Fakir, T.; Babaee, S.; Naidoo, P. Application of Gas Hydrate for the Treatment of Vinasse: Phase Equilibrium and Kinetic Investigations. J. Chem. Eng. Data 2021, 66, 504–514. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef]
- Yu, C.; Fan, S.; Lang, X.; Wang, Y.; Li, G.; Wang, S. Hydrogen and chemical energy storage in gas hydrate at mild conditions. Int. J. Hydrogen Energy 2020, 45, 14915–14921. [Google Scholar] [CrossRef]
- Filarsky, F.; Schmuck, C.; Schultz, H.J. Development of a Gas Hydrate Absorption for Energy storage and Gas separation–Proof of Concept based on Natural Gas. Energy Procedia 2019, 158, 5367–5373. [Google Scholar] [CrossRef]
- Eslamimanesh, A.; Mohammadi, A.H.; Richon, D.; Naidoo, P.; Ramjugernath, D. Application of gas hydrate formation in separation processes: A review of experimental studies. J. Chem. Thermodyn. 2012, 46, 62–71. [Google Scholar] [CrossRef]
- Babu, P.; Linga, P.; Kumar, R.; Englezos, P. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture. Energy 2015, 85, 261–279. [Google Scholar] [CrossRef]
- Babaee, S.; Hashemi, H.; Naidoo, P.; Ramjugernath, D. Application of Gas Hydrates in the Separation and Purification of Xenon from a Mixture of Xenon and Argon. J. Chem. Eng. Data 2021, 66, 3815–3825. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas Hydrate-Based Process for Desalination of Heavy Metal Ions from an Aqueous Solution: Kinetics and Rate of Recovery. ACS EST Water 2021, 1, 134–144. [Google Scholar] [CrossRef]
- Khan, M.N.; Peters, C.J.; Koh, C.A. Desalination using gas hydrates: The role of crystal nucleation, growth and separation. Desalination 2019, 468, 114049. [Google Scholar] [CrossRef]
- Niu, M.; Wu, G.; Yin, Z.; Sun, Y.; Liu, K.; Chen, D. Effectiveness of CO2-N2 injection for synergistic CH4 recovery and CO2 sequestration at marine gas hydrates condition. Chem. Eng. J. 2021, 420, 129615. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, Y.; Yu, C.; Li, G.; Lang, X.; Wang, S.; Fan, S. Promoting methane hydrate formation with expanded graphite additives: Application to solidified natural gas storage. Fuel 2021, 299, 120867. [Google Scholar] [CrossRef]
- Schicks, J.M.; Haeckel, M.; Janicki, G.; Spangenberg, E.; Thaler, J.; Giese, R.; Strauch, B.; Heeschen, K.; Priegnitz, M.; Luzi-Helbing, M.; et al. Development, test, and evaluation of exploitation technologies for the application of gas production from natural gas hydrate reservoirs and their potential application in the Danube Delta, Black Sea. Mar. Pet. Geol. 2020, 120, 104488. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Lal, B. Seawater and produced water treatment via gas hydrate: Review. J. Environ. Chem. Eng. 2021, 9, 105053. [Google Scholar] [CrossRef]
- Hammerschmidt, E.G. Formation of Gas Hydrates in Natural Gas Transmission Lines. Ind. Eng. Chem. 1934, 26, 851–855. [Google Scholar] [CrossRef]
- Zhang, Z.-c.; Wu, N.-y.; Liu, C.-l.; Hao, X.-l.; Zhang, Y.-c.; Gao, K.; Peng, B.; Zheng, C.; Tang, W.; Guo, G.-j. Molecular simulation studies on natural gas hydrates nucleation and growth: A review. China Geol. 2022, 5, 330–344. [Google Scholar] [CrossRef]
- Salmin, D.C.; Estanga, D.; Koh, C.A. Review of gas hydrate anti-agglomerant screening techniques. Fuel 2022, 319, 122862. [Google Scholar] [CrossRef]
- Rajnauth, J.; Barrufet, M.; Falcone, G. Hydrate Formation: Considering the effects of Pressure, Temperature, Composition and Water. Energy Sci. Technol. 2012, 4, 60–67. [Google Scholar] [CrossRef]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M. A perspective on dual purpose gas hydrate and corrosion inhibitors for flow assurance. J. Pet. Sci. Eng. 2019, 183, 106418. [Google Scholar] [CrossRef]
- Ruan, X.; Li, X.-S.; Xu, C.-G. A review of numerical research on gas production from natural gas hydrates in China. J. Nat. Gas Sci. Eng. 2021, 85, 103713. [Google Scholar] [CrossRef]
- Khokhar, A.A.; Gudmundsson, J.S.; Sloan, E.D. Gas storage in structure H hydrates. Fluid Phase Equilibria 1998, 150–151, 383–392. [Google Scholar] [CrossRef]
- Kavianpour, B.; Shariati, A.; Moshfeghian, M. Thermodynamic investigation and hydrate inhibition of real gas flow through orifice during depressurization. Process Saf. Environ. Prot. 2014, 92, 224–230. [Google Scholar] [CrossRef]
- Chen, B.; Sun, H.; Zhao, G.; Wang, B.; Zhao, Y.; Yang, M. Experimental observation of methane hydrate dissociation via different depressurization modes under water phase flow. Fuel 2021, 283, 118908. [Google Scholar] [CrossRef]
- Yang, C.; Zi, M.; Wu, G.; Zou, X.; Liu, K.; Chen, D. Concentration effect of kinetic hydrate inhibitor on hydrate formation and inhibition. Fuel 2022, 323, 124448. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Lang, C.; Zhao, J.; Lv, X.; Ge, Y.; Jiang, L. Experimental study on the gas hydrates blockage and evaluation of kinetic inhibitors using a fully visual rocking cell. J. Nat. Gas Sci. Eng. 2021, 96, 104331. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/ inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Farhadian, A.; Go, W.; Yun, S.; Rahimi, A.; Reza Nabid, M.; Iravani, D.; Seo, Y. Efficient dual-function inhibitors for prevention of gas hydrate formation and CO2/H2S corrosion inside oil and gas pipelines. Chem. Eng. J. 2022, 431, 134098. [Google Scholar] [CrossRef]
- Fakher, S.; Elgahawy, Y.; Abdelaal, H.; El Tonbary, A.; Imqam, A. Reducing Excessive Water Production Associated with Gas Hydrate Reservoirs Using a Thermal In-Situ Heating-Inhibitor Method. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar] [CrossRef]
- Cranganu, C. In-situ thermal stimulation of gas hydrates. J. Pet. Sci. Eng. 2009, 65, 76–80. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J. Thermal Stimulation Based Methane Production from Hydrate Bearing Quartz Sediment. Ind. Eng. Chem. Res. 2013, 52, 6571–6581. [Google Scholar] [CrossRef]
- Kou, X.; Li, X.-S.; Wang, Y.; Chen, Z.-Y. Hydrate decomposition front within porous media under thermal stimulation and depressurization conditions: Macroscale to microscale. Int. J. Heat Mass Transf. 2022, 188, 122653. [Google Scholar] [CrossRef]
- Wan, Q.-C.; Si, H.; Li, B.; Yin, Z.-Y.; Gao, Q.; Liu, S.; Han, X.; Chen, L.-L. Energy recovery enhancement from gas hydrate based on the optimization of thermal stimulation modes and depressurization. Appl. Energy 2020, 278, 115612. [Google Scholar] [CrossRef]
- Nair, V.C.; Prasad, S.K.; Kumar, R.; Sangwai, J.S. Energy recovery from simulated clayey gas hydrate reservoir using depressurization by constant rate gas release, thermal stimulation and their combinations. Appl. Energy 2018, 225, 755–768. [Google Scholar] [CrossRef]
- Roostaie, M.; Leonenko, Y. Gas production from methane hydrates upon thermal stimulation; an analytical study employing radial coordinates. Energy 2020, 194, 116815. [Google Scholar] [CrossRef] [Green Version]
- Wan, Q.-C.; Si, H.; Li, B.; Li, G. Heat transfer analysis of methane hydrate dissociation by depressurization and thermal stimulation. Int. J. Heat Mass Transf. 2018, 127, 206–217. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Lv, X.; Ge, K.; Cheng, C.; Dong, H. Numerical analysis of the gas recovery performance in hydrate reservoirs with various parameters by stepwise depressurization. J. Pet. Sci. Eng. 2021, 203, 108670. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, L.; Zhang, Q.; Wang, S.; Wang, K.; Xu, R. Numerical study on gas production from methane hydrate reservoir by depressurization in a reactor. Renew. Sustain. Energy Rev. 2020, 134, 110330. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, J.-N.; Gao, Y.; Ma, Z.; Lv, X.; Song, Y. Dissociation characteristics of methane hydrates in South China Sea sediments by depressurization. Appl. Energy 2019, 243, 266–273. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, X.; Xiang, W.; Zhang, J.; Cao, C.; Wang, H.; Hu, X.; Gao, B. Ball milling biochar with ammonia hydroxide or hydrogen peroxide enhances its adsorption of phenyl volatile organic compounds (VOCs). J. Hazard. Mater. 2021, 403, 123540. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-S.; Chen, Z.-Y.; Ruan, X.-K.; Huang, N.-S. Methane Hydrate Dissociation by Depressurization in Sediments with Different Hydrate Saturations in Cubic Hydrate Simulator. Energy Procedia 2014, 61, 990–994. [Google Scholar] [CrossRef]
- Singh, A.; Suri, A. A review on gas hydrates and kinetic hydrate inhibitors based on acrylamides. J. Nat. Gas Sci. Eng. 2020, 83, 103539. [Google Scholar] [CrossRef]
- Semenov, A.P.; Mendgaziev, R.I.; Stoporev, A.S.; Istomin, V.A.; Sergeeva, D.V.; Tulegenov, T.B.; Vinokurov, V.A. Dimethyl sulfoxide as a novel thermodynamic inhibitor of carbon dioxide hydrate formation. Chem. Eng. Sci. 2022, 255, 117670. [Google Scholar] [CrossRef]
- Najibi, H.; Azimi, A.; Javanmardi, J.; Roozbahani, R.; Mohammadi, A.H. Natural gas hydrate stability conditions and water activity in aqueous solutions containing mono ethylene glycol (MEG) and salt: Experimental measurements and thermodynamic modeling. Fluid Phase Equilibria 2022, 554, 113322. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Øvsthus, J.; Tomita, T.; Chosa, J.-I. Studies on some zwitterionic surfactant gas hydrate anti-agglomerants. Chem. Eng. Sci. 2006, 61, 4048–4059. [Google Scholar] [CrossRef]
- Xiao, C.; Adidharma, H. Dual function inhibitors for methane hydrate. Chem. Eng. Sci. 2009, 64, 1522–1527. [Google Scholar] [CrossRef]
- Lederhos, J.P.; Long, J.P.; Sum, A.; Christiansen, R.L.; Sloan, E.D. Effective kinetic inhibitors for natural gas hydrates. Chem. Eng. Sci. 1996, 51, 1221–1229. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, C.; Wang, H.; Yang, L.; Zhang, L.; Zhao, J.; Song, Y. Atomistic insights into the performance of thermodynamic inhibitors in the nucleation of methane hydrate. Chem. Eng. J. 2022, 431, 133479. [Google Scholar] [CrossRef]
- Semenov, A.P.; Mendgaziev, R.I.; Stoporev, A.S.; Istomin, V.A.; Sergeeva, D.V.; Ogienko, A.G.; Vinokurov, V.A. The pursuit of a more powerful thermodynamic hydrate inhibitor than methanol. Dimethyl sulfoxide as a case study. Chem. Eng. J. 2021, 423, 130227. [Google Scholar] [CrossRef]
- Kvamme, B.; Wei, N.; Zhao, J.; Zhou, S.; Zhang, L.; Sun, W.; Saeidi, N. Alcohols for hydrate inhibition–Different alcohols and different mechanisms. Petroleum 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Semenov, A.P.; Stoporev, A.S.; Mendgaziev, R.I.; Gushchin, P.A.; Khlebnikov, V.N.; Yakushev, V.S.; Istomin, V.A.; Sergeeva, D.V.; Vinokurov, V.A. Synergistic effect of salts and methanol in thermodynamic inhibition of sII gas hydrates. J. Chem. Thermodyn. 2019, 137, 119–130. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Mohammadi, A.H.; Kamran-Pirzaman, A. Thermodynamic modeling of the dissociation conditions of hydrogen sulfide clathrate hydrate in the presence of aqueous solution of inhibitor (alcohol, salt or ethylene glycol). Chem. Eng. Res. Des. 2014, 92, 2283–2293. [Google Scholar] [CrossRef]
- Qasim, A.; Khan, M.S.; Lal, B.; Ismail, M.C.; Rostani, K. Quaternary ammonium salts as thermodynamic hydrate inhibitors in the presence and absence of monoethylene glycol for methane hydrates. Fuel 2020, 259, 116219. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Chen, Z.; Li, W.; Qin, Y.; Dong, B. The growth of methane hydrate with impingement influenced by thermodynamic inhibitor. Fuel 2021, 304, 121390. [Google Scholar] [CrossRef]
- Sun, J.; Sun, R.; Ming Chou, I.; Nguyen, A.V.; Jiang, L. Experimental measurement and thermodynamic modeling of dissociation conditions of hydrogen sulfide hydrate in the presence of electrolyte solutions. Chem. Eng. J. 2022, 431, 133821. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Lv, X.; Ge, K.; Jiang, Z.; Li, Y. Experimental measurement and thermodynamic modeling of methane hydrate phase equilibria in the presence of chloride salts. Chem. Eng. J. 2020, 395, 125126. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Dybvik, L. Studies on New Gas Hydrate Inhibitors. In Proceedings of the SPE Offshore Europe, Aberdeen, UK, 5–8 September 1995. [Google Scholar]
- Sloan, E.D. Clathrate Hydrates: The Other Common Solid Water Phase. Ind. Eng. Chem. Res. 2000, 39, 3123–3129. [Google Scholar] [CrossRef]
- Englezos, P. Nucleation and Growth of Gas Hydrate Crystals in Relation to Kinetic Inhibition. Rev. Inst. Fr. Pét. 1996, 51, 789–795. [Google Scholar] [CrossRef]
- Fu, S.B.; Cenegy, L.M.; Neff, C.S. A Summary of Successful Field Applications of a Kinetic Hydrate Inhibitor. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001. [Google Scholar]
- Earle, S.; Sloan, J.J.C.S.o.M. Method for Controlling Clathrate Hydrates in Fluid Systems. 1998. Available online: https://data.epo.org/publication-server/document?iDocId=1556721&iFormat=0 (accessed on 2 September 2022).
- Safo, I.A.; Werheid, M.; Dosche, C.; Oezaslan, M. The role of polyvinylpyrrolidone (PVP) as a capping and structure-directing agent in the formation of Pt nanocubes. Nanoscale Adv. 2019, 1, 3095–3106. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Kelland, M.A. Study of the Kinetic Hydrate Inhibitor Performance of Poly(N-vinylcaprolactam) and poly(N-isopropylmethacrylamide) with Varying End Caps. Energy Fuels 2018, 32, 9211–9219. [Google Scholar] [CrossRef]
- Erfani, A.; Varaminian, F.; Mohammadi, M. Gas Hydrate Formation Inhibition Using Low Dosage Hydrate Inhibitors. In Proceedings of the 2nd National Iranian Conference on Gas Hydrate (NICGH), Semnan, Iran, 15 May 2013. [Google Scholar]
- Ke, W.; Svartaas, T.M.; Abay, H.K. Effects of Low Concentration Methanol, PVP and PVCap on Structure-I Methane Hydrate Formation. Energy Power Eng. 2013, 7, 432–439. [Google Scholar]
- Cha, M.; Shin, K.; Seo, Y.; Shin, J.Y.; Kang, S.P. Catastrophic growth of gas hydrates in the presence of kinetic hydrate inhibitors. J. Phys. Chem. A 2013, 117, 13988–13995. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Q.; Long, Z.; Liang, D. In situ PXRD analysis on the kinetic effect of PVP-K90 and PVCap on methane hydrate dissociation below ice point. Fuel 2021, 286, 119491. [Google Scholar] [CrossRef]
- Choudhary, N.; Das, S.; Roy, S.; Kumar, R. Effect of polyvinylpyrrolidone at methane hydrate-liquid water interface. Application in flow assurance and natural gas hydrate exploitation. Fuel 2016, 186, 613–622. [Google Scholar] [CrossRef]
- Gulbrandsen, A.C.; Svartås, T.M. Effects of PVCap on Gas Hydrate Dissociation Kinetics and the Thermodynamic Stability of the Hydrates. Energy Fuels 2017, 31, 9863–9873. [Google Scholar] [CrossRef]
- O’Reilly, R.; Ieong, N.S.; Chua, P.C.; Kelland, M.A. Missing Poly(N-vinyl lactam) Kinetic Hydrate Inhibitor: High-Pressure Kinetic Hydrate Inhibition of Structure II Gas Hydrates with Poly(N-vinyl piperidone) and Other Poly(N-vinyl lactam) Homopolymers. Energy Fuels 2011, 25, 4595–4599. [Google Scholar] [CrossRef]
- Ke, W.; Chen, D. A short review on natural gas hydrate, kinetic hydrate inhibitors and inhibitor synergists. Chin. J. Chem. Eng. 2019, 27, 2049–2061. [Google Scholar] [CrossRef]
- Wang, R.; Sun, H.; Sun, J.; Qu, Y.; Zhang, J.; Shi, X.; Zhang, L.; Guo, D. Effect of Dissolution and Dispersion Conditions of VC-713 on the Hydrate Inhibition. J. Chem. 2019, 2019, 3547518. [Google Scholar] [CrossRef]
- Ghosh, R.; Kelland, M.A. Nonpolymeric Citramide-Based Kinetic Hydrate Inhibitors: Good Performance with Just Six Alkylamide Groups. ACS Omega 2022, 7, 13953–13962. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, H.; Li, Y.; Zou, B.; Xiao, A.; Ma, M. Synthesis and Evaluation of a New Kind of Kinetic Hydrate Inhibitor. Key Eng. Mater. 2017, 727, 781–790. [Google Scholar] [CrossRef]
- Wu, R.; Kozielski, K.; Hartley, P.; May, E.; Boxall, J.; Maeda, N. Methane–Propane Mixed Gas Hydrate Film Growth on the Surface of Water and Luvicap EG Solutions. Energy Fuels 2013, 27, 2548–2554. [Google Scholar] [CrossRef]
- Xu, S.; Fan, S.; Wang, Y.; Lang, X. An Investigation of Kinetic Hydrate Inhibitors on the Natural Gas from the South China Sea. J. Chem. Eng. Data 2015, 60, 311–318. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S.; von Solms, N. Application of various water soluble polymers in gas hydrate inhibition. Renew. Sustain. Energy Rev. 2016, 60, 206–225. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zheng, Y. Experimental Study on Inhibition of Hydrate Formation by Compound Solution of PVP and Ethylene Glycol Ether. IOP Conf. Ser. Earth Environ. Sci. 2020, 453, 012094. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, L.; Li, Z.; Liu, B.; Chen, G. Inhibition Effect of Kinetic Hydrate Inhibitors on the Growth of Methane Hydrate in Gas–Liquid Phase Separation State. Energies 2019, 12, 4482. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Cui, J.; Li, J.; Zhu, R.; Liu, B.; Ban, S.; Chen, G. High efficient development of green kinetic hydrate inhibitors via combined molecular dynamic simulation and experimental test approach. Green Chem. Eng. 2022, 3, 34–43. [Google Scholar] [CrossRef]
- Aghajanloo, M.; Reza Ehsani, M.; Taheri, Z.; Jafari Behbahani, T.; Mohammadi, A.H.; Mohammad Taheri, M. Kinetics of methane + hydrogen sulfide clathrate hydrate formation in the presence/absence of poly N-vinyl pyrrolidone (PVP) and L-tyrosine: Experimental study and modeling of the induction time. Chem. Eng. Sci. 2022, 250, 117384. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, J.; Lv, K.; Sun, J.; Wang, R.; Wang, J.; Guo, X.; Hou, Q.; Liu, J.; Bai, Y.; et al. Effects of PVP and NaCl on the decomposition of methane hydrate by MD simulation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127817. [Google Scholar] [CrossRef]
- Ke, W.; Svartaas, T.M.; Kvaløy, J.T.; Kosberg, B.R. Inhibition–Promotion: Dual Effects of Polyvinylpyrrolidone (PVP) on Structure-II Hydrate Nucleation. Energy Fuels 2016, 30, 7646–7655. [Google Scholar] [CrossRef]
- Qin, H.-B.; Zhang, Z.-Y.; Sun, C.-Y.; Chen, G.-J.; Ma, Q.-L.; Ning, Z.-F. Interfacial Tension between Methane and Water Containing Kinetic Hydrate Inhibitor PVP Ramification and Its Emulsification Property. J. Chem. Eng. Data 2017, 62, 2770–2775. [Google Scholar] [CrossRef]
- Karaaslan, U.; Parlaktuna, M. PEOA New Hydrate Inhibitor Polymer. Energy Fuels 2002, 16, 1387–1391. [Google Scholar] [CrossRef]
- Ivall, J.; Pasieka, J.; Posteraro, D.; Servio, P. Profiling the Concentration of the Kinetic Inhibitor Polyvinylpyrrolidone throughout the Methane Hydrate Formation Process. Energy Fuels 2015, 29, 2329–2335. [Google Scholar] [CrossRef]
- He, Y.; Long, Z.; Lu, J.; Shi, L.; Yan, W.; Liang, D. Investigation on Methane Hydrate Formation in Water-based Drilling Fluid. Energy Fuels 2021, 35, 5264–5270. [Google Scholar] [CrossRef]
- Kang, H.; Koh, D.-Y.; Ahn, Y.-H.; Jung, S.; Park, J.; Lee, J.; Lee, H. Optical Properties of Tetrahydrofuran Clathrate Hydrates with Polyvinylpyrrolidone (THF + H2O + PVP) Revealed by Terahertz (THz) Time-Domain Spectroscopy. J. Chem. Eng. Data 2015, 60, 238–246. [Google Scholar] [CrossRef]
- Daraboina, N.; Linga, P.; Ripmeester, J.; Walker, V.K.; Englezos, P. Natural Gas Hydrate Formation and Decomposition in the Presence of Kinetic Inhibitors. 2. Stirred Reactor Experiments. Energy Fuels 2011, 25, 4384–4391. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Ke, W.; Zhao, C.; Chen, D. Experimental Evaluation of Kinetic Hydrate Inhibitors and Mixed Formulations with Monoethylene Glycol for Hydrate Prevention in Pure Water and Brine–Oil Systems. Energy Fuels 2020, 34, 12274–12290. [Google Scholar] [CrossRef]
- Anderson, B.J.; Tester, J.W.; Borghi, G.P.; Trout, B.L. Properties of Inhibitors of Methane Hydrate Formation via Molecular Dynamics Simulations. J. Am. Chem. Soc. 2005, 127, 17852–17862. [Google Scholar] [CrossRef]
- Kirsh, Y.E.; Yanul, N.A.; Kalninsh, K.K. Structural transformations and water associate interactions in poly-N-vinylcaprolactam–water system. Eur. Polym. J. 1999, 35, 305–316. [Google Scholar] [CrossRef]
- Makogon, T.Y.; Larsen, R.; Knight, C.A.; Dendy Sloan, E. Melt growth of tetrahydrofuran clathrate hydrate and its inhibition: Method and first results. J. Cryst. Growth 1997, 179, 258–262. [Google Scholar] [CrossRef]
- Sharifi, H.; Hatzikiriakos, S.G.; Englezos, P. Rheological evaluation of kinetic hydrate inhibitors in NaCl/n-heptane solutions. AIChE J. 2014, 60, 2654–2659. [Google Scholar] [CrossRef]
- Wan, L.; Liang, D.-Q.; Ding, Q.; Hou, G. Investigation into the inhibition of methane hydrate formation in the presence of hydroxy-terminated poly(N-vinylcaprolactam). Fuel 2019, 239, 173–179. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Guo, J.; Chen, G.; Zhong, J.; Yan, Y.; Zhang, J. Molecular insights into the kinetic hydrate inhibition performance of Poly(N-vinyl lactam) polymers. J. Nat. Gas Sci. Eng. 2020, 83, 103504. [Google Scholar] [CrossRef]
- Wu, R.; Aman, Z.M.; May, E.F.; Kozielski, K.A.; Hartley, P.G.; Maeda, N.; Sum, A.K. Effect of Kinetic Hydrate Inhibitor Polyvinylcaprolactam on Cyclopentane Hydrate Cohesion Forces and Growth. Energy Fuels 2014, 28, 3632–3637. [Google Scholar] [CrossRef]
- Mardani, M.; Azimi, A.; Javanmardi, J.; Mohammadi, A.H. Effect of EMIM-BF4 Ionic Liquid on Dissociation Temperature of Methane Hydrate in the Presence of PVCap: Experimental and Modeling Studies. Energy Fuels 2019, 33, 50–57. [Google Scholar] [CrossRef]
- Gulbrandsen, A.C.; Svartaas, T.M. Effect of Poly Vinyl Caprolactam Concentration on the Dissociation Temperature for Methane Hydrates. Energy Fuels 2017, 31, 8505–8511. [Google Scholar] [CrossRef]
- Gulbrandsen, A.C.; Svartaas, T.M. Influence on Hydrate Dissociation for Methane Hydrates Formed in the Presence of PolyVinylCaprolactam versus PolyVinylCaprolactam + Butyl Glycol Ether. Energy Fuels 2017, 31, 6352–6357. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Y.; Liang, D. Investigation into the Inhibition of Methane Hydrate Formation in the Presence of Hydroxy- and Esteryl-Terminated Poly(N-vinylcaprolactam). Energy Fuels 2022, 36, 3848–3856. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Sheng, Q.; Wood, C.D.; Seo, Y. Kinetic Hydrate Inhibition Performance of Poly(vinyl caprolactam) Modified with Corrosion Inhibitor Groups. Energy Fuels 2017, 31, 9363–9373. [Google Scholar] [CrossRef]
- Long, Z.; Lu, Z.; Ding, Q.; Zhou, X.; Lei, J.; Liang, D. Evaluation of Kinetic Inhibition of Methane Hydrate Formation by a Copolymer of N-Vinylcaprolactam with 1-Vinylimidazole. Energy Fuels 2019, 33, 10133–10142. [Google Scholar] [CrossRef]
- Kelland, M.A.; Dirdal, E.G.; Ree, L.H.S. Solvent Synergists for Improved Kinetic Hydrate Inhibitor Performance of Poly(N-vinylcaprolactam). Energy Fuels 2020, 34, 1653–1663. [Google Scholar] [CrossRef]
- Mady, M.F.; Kelland, M.A. Fluorinated Quaternary Ammonium Bromides: Studies on Their Tetrahydrofuran Hydrate Crystal Growth Inhibition and as Synergists with Polyvinylcaprolactam Kinetic Gas Hydrate Inhibitor. Energy Fuels 2013, 27, 5175–5181. [Google Scholar] [CrossRef]
- Chua, P.C.; Kelland, M.A. Tetra(iso-hexyl)ammonium Bromide—The Most Powerful Quaternary Ammonium-Based Tetrahydrofuran Crystal Growth Inhibitor and Synergist with Polyvinylcaprolactam Kinetic Gas Hydrate Inhibitor. Energy Fuels 2012, 26, 1160–1168. [Google Scholar] [CrossRef]
- Kim, J.; Shin, K.; Seo, Y.; Cho, S.J.; Lee, J.D. Synergistic Hydrate Inhibition of Monoethylene Glycol with Poly(vinylcaprolactam) in Thermodynamically Underinhibited System. J. Phys. Chem. B 2014, 118, 9065–9075. [Google Scholar] [CrossRef]
- Magnusson, C.D.; Kelland, M.A. Study on the Synergistic Properties of Quaternary Phosphonium Bromide Salts with N-Vinylcaprolactam Based Kinetic Hydrate Inhibitor Polymers. Energy Fuels 2014, 28, 6803–6810. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.S.; Kim, H.; Kim, S.-H.; Seo, Y. Hydrate Growth Inhibition by Poly(vinyl caprolactam) Released from Microcarriers under Turbulent Mixing Conditions. Energy Fuels 2018, 32, 9001–9009. [Google Scholar] [CrossRef]
- Kelland, M.A.; Moi, N.; Howarth, M. Breakthrough in Synergists for Kinetic Hydrate Inhibitor Polymers, Hexaalkylguanidinium Salts: Tetrahydrofuran Hydrate Crystal Growth Inhibition and Synergism with Polyvinylcaprolactam. Energy Fuels 2013, 27, 711–716. [Google Scholar] [CrossRef]
- Shao, Z.; Sun, J.; Wang, J.; Lv, K.; Liao, B.; Wang, R.; Jiang, H. Effects of Modified Cellulose on Methane Hydrate Decomposition: Experiments and Molecular Dynamics Simulations. ACS Sustain. Chem. Eng. 2021, 9, 9689–9697. [Google Scholar] [CrossRef]
- Lee, W.; Shin, J.-Y.; Kim, K.-S.; Kang, S.-P. Synergetic Effect of Ionic Liquids on the Kinetic Inhibition Performance of Poly(N-vinylcaprolactam) for Natural Gas Hydrate Formation. Energy Fuels 2016, 30, 9162–9169. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.-S.; Kang, S.-P.; Kim, J.-N. Synergetic Performance of the Mixture of Poly(N-vinylcaprolactam) and a Pyrrolidinium-Based Ionic Liquid for Kinetic Hydrate Inhibition in the Presence of the Mineral Oil Phase. Energy Fuels 2018, 32, 4932–4941. [Google Scholar] [CrossRef]
- Imran, M.; Saleem, Q.; Ajwad, H.A.; Makogon, T.Y.; Ali, S.A.; Rushaid, A.; Panda, S.K.; Al-Eid, M.; Alawani, N.A.; Aleisa, R.M.; et al. Design and development of N-vinylcaprolactam copolymers as kinetic hydrate inhibitors for sour gas environments. Fuel 2022, 311, 122497. [Google Scholar] [CrossRef]
- Yang, T.; Yin, S.; Xie, M.; Chen, F.; Su, B.; Lin, H.; Xue, Y.; Han, S. Effects of N-containing pour point depressants on the cold flow properties of diesel fuel. Fuel 2020, 272, 117666. [Google Scholar] [CrossRef]
- O’Reilly, R.; Ieong, N.S.; Chua, P.C.; Kelland, M.A. Crystal growth inhibition of tetrahydrofuran hydrate with poly(N-vinyl piperidone) and other poly(N-vinyl lactam) homopolymers. Chem. Eng. Sci. 2011, 66, 6555–6560. [Google Scholar] [CrossRef]
- Cohen, J.M.; Young, W.D. Method for Inhibiting the Formation of Gas Hydrates. WIPO (PCT). Google Patents WO1996041786A1, 2000. [Google Scholar]
- Aman, Z.M.; Koh, C.A. Interfacial phenomena in gas hydrate systems. Chem. Soc. Rev. 2016, 45, 1678–1690. [Google Scholar] [CrossRef]
- Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Fundamentals and Applications of Gas Hydrates. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Anderson, R.; Mozaffar, H.; Tohidi, F. The Return of Kinetic Hydrate Inhibitors. Energy Fuels 2015, 29, 8254–8260. [Google Scholar] [CrossRef]
- Ning, F.; Guo, D.; Din, S.U.; Zhang, H.; Ou, W.; Fang, B.; Liang, Y.; Zhang, L.; Lee, K.; Koh, C.A. The kinetic effects of hydrate anti-agglomerants/surfactants. Fuel 2022, 318, 123566. [Google Scholar] [CrossRef]
- Song, G.; Li, Y.; Wang, W.; Jiang, K.; Shi, Z.; Yao, S. Hydrate formation in oil–water systems: Investigations of the influences of water cut and anti-agglomerant. Chin. J. Chem. Eng. 2020, 28, 369–377. [Google Scholar] [CrossRef]
- Zhao, X.; Fang, Q.; Qiu, Z.; Mi, S.; Wang, Z.; Geng, Q.; Zhang, Y. Experimental investigation on hydrate anti-agglomerant for oil-free systems in the production pipe of marine natural gas hydrates. Energy 2022, 242, 122973. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, Y.; Liu, C.; Kang, M.; Chen, G.; Deng, B.; Zeng, F. Methane hydrate dissociation from anti-agglomerants containing oil dominated dispersed systems. Fuel 2021, 294, 120561. [Google Scholar] [CrossRef]
- Sa, J.-H.; Melchuna, A.; Zhang, X.; Rivero, M.; Glénat, P.; Sum, A.K. Investigating the effectiveness of anti-agglomerants in gas hydrates and ice formation. Fuel 2019, 255, 115841. [Google Scholar] [CrossRef]
- Brown, E.P.; Koh, C.A. Competitive Interfacial Effects of Surfactant Chemicals on Clathrate Hydrate Particle Cohesion. Energy Fuels 2016, 30, 8065–8071. [Google Scholar] [CrossRef]
- Bellucci, M.A.; Walsh, M.R.; Trout, B.L. Molecular Dynamics Analysis of Anti-Agglomerant Surface Adsorption in Natural Gas Hydrates. J. Phys. Chem. C 2018, 122, 2673–2683. [Google Scholar] [CrossRef]
- Chua, P.C.; Kelland, M.A. Study of the Gas Hydrate Antiagglomerant Performance of a Series of Mono- and Bis-amine Oxides: Dual Antiagglomerant and Kinetic Hydrate Inhibition Behavior. Energy Fuels 2018, 32, 1674–1684. [Google Scholar] [CrossRef]
- Hu, S.; Vo, L.; Monteiro, D.; Bodnar, S.; Prince, P.; Koh, C.A. Structural Effects of Gas Hydrate Antiagglomerant Molecules on Interfacial Interparticle Force Interactions. Langmuir 2021, 37, 1651–1661. [Google Scholar] [CrossRef]
- Nazari, K.; Ahmadi, A.N.; Moradi, M.R.; Sahraei, V.; Taghikhani, V.; Ghobti, C. A thermodynamic study of methane hydrate formation in the presence of [Bmim][Bf 4] and [Bmim][Ms] ionic liquids. In Proceedings of the 7th International Conference on Gas Hydrates (ICGH 2011), Edinburgh, UK, 17–21 July 2011; pp. 17–21. [Google Scholar]
- Xiao, C.; Wibisono, N.; Adidharma, H. Dialkylimidazolium halide ionic liquids as dual function inhibitors for methane hydrate. Chem. Eng. Sci. 2010, 65, 3080–3087. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Y.; Zeng, P.; Yang, W.; Liang, Q.; Peng, X.; Liu, Y.; Hu, Y. Effect of 1-butyl-3-methylimidazolium tetrafluoroborate on the formation rate of CO2 hydrate. J. Nat. Gas Chem. 2008, 17, 264–267. [Google Scholar] [CrossRef]
- Makino, T.; Matsumoto, Y.; Sugahara, T.; Ohgaki, K.; Masuda, H. Effect of ionic liquid on hydrate formation rate in carbon dioxide hydrates. In Proceedings of the 7th International Conference on Gas Hydrates, Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Lal, B.; Bustam, M.A.; Shariff, A.M. Thermodynamic effect of ammonium based ionic liquids on CO2 hydrates phase boundary. J. Mol. Liq. 2017, 238, 533–539. [Google Scholar] [CrossRef]
- Khan, M.S.; Partoon, B.; Bavoh, C.B.; Lal, B.; Mellon, N.B. Influence of tetramethylammonium hydroxide on methane and carbon dioxide gas hydrate phase equilibrium conditions. Fluid Phase Equilibria 2017, 440, 1–8. [Google Scholar] [CrossRef]
- Kim, K.-S.; Kang, J.W.; Kang, S.-P. Tuning ionic liquids for hydrate inhibition. Chem. Commun. 2011, 47, 6341–6343. [Google Scholar] [CrossRef]
- Kim, N.-J.; Park, S.-S.; Kim, H.T.; Chun, W. A comparative study on the enhanced formation of methane hydrate using CM-95 and CM-100 MWCNTs. Int. Commun. Heat Mass Transf. 2011, 38, 31–36. [Google Scholar] [CrossRef]
- Nazari, K.; Moradi, M.R.; Ahmadi, A.N. Kinetic Modeling of Methane Hydrate Formation in the Presence of Low-Dosage Water-Soluble Ionic Liquids. Chem. Eng. Technol. 2013, 36, 1915–1923. [Google Scholar] [CrossRef]
- Menezes, D.É.S.d.; Pessôa Filho, P.d.A.; Robustillo Fuentes, M.D. Use of 1-butyl-3-methylimidazolium-based ionic liquids as methane hydrate inhibitors at high pressure conditions. Chem. Eng. Sci. 2020, 212, 115323. [Google Scholar] [CrossRef]
- Nasrollahebrahim, M.E.H.; Abareshi, B.; Ghotbi, C.; Taghikhani, V.; Amir, H. Investigation of Six Imidazolium-Based Ionic Liquids as Thermo-Kinetic Inhibitors for Methane Hydrate by Molecular Dynamics Simulation. In Proceedings of the National Iranian Conference on Gas Hydrate (Nicgh), Tehran, Iran, 15 May 2013. ffhal-01562325f. [Google Scholar]
- Lee, D.; Go, W.; Seo, Y. Experimental and computational investigation of methane hydrate inhibition in the presence of amino acids and ionic liquids. Energy 2019, 182, 632–640. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Shariff, A.M.; Mukhtar, H. Ammonium hydroxide ILs as dual-functional gas hydrate inhibitors for binary mixed gas (carbon dioxide and methane) hydrates. J. Mol. Liq. 2019, 274, 33–44. [Google Scholar] [CrossRef]
- Khan, M.S.; Liew, C.S.; Kurnia, K.A.; Cornelius, B.; Lal, B. Application of COSMO-RS in Investigating Ionic Liquid as Thermodynamic Hydrate Inhibitor for Methane Hydrate. Procedia Eng. 2016, 148, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Sulaimon, A.A.; Tajuddin, M.Z.M. Application of COSMO-RS for pre-screening ionic liquids as thermodynamic gas hydrate inhibitor. Fluid Phase Equilibria 2017, 450, 194–199. [Google Scholar] [CrossRef]
- Nashed, O.; Dadebayev, D.; Khan, M.S.; Bavoh, C.B.; Lal, B.; Shariff, A.M. Experimental and modelling studies on thermodynamic methane hydrate inhibition in the presence of ionic liquids. J. Mol. Liq. 2018, 249, 886–891. [Google Scholar] [CrossRef]
- Zare, M.; Haghtalab, A.; Ahmadi, A.N.; Nazari, K. Experiment and thermodynamic modeling of methane hydrate equilibria in the presence of aqueous imidazolium-based ionic liquid solutions using electrolyte cubic square well equation of state. Fluid Phase Equilibria 2013, 341, 61–69. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Nashed, O.; Rehman, A.N.; Othaman, N.A.A.B.; Lal, B.; Sabil, K.M. Ionic Liquids as Gas Hydrate Thermodynamic Inhibitors. Ind. Eng. Chem. Res. 2021, 60, 15835–15873. [Google Scholar] [CrossRef]
- Peng, X.; Hu, Y.; Liu, Y.; Jin, C.; Lin, H. Separation of ionic liquids from dilute aqueous solutions using the method based on CO2 hydrates. J. Nat. Gas Chem. 2010, 19, 81–85. [Google Scholar] [CrossRef]
- Li, X.-S.; Liu, Y.-J.; Zeng, Z.-Y.; Chen, Z.-Y.; Li, G.; Wu, H.-J. Equilibrium Hydrate Formation Conditions for the Mixtures of Methane + Ionic Liquids + Water. J. Chem. Eng. Data 2011, 56, 119–123. [Google Scholar] [CrossRef]
- Tumba, K.; Reddy, P.; Naidoo, P.; Ramjugernath, D.; Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Phase Equilibria of Methane and Carbon Dioxide Clathrate Hydrates in the Presence of Aqueous Solutions of Tributylmethylphosphonium Methylsulfate Ionic Liquid. J. Chem. Eng. Data 2011, 56, 3620–3629. [Google Scholar] [CrossRef]
- Richard, A.R.; Adidharma, H. The performance of ionic liquids and their mixtures in inhibiting methane hydrate formation. Chem. Eng. Sci. 2013, 87, 270–276. [Google Scholar] [CrossRef]
- Partoon, B.; Wong, N.M.S.; Sabil, K.M.; Nasrifar, K.; Ahmad, M.R. A study on thermodynamics effect of [EMIM]-Cl and [OH-C2MIM]-Cl on methane hydrate equilibrium line. Fluid Phase Equilibria 2013, 337, 26–31. [Google Scholar] [CrossRef]
- Keshavarz, L.; Javanmardi, J.; Eslamimanesh, A.; Mohammadi, A.H. Experimental measurement and thermodynamic modeling of methane hydrate dissociation conditions in the presence of aqueous solution of ionic liquid. Fluid Phase Equilibria 2013, 354, 312–318. [Google Scholar] [CrossRef]
- Sabil, K.M.; Nashed, O.; Lal, B.; Ismail, L.; Japper-Jaafar, A. Experimental investigation on the dissociation conditions of methane hydrate in the presence of imidazolium-based ionic liquids. J. Chem. Thermodyn. 2015, 84, 7–13. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Partoon, B.; Keong, L.K.; Bustam, A.B.; Mellon, N.B. Experimental Evaluation of a Novel Thermodynamic Inhibitor for CH4 and CO2 Hydrates. Procedia Eng. 2016, 148, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Altamash, T.; Khraisheh, M.; Qureshi, M.F.; Saad, M.A.; Aparicio, S.; Atilhan, M. Cost-effective alkylammonium formate-based protic ionic liquids for methane hydrate inhibition. J. Nat. Gas Sci. Eng. 2018, 58, 59–68. [Google Scholar] [CrossRef]
- Gupta, P.; Sakthivel, S.; Sangwai, J.S. Effect of aromatic/aliphatic based ionic liquids on the phase behavior of methane hydrates: Experiments and modeling. J. Chem. Thermodyn. 2018, 117, 9–20. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Nashed, O.; Lal, B.; Mellon, N.B. Impacts of ammonium based ionic liquids alkyl chain on thermodynamic hydrate inhibition for carbon dioxide rich binary gas. J. Mol. Liq. 2018, 261, 283–290. [Google Scholar] [CrossRef]
- Kazemi, F.; Javanmardi, J.; Aftab, S.; Mohammadi, A.H. Experimental study and thermodynamic modeling of the stability conditions of methane clathrate hydrate in the presence of TEACl and/or BMIM-BF4 in aqueous solution. J. Chem. Thermodyn. 2019, 130, 95–103. [Google Scholar] [CrossRef]
- Del Villano, L.; Kelland, M.A. An investigation into the kinetic hydrate inhibitor properties of two imidazolium-based ionic liquids on Structure II gas hydrate. Chem. Eng. Sci. 2010, 65, 5366–5372. [Google Scholar] [CrossRef]
- Lee, W.; Shin, J.-Y.; Cha, J.-H.; Kim, K.-S.; Kang, S.-P. Inhibition effect of ionic liquids and their mixtures with poly(N-vinylcaprolactam) on methane hydrate formation. J. Ind. Eng. Chem. 2016, 38, 211–216. [Google Scholar] [CrossRef]
- Kitajima, T.; Ohtsubo, N.; Hashimoto, S.; Makino, T.; Kodama, D.; Ohgaki, K. Study on Prompt Methane Hydration Derived by Addition of Ionic Liquid. Am. Chem. Sci. J. 2012, 2, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Rasoolzadeh, A.; Javanmardi, J.; Eslamimanesh, A.; Mohammadi, A.H. Experimental study and modeling of methane hydrate formation induction time in the presence of ionic liquids. J. Mol. Liq. 2016, 221, 149–155. [Google Scholar] [CrossRef]
- Lee, W.; Shin, J.-Y.; Kim, K.-S.; Kang, S.-P. Kinetic Promotion and Inhibition of Methane Hydrate Formation by Morpholinium Ionic Liquids with Chloride and Tetrafluoroborate Anions. Energy Fuels 2016, 30, 3879–3885. [Google Scholar] [CrossRef]
- Rasoolzadeh, A.; Javanmardi, J.; Mohammadi, A.H. An experimental study of the synergistic effects of BMIM-BF4, BMIM-DCA and TEACl aqueous solutions on methane hydrate formation. Pet. Sci. 2019, 16, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Lv, R.; Yan, Z.; Huang, F.; Zhang, X.; Chen, G.-J.; Yue, T. Design of Alanine-Rich Short Peptides as a Green Alternative of Gas Hydrate Inhibitors: Dual Methyl Group Docking for Efficient Adsorption on the Surface of Gas Hydrates. ACS Sustain. Chem. Eng. 2020, 8, 4256–4266. [Google Scholar] [CrossRef]
- Lee, D.; Go, W.; Ko, G.; Seo, Y. Inhibition synergism of glycine (an amino acid) and [BMIM][BF4] (an ionic liquid) on the growth of CH4 hydrate. Chem. Eng. J. 2020, 393, 124466. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; Solms, N.v. Screening of Amino Acids and Surfactant as Hydrate Promoter for CO2 Capture from Flue Gas. Processes 2020, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Koyama, Y.; Ihsan, A.B.; Kelland, M.A. Kinetic Hydrate Inhibition of Glycyl-valine-Based Alternating Peptoids with Tailor-Made N-Substituents. Energy Fuels 2020, 34, 4849–4854. [Google Scholar] [CrossRef]
- Abbasian Rad, S.; Rostami Khodaverdiloo, K.; Karamoddin, M.; Varaminian, F.; Peyvandi, K. Kinetic study of amino acids inhibition potential of Glycine and l-leucine on the ethane hydrate formation. J. Nat. Gas Sci. Eng. 2015, 26, 819–826. [Google Scholar] [CrossRef]
- Qureshi, M.F.; Khraisheh, M.; Almomani, F. Doping amino acids with classical gas hydrate inhibitors to facilitate the hydrate inhibition effect at low dosages. Greenh. Gases Sci. Technol. 2020, 10, 783–794. [Google Scholar] [CrossRef]
- Roosta, H.; Dashti, A.; Mazloumi, S.H.; Varaminian, F. Inhibition properties of new amino acids for prevention of hydrate formation in carbon dioxide–water system: Experimental and modeling investigations. J. Mol. Liq. 2016, 215, 656–663. [Google Scholar] [CrossRef]
- Altamash, T.; Qureshi, M.F.; Aparicio, S.; Aminnaji, M.; Tohidi, B.; Atilhan, M. Gas hydrates inhibition via combined biomolecules and synergistic materials at wide process conditions. J. Nat. Gas Sci. Eng. 2017, 46, 873–883. [Google Scholar] [CrossRef]
- Reitzer, L. Amino Acid Synthesis. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Sa, J.H.; Kwak, G.H.; Lee, B.R.; Park, D.H.; Han, K.; Lee, K.H. Hydrophobic amino acids as a new class of kinetic inhibitors for gas hydrate formation. Sci. Rep. 2013, 3, 2428. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.S.R.; Kiran, B.S. Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates? J. Nat. Gas Sci. Eng. 2018, 52, 461–466. [Google Scholar] [CrossRef]
- Sa, J.-H.; Kwak, G.-H.; Han, K.; Ahn, D.; Cho, S.J.; Lee, J.D.; Lee, K.-H. Inhibition of methane and natural gas hydrate formation by altering the structure of water with amino acids. Sci. Rep. 2016, 6, 31582. [Google Scholar] [CrossRef]
- Qureshi, M.F.; Khraisheh, M.; AlMomani, F. Probing the effect of various water fractions on methane (CH4) hydrate phase equilibria and hydrate inhibition performance of amino acid L-proline. J. Mol. Liq. 2021, 333, 115888. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Lee, P.Y.; Premasinghe, K.; Linga, P. Effect of Biofriendly Amino Acids on the Kinetics of Methane Hydrate Formation and Dissociation. Ind. Eng. Chem. Res. 2017, 56, 6145–6154. [Google Scholar] [CrossRef]
- Longinos, S.N.; Parlaktuna, M. Examination of asparagine, aspartic acid and threonine in methane (95%)-propane (5%) gas hydrates as kinetic inhibitors. React. Kinet. Mech. Catal. 2021, 134, 87–94. [Google Scholar] [CrossRef]
- Masri, A.N.; Sulaimon, A.A. Amino acid-based ionic liquids as dual kinetic-thermodynamic methane hydrate inhibitor. J. Mol. Liq. 2022, 349, 118481. [Google Scholar] [CrossRef]
- Mehrabi, K.; Javanmardi, J.; Rasoolzadeh, A.; Mohammadi, A.H. Thermodynamic modeling of clathrate hydrate stability conditions in the presence of amino acid aqueous solution. J. Mol. Liq. 2020, 313, 113488. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Khan, M.S.; Lal, B.; Bt Abdul Ghaniri, N.I.; Sabil, K.M. New methane hydrate phase boundary data in the presence of aqueous amino acids. Fluid Phase Equilibria 2018, 478, 129–133. [Google Scholar] [CrossRef]
- Long, Z.; Zhou, X.; He, Y.; Li, D.; Liang, D. Performance of mixture of ethylene glycol and glycine in inhibiting methane hydrate formation. J. Nat. Gas Sci. Eng. 2018, 56, 134–140. [Google Scholar] [CrossRef]
- Mannar, N.; Bavoh, C.B.; Baharudin, A.H.; Lal, B.; Mellon, N.B. Thermophysical properties of aqueous lysine and its inhibition influence on methane and carbon dioxide hydrate phase boundary condition. Fluid Phase Equilibria 2017, 454, 57–63. [Google Scholar] [CrossRef]
- Maddah, M.; Maddah, M.; Peyvandi, K. Molecular dynamics simulation of methane hydrate formation in presence and absence of amino acid inhibitors. J. Mol. Liq. 2018, 269, 721–732. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Kok Keong, L. Methane hydrate-liquid-vapour-equilibrium phase condition measurements in the presence of natural amino acids. J. Nat. Gas Sci. Eng. 2017, 37, 425–434. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Gonfa, G.; Foo Khor, S.; Sharif, A.M. Inhibition effect of amino acids on carbon dioxide hydrate. Chem. Eng. Sci. 2017, 171, 331–339. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; He, Y. Interaction of amino acid functional group with water molecule on methane hydrate growth. J. Nat. Gas Sci. Eng. 2021, 93, 104066. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Wang, R.; Lv, K.; Wang, J.; Liao, B.; Shi, X.; Wang, Q.; Qu, Y.; Huang, H. Mechanisms of synergistic inhibition of hydrophilic amino acids with kinetic inhibitors on hydrate formation. Fuel 2022, 321, 124012. [Google Scholar] [CrossRef]
- Wang, R.; Li, R.; Zhang, L.; Sun, J.; Sun, H.; Shi, X. Kinetic mechanism of hydrophilic amino acid inhibiting the formation of tetrahydrofuran (THF) hydrate. Nat. Gas Ind. B 2020, 7, 184–189. [Google Scholar] [CrossRef]
- Naeiji, P.; Arjomandi, A.; Varaminian, F. Amino acids as kinetic inhibitors for tetrahydrofuran hydrate formation: Experimental study and kinetic modeling. J. Nat. Gas Sci. Eng. 2014, 21, 64–70. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Yang, X.; He, Y. Examination of amino acid inhibitor effect in methane hydrate dissociation via molecular dynamics simulation. J. Mol. Liq. 2021, 325, 115205. [Google Scholar] [CrossRef]
- Lee, D.; Go, W.; Seo, Y. Synergistic kinetic inhibition of amino acids and ionic liquids on CH4 hydrate for flow assurance. Fuel 2020, 263, 116689. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Liu, Z.; Sun, X.; Zhao, L.; Shi, Y.; Zhou, G.; Rui, Z.; Lu, G. Effect of biofriendly amino acids on methane hydrate decomposition: Insights from molecular dynamics simulations. Fuel 2022, 325, 124919. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Nashed, O.; Khan, M.S.; Partoon, B.; Lal, B.; Sharif, A.M. The impact of amino acids on methane hydrate phase boundary and formation kinetics. J. Chem. Thermodyn. 2018, 117, 48–53. [Google Scholar] [CrossRef]
- Sa, J.-H.; Kwak, G.-H.; Han, K.; Ahn, D.; Lee, K.-H. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 2015, 5, 11526. [Google Scholar] [CrossRef] [Green Version]
- Perfeldt, C.M.; Chua, P.C.; Daraboina, N.; Friis, D.; Kristiansen, E.; Ramløv, H.; Woodley, J.M.; Kelland, M.A.; von Solms, N. Inhibition of Gas Hydrate Nucleation and Growth: Efficacy of an Antifreeze Protein from the Longhorn Beetle Rhagium mordax. Energy Fuels 2014, 28, 3666–3672. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Choudhary, N.; Kumar, A.; Chakrabarty, S.; Kumar, R. Effect of the amino acid l-histidine on methane hydrate growth kinetics. J. Nat. Gas Sci. Eng. 2016, 35, 1453–1462. [Google Scholar] [CrossRef]
- Kakati, H.; Mandal, A.; Laik, S. Promoting effect of Al2O3/ZnO-based nanofluids stabilized by SDS surfactant on CH4+C2H6+C3H8 hydrate formation. J. Ind. Eng. Chem. 2016, 35, 357–368. [Google Scholar] [CrossRef]
- Kakati, H.; Mandal, A.; Laik, S. Synergistic effect of Polyvinylpyrrolidone (PVP) and L-tyrosine on kinetic inhibition of CH4 + C2H4 + C3H8 hydrate formation. J. Nat. Gas Sci. Eng. 2016, 34, 1361–1368. [Google Scholar] [CrossRef]
- Talaghat, M.R. Experimental investigation of induction time for double gas hydrate formation in the simultaneous presence of the PVP and l-Tyrosine as kinetic inhibitors in a mini flow loop apparatus. J. Nat. Gas Sci. Eng. 2014, 19, 215–220. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Formation and Dissociation of CH4 and CO2 Hydrates in Presence of a Sediment Composed by Pure Quartz Mixed with Ti23 Particles. Materials 2022, 15, 1470. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Kang, D.W.; Ahn, Y.-H.; Lee, W.; Cha, M.; Lee, J.W. Recoverable magnetic nanoparticles as hydrate inhibitors. Chem. Eng. J. 2020, 389, 124461. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Shi, L.; Long, Z.; Lu, J.; Liang, D. Study on the hydrate inhibition effect of nano-silica in drilling fluids. J. Nat. Gas Sci. Eng. 2022, 105, 104688. [Google Scholar] [CrossRef]
- Javidani, A.; Abedi Farizhendi, S.; Mohammadi, A.; Hassan, H.; Mohammadi, A.; Manteghian, M. The effects of graphene oxide nanosheets and Al2O3 nanoparticles on the kinetics of methane + THF hydrate formation at moderate conditions. J. Mol. Liq. 2020, 316, 113872. [Google Scholar] [CrossRef]
- Nesterov, A.N.; Reshetnikov, A.M.; Manakov, A.Y.; Rodionova, T.V.; Paukshtis, E.A.; Asanov, I.P.; Bardakhanov, S.P.; Bulavchenko, A.I. Promotion and inhibition of gas hydrate formation by oxide powders. J. Mol. Liq. 2015, 204, 118–125. [Google Scholar] [CrossRef]
- Park, S.-S.; Lee, S.-B.; Kim, N.-J. Effect of multi-walled carbon nanotubes on methane hydrate formation. J. Ind. Eng. Chem. 2010, 16, 551–555. [Google Scholar] [CrossRef]
- Li, J.; Liang, D.; Guo, K.; Wang, R.; Fan, S. Formation and dissociation of HFC134a gas hydrate in nano-copper suspension. Energy Convers. Manag. 2006, 47, 201–210. [Google Scholar] [CrossRef]
- Yu, Y.-S.; Zhou, S.-D.; Li, X.-S.; Wang, S.-L. Effect of graphite nanoparticles on CO2 hydrate phase equilibrium. Fluid Phase Equilibria 2016, 414, 23–28. [Google Scholar] [CrossRef]
- Mohammadi, M.; Haghtalab, A.; Fakhroueian, Z. Experimental study and thermodynamic modeling of CO2 gas hydrate formation in presence of zinc oxide nanoparticles. J. Chem. Thermodyn. 2016, 96, 24–33. [Google Scholar] [CrossRef]
- Wang, R.; Liu, T.; Ning, F.; Ou, W.; Zhang, L.; Wang, Z.; Peng, L.; Sun, J.; Liu, Z.; Li, T.; et al. Effect of hydrophilic silica nanoparticles on hydrate formation: Insight from the experimental study. J. Energy Chem. 2019, 30, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, M.H.; Manteghian, M.; Naeiji, P. Study the effect of Ag nanoparticles on the kinetics of CO2 hydrate growth by molecular dynamics simulation. J. Mol. Liq. 2021, 343, 117668. [Google Scholar] [CrossRef]
- Lee, D.; Jeoung, S.; Moon, H.R.; Seo, Y. Recoverable and recyclable gas hydrate inhibitors based on magnetic nanoparticle-decorated metal–organic frameworks. Chem. Eng. J. 2020, 401, 126081. [Google Scholar] [CrossRef]
- Xu, M.; Fang, X.; Ning, F.; Ou, W.; Zhang, L.; Wang, D. Effect of hydrophilic silica nanoparticles on hydrate formation during methane gas migration in a simulated wellbore. Petroleum 2021, 7, 485–495. [Google Scholar] [CrossRef]
- Golpour, M.; Pakizeh, M. Development of a new nanofiltration membrane for removal of kinetic hydrate inhibitor from water. Sep. Purif. Technol. 2017, 183, 237–248. [Google Scholar] [CrossRef]











| Hydrate Inhibitor | Pros | Cons |
|---|---|---|
| Methanol |
|
|
| MEG |
|
|
| Polymer | Chemical Structure | Molecular Weight (Da) | Ref |
|---|---|---|---|
| PVP |  | 5000–16,000 | [77] |
| PVCap |  | 20,000 | [78] |
| PVPip |  | 4000–85,000 | [79] |
| PVP-VA73 |  | 38,000–40,000 | [80] |
| VC-713 |  | 82,700 | [81] |
| VCap/VP |  | 1000–6,000,000 | [82] |
| Inhibex 501 |  | 5000–8000 | [83] |
| Luvicap EG |  | 2000 | [84] |
| PVP Type | Monomer | Monomer | Molecular Structure | Reference |
|---|---|---|---|---|
| PVP | Vinylpyrrolidone |  | [87] | |
| PVP-A | Vinylpyrrolidone | Butyl acrylate |  | [88] |
| PVP-ME | Vinylpyrrolidone | Methyl acrylate |  | [89] |
| PVP-PE | Vinylpyrrolidone | Propyl acrylate |  | [89] |
| PVP-EE | Vinylpyrrolidone | Ethyl acrylate |  | [89] |
| Hydrate Inhibitor | Hydrate Type | Function | Inhibition Effect | Reference |
|---|---|---|---|---|
| PVP | Methane–propane hydrate | KHI | The methane–propane hydrate was successfully inhibited by increasing the induction time of the hydrate formation. | [92] |
| PVP ramification (PVP-BP) | Methane hydrate | KHI | Hindered the formation of methane gas hydrate. | [93] |
| PVP | Methane hydrate | KHI | The hydrate was successfully inhibited. | [94] |
| PVP | Methane hydrate | KHI | The formation of methane hydrate was prohibited successfully. | [95] |
| PVP-K90 | Methane hydrate | KHI | The hydrate nucleation time was prolonged. Thus, the methane hydrate growth was hindered. | [96] |
| PVP | Structure II (SII) gas hydrate | KHI | Prolonged the development of gas hydrate crystals | [79] |
| PVP | Tetrahydrofuran (THF) clathrate hydrate | KHI | The formation of tetrahydrofuran (THF) was prohibited successfully | [97] |
| PVP | Natural gas hydrate | KHI | Prolonged the development of natural gas hydrate | [98] |
| PVP | Structure II methane–propane hydrate | KHI | Prevented the methane–propane hydrate growth | [99] |
| PVP | Natural gas hydrate | KHI | Successfully inhibited the formation of hydrate | [100] |
| Inhibitor Type | Hydrate Type | Function | Inhibition Effect | Reference |
|---|---|---|---|---|
| PVCap | Methane hydrate | KHI | Increased the dissociation rate of methane hydrate. | [78] |
| PVCap | Cyclopentane Hydrate | KHI | Hydrate film growth was significantly slowed, requiring over 2 h for complete conversion. | [106] |
| PVCap | Methane hydrate | KHI | Sped up the methane hydrate dissociation | [107] |
| PVCap | Methane hydrate | KHI | Increased the methane hydrate dissociation temperature. | [108] |
| PVCap | Methane hydrate | KHI | Increased dissociation temperature | [109] |
| PVCap | Methane hydrate | KHI | Increased the induction time of methane hydrate formation by approximately 1400 min. | [110] |
| PVCap | Natural gas hydrate | KHI | Hindered the formation of the hydrate | [111] |
| PVCap | Methane hydrate | KHI | Suppressed the methane hydrate formation by hindering the nucleation of methane hydrate crystals | [112] |
| PVCap | Methane hydrate | KHI | Hindered the formation of methane gas hydrate. | [100] |
| PVCap | Natural gas hydrate | KHI | Stopped the formation of the gas hydrate | [113] |
| PVCap | Tetrahydrofuran hydrate | KHI | Inhibited the formation of tetrahydrofuran hydrate. | [114] |
| PVCap | Tetrahydrofuran hydrate | KHI | Inhibited the formation of THF hydrate by delaying the nucleation process of the hydrate. | [115] |
| PVCap | Natural gas hydrate | KHI | Hindered the formation of gas hydrate by delaying the onset time of the hydrate significantly. | [116] |
| PVCap | Tetrahydrofuran hydrate | KHI | Inhibited the formation of THF hydrate. | [117] |
| PVCap | Natural gas hydrate | KHI | Successfully inhibited the natural gas hydrate formation, by delaying the hydrate onset time. | [118] |
| PVCap | Tetrahydrofuran hydrate | KHI | Inhibited the formation of THF hydrate. | [119] |
| PVCap | Methane hydrate | KHI | Hindered the formation of methane gas hydrate. | [120] |
| PVCap | Natural gas hydrate | KHI | Successfully inhibited the development of natural gas hydrate. | [121] |
| PVCap | Methane hydrate | KHI | Hindered the formation of methane gas hydrate. | [122] |
| Inhibitor Type | Hydrate Type | Function | Inhibition Effect | References |
|---|---|---|---|---|
| Quaternary ammonium salt | Cyclopentane hydrates | AAs | Decreased the hydrate slurry. | [135] |
| Fluorinated Quaternary Ammonium Bromides | Tetrahydrofuran hydrate | AAs | Successfully reduced tetrahydrofuran hydrate slurry. | [114] |
| Quaternary ammonium salt | Methane–propane sII hydrate | AAs | Decreased the slurry of methane–propane sII hydrate. | [136] |
| Quaternary ammonium surfactants | Natural gas sII hydrate | AAs | Inhibited the hydrate successfully. | [137] |
| Quaternary ammonium salts | Natural gas hydrate | AAs | Decreased the hydrate slurry and inter-particle interactions. | [138] |
| Ionic Liquid | Hydrate Type | Function | Concentration | References |
|---|---|---|---|---|
| [C4C1im][BF4] [C4C1im][BF4] [C2C1im][N(CN)2] [C2C1][CF3SO3] [C2C1im][C2SO4] | CH4 | KHI/THI | 0.1–10 10 10 10 10 | [55] |
| [C4C1im][BF4] [C4C1im][C1SO4] [OHC2C1Pyrr]Cl [OHC2C1Pyrr][BF4] [C4C1Pyrr]Br [C4C1Pyrr][BF4] [2-(OHC2)C1Mor]Br [2-(OHC2)C1Mor] [BF4] | CH4 | THI/KHI | 0.6–7 wt% & 0.1–10 wt% | [139] |
| [C2C1im]Cl [C4C1im]Cl [C2C1im]Br [C4C1im]Br [C3C1im]I [C4C1im]I | CH4 | KHI/THI | 10 | [140] |
| [C4C1im][BF4] | CO2 | KHI/THI | 0.0008–0.1 mol% | [141] |
| [2-(OHC2)C1Mor][BF4] [C2C1im][NO3] [C2C1im][BF4] [C2C1im] [CF3SO3] | CO2 | THI/KHI | 0.10–1.04 mol% | [142] |
| TMACl TEAOH TPrAOH | CO2 | THI | 1 wt% 5 wt% 10 wt% | [143,144,145] |
| [C2C1im][BF4] [2-(OHC2)C1Pyrr][BF4] [C4C1Pyrr][BF4] | CH4 | THI/KHI | 0.1–10 wt% | [146,147] |
| [BMIM][BF4] BMIM][MeSO4] [BMIM][HSO4] | CH4 | THI/KHI | 0.6 wt% | [148] |
| [BMIM][Cl], [BMIM][Br] | CH4 | THI/KHI | 1 wt%, 5 wt%, 10 wt%, 15 wt% | [149] |
| [2,3-(OHC3)2C1im][f2N] [2-(OHC2)C1im][f2N] [C2C1im][BF4] [C4C1im] [BF4] [C4C1im][OAc] [C2C1im][C2SO4] | CH4 | THI/KHI | 0.5–1.0 wt% | [150] |
| [BMIM][BF4], [BMIM][I] | CH4 | THI/KHI | 3 mol% | [151] |
| TEAOH, TPrAOH, TBAOH | CO2/CH4 | THI/KHI | 40 wt% | [152] |
| TMAOH, | CO2/CH4 | THI/KHI | 25 wt% | [152] |
| Ionic Liquid | Hydrate Type | Function | Concentration | References |
|---|---|---|---|---|
| TMACl TEAOH TPrAOH | CO2 | THI | 1 wt% 5 wt% 10 wt% | [143,144,145] |
| [C4C1im]Cl [C6C1im]Cl [C8C1im]Cl [C4C1im][BF4] [C6C1im][BF4] [C4C1im][TFA] [C4C1im][PF6] | CO2 | THI | - | [158] |
| [C1C1im]I [C2C1im]I [OHC2C1im]Cl [N1111]Cl [N111C2OH]Cl | CH4 | THI | 0.1 MF | [159] |
| [C4C1im][C1SO4] [C2C1im][HSO4] [C2C1im][C2SO4] [C4C1im][BF4] [2- (OHC2)C1im][BF4] | CH4 | THI | 10 wt% 10 wt% 8–10 wt% 10 wt% 10–20 wt% | [156] |
| [P4441][C1SO4] | CH4, CO2 | THI | 0.2611–0.5007 MF | [160] |
| [C2C1im]Cl [C2C1im]Br [C2C1im]Br + [C2C1im]Cl (1:1) [C2C1im]Cl + MEG (1:1 [C2C1im]Cl + NaCl (1:1) | CH4 | THI | 5–40 wt% 20 wt% 20 wt% 10–30 wt% 10 wt% | [161] |
| [C2C1im]Cl [(OHC2)C1im]Cl | CH4 | THI | wt% | [162] |
| [C4C1im][BF4] [C4C1im][N(CN)2] [N2222]Cl | CH4 | THI | 0.10–0.20 MF 0.1 MF 0.1 MF | [163] |
| [OH-EMIM][Cl] [OH-EMIM][Br] [BMIM][Br] [BMIM][Cl] [BMIM] [ClO4] [BMIM][N(CN)2] [BMIM][HSO4] [BMIM][CF3SO3] [BMIM] [CH3SO4] | CH4 | THI | 0.1 MF | [164] |
| TMAOH | CH4/CO2 | THI | 10 wt% | [165] |
| [EA][Of], [DMEA][Of], [DMA][Of] | CH4 | THI | 5 wt%, 10 wt% | [166] |
| [BMIM][Cl] [OMIM][Cl] [BMIM][Br] [HMIM][Br] [BMIM][HSO4] [ET2NH2][HSO4] [Pr3NH][HSO4] [Bu3NH][HSO4] | CH4 | THI | 0.01 MF | [167] |
| TMAOH | CO2 (70%) + CH4 (30%) | THI | 25 wt% | [168] |
| TEAOH, TPrAOH, TBAOH | CO2 (70%) + CH4 (30%) | THI | 40 wt% | [168] |
| TEACl + BMIM-BF4 | CH4 | THI | 4.55 + 4.85 wt% | [169] |
| Ionic Liquid | Hydrate Type | Function | Concentration | References |
|---|---|---|---|---|
| [C2C1im][BF4] [C4C1im][BF4] | SNG | KHI | 5000–10,000 ppm 10,000 ppm | [170] |
| [C4C1im][PF6] | CH4 | KHI | 0–1000 ppm | [172] |
| [BMIM][BF4] | CH4 | KHI | 1 wt%, 10 wt%, 15 wt%, 20 wt% | [173] |
| BMIM-DCA, TEACL | CH4 | KHI | 10 wt% | [173] |
| EMIM-BF4 EMP-BF4 EMP-Br EMP-Cl BMP-BF4 BMP-Br BMP-ClHEMP-BF4 HEMP-Cl | CH4 | KHI | 0.5 wt%, 1.0 wt%, 2.0 wt%, 3.0 wt% | [171,174] |
| [OH-EMIM][Br], [BMIM][Br] [OH-EMIM][Cl] [BMIM][Cl], [BMIM][CF3SO3] [BMIM][CiO4] [BMIM][N(CN)2], [BMIM][HSO4] | CH4 | KHI | 0.97 MF 0.09 MF 0.95 MF 0.93 MF 0.94 MF | [154] |
| [BMIM][BF4] [BMIM][DCA] TEACl | CH4 | KHI | 1–20 wt% | [175] |
| Amino Acid | Structure | Weight (g/mol) | References |
|---|---|---|---|
| Alanine |  | 89.09 | [176] |
| Glycine |  | 75.07 | [177] |
| Valine |  | 117.151 | [178,179] |
| Leucine |  | 131.17 | [180] |
| Histidine |  | 155.1546 | [181] |
| Glutamine |  | 146.14 | [182] |
| Phenylalanine |  | 165.19 | [183] |
| Isoleucine |  | 131.17 | [184,185] |
| Threonine |  | 119.1192 | [186] |
| Serine |  | 105.09 | [187] |
| Proline |  | 115.13 | [188,189] |
| Asparagine |  | 132.12 | [190] |
| Amino Acid | Hydrate Type | Function | Concentration | References |
|---|---|---|---|---|
| Glycine L-Alanine L-Valine | CO2 | THI | 0.1–3.0 mol% 0.1–2.2 mol% 0.1–0.5 mol% | [176] |
| Glycine Alanine Serine Proline | CH4 | THI | 0.5–3 wt% 0.5–2.2 wt% 1.3–3 wt% 1.3–9 wt% | [187] |
| Glycine Alanine Serine Proline Arginine | CH4 | THI | 5–20 wt% 10 wt% 10 wt% 10 wt% 10 wt% | [197,198] |
| Glycine Alanine Serine Proline Arginine | CO2 | THI | 5–20 wt% 10 wt% 10 wt% 10 wt% 10 wt% | [197,198] |
| Lysine | CH4 | THI | 5–10 wt% | [195] |
| Lysine | CO2 | THI | 5–10 wt% | [195] |
| Amino Acid | Hydrate Type | Function | Concentration | References |
|---|---|---|---|---|
| Glycine L-Alanine L-Valine Leucine Isoleucine | CO2 | KHI | 0.01–1.0 mol% 0.1 mol% 0.1 mol% 0.1 mol% 0.1 mol% | [179] |
| L-Alanine Aspartic acid Asparagine Phenylalanine Histidine | CO2 | KHI | 0.01–0.1 mol% 0.01 mol% 0.01 mol% 0.1 mol% 0.1 mol% | [207] |
| Glycine Leucine | C2H6 | KHI | 0.05–3 wt% | [180] |
| Glycine Leucine | THF | KHI | 0.05–1.5 wt% | [202] |
| L-threonine L-valine | CH4 | KHI | 2770–1385 ppm | [208] |
| L-histidine | CH4 | KHI | 0.1–1 wt% | [209] |
| PVP and L-Tyrosine | NG | KHI | 1 wt% | [210,211] |
| PVP and L-Tyrosine | NG | KHI | 100–275 ppm | [212] |
| Glycine Alanine Serine Proline | CH4 | KHI | 0.1 wt% | [187] |
| Glycine Proline Serine Threonine Glutamine Histidine | CO2 | KHI | 0.5–2 wt% | [182] |
| Nanoparticle | Three Dimensional Structure (3D) | Hydrate Inhibition Effect | Reference |
|---|---|---|---|
| Zinc oxide |  | Acts as a THI by shifting the hydrate equilibrium curve to a region with higher pressure and lower temperature | [213] |
| Magnetite |  | Functions as both kinetic inhibitor and anti-agglomerate. | [214] |
| Silica |  | Acts as KHI by increasing the induction time of the gas hydrate. | [215] |
| Graphene |  | Effectively inhibits hydrates formation. | [216] |
| TiO2 |  | Inhibits the hydrate formation by increasing the induction time. | [217] |
| Carbon nanotubes (CNTs) |  | Inhibits the hydrate formation by increasing the induction time. | [218] |
| THI Type | Drawback | Reason |
|---|---|---|
| THIs | High concentration is required to maintain high hydrate inhibition efficiency. | THIs have high water solubility and volatility. |
| THIs | High overall cost. | High volumes of THIs are required to efficiently inhibit hydrates. |
| Alcohols and glycols | Health hazard. Therefore, strict environmental regulations are required for their usage. | Alcohols and glycols are highly toxic. |
| Methanol | Health hazard. Hence, rigorous environmental laws are required for its application. | Highly flammable, due to its low flash point (11 °C). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhenawy, S.; Khraisheh, M.; Almomani, F.; Al-Ghouti, M.A.; Hassan, M.K.; Al-Muhtaseb, A. Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques. Energies 2022, 15, 8551. https://doi.org/10.3390/en15228551
Elhenawy S, Khraisheh M, Almomani F, Al-Ghouti MA, Hassan MK, Al-Muhtaseb A. Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques. Energies. 2022; 15(22):8551. https://doi.org/10.3390/en15228551
Chicago/Turabian StyleElhenawy, Salma, Majeda Khraisheh, Fares Almomani, Mohammad A. Al-Ghouti, Mohammad K. Hassan, and Ala’a Al-Muhtaseb. 2022. "Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques" Energies 15, no. 22: 8551. https://doi.org/10.3390/en15228551