A Review of the Emulsification Method for Alternative Fuels Used in Diesel Engines
Abstract
:1. Introduction
2. Biofuel Future and Present
2.1. The Use of Biofuel in Internal Combustion (IC) Engines
2.2. Alternative Fuels Performance and Emissions Behaviour in CI Engines
3. Background of Emulsified Biofuel
3.1. Dispersed System in the Emulsified Biofuel
3.2. The Micro-Explosion Phenomenon
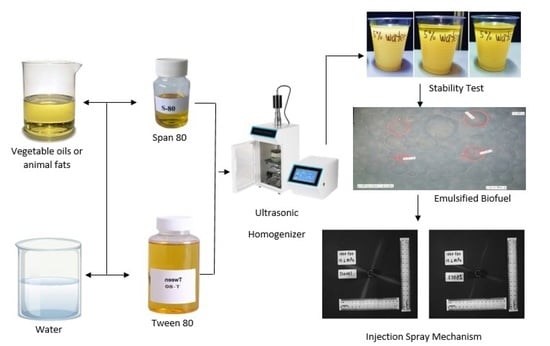
4. Formulation of Emulsified Biofuels: Materials and Procedures
4.1. Preparation of Emulsified Biofuels
| Chemical Name | Molecular Formula | HLB Number | Reference |
|---|---|---|---|
| Sorbitan monooleate (Span 80) | C24H44O6 | 4.3 | [91] |
| Sorbitan monooleate (Span 80) | C64H124O26 | 15.0 | [91] |
| Sorbitan monostearate (Span 60) | C24H46O6 | 4.7 | [92] |
| Tetraethylene glycol dodecyl ether (Brij 30) | C20H42O5 | 9.7 | [93] |
| Sorbitan monopalmitate (Span 40) | C22H42O6 | 6.7 | [94] |
| Sodium bis-[2-ethylhexyl] sulfosuccinate (AOT) | C20H37O7SNa | 10.2 | [93] |
| Sorbitan sesquiolate (Span 83) | C66H130O18 | 3.7 | [95] |
| Oleic acid | C18H34O2 | 1.0 | [92] |
| Sorbitan trioleate (Span 85) | C60H108O8 | 1.8 | [91] |
4.2. Physicochemical Characteristics of Stability
4.2.1. Stability
4.2.2. Stability Classes
4.2.3. Factor Affecting Biofuel Emulsion Stability
HLB Value Effect on the Emulsion Stability
Effect of Additives
Effect of Emulsifier Type Used on Emulsion Stability
Co-Surfactant Effect on the Emulsion Stability
The Effect of WATER Concentration on the Stability of the Emulsion
The Effect of the Type of Emulsion Used
5. Evaluation of the Performance of Diesel Engines Running on Emulsified Biofuels
5.1. Emission and Overall Engine Performance
5.2. Effect of Emulsified Biofuels on Friction and Wear, Lubricant, and Endurance of Engine Components
6. Conclusions
- Despite the various feedstocks, emulsified biofuels are possibly formulated from vegetable oils or animal fats. Some factors affect emulsified biofuel stability, such as base biofuel, surfactant, co-surfactant, HLB value of surfactant, and alcohol. A surfactant, co-surfactant, and water composition optimised for a particular base biofuel may result in the greatest stability, as has been documented. Additionally, microemulsions have a greater degree of stability than other kinds of emulsions.
- The viscosity, density, and cetane number of an emulsified biofuel system increase as the water concentration increases. On the other hand, amplified water concentration decreases the heating value and flash point of emulsified biofuel. Nevertheless, emulsified biofuel is capable of improving characteristics such as the combustion rate and emission levels.
- The reduced calorific value of emulsified biofuel results in a rise in BSFC, BP, and torque, although the gain is not substantial in comparison to diesel.
- As a result of water presence in emulsified biofuel, ignition is delayed. At low loads, this effect reduces the peak pressure of combustion.
- The water vaporisation in the combustion chamber operates as a heat sink. Consequently, the combustion chamber’s temperature will be decreased, and accordingly, NOx will be reduced.
- The phenomenon of micro-explosion has been found to lower the BSFC and improve the engine brake thermal efficiency (BTE).
- Using emulsified biofuel improves injection and atomisation due to the micro-explosion effect the instant emulsified biofuel droplets make their way into the combustion chamber.
- The emulsified biofuel reduced the emissions of HC, CO, PM, CO2, and NOx in comparison to conventional diesel fuel.
7. Recommendations
- The method and procedure for making emulsified biofuel ought to be cost-effective, simple to manage, efficient, and stable in formulation.
- The major drawback of emulsified biofuel lies in its reduced calorific value. This problem needs to be addressed by an extensive study on additional additives to improve energy efficiency.
- The advantages and disadvantages should be clearly emphasised in the areas of ignition delay and heat sink effectiveness.
- Additional research is needed to overcome the constraints associated with reduced BP and torque with regard to emulsified biofuels.
- Despite the emulsified biofuel being irrelevant to engine corrosion, further explanation is still needed regarding the phenomenon of water vapour formation when it condenses in the combustion chamber. Thus, corrosion analysis must be performed on the cylinder wall.
- Due to differences in the density of water and biofuel, emulsified biofuels result in better injection. Thus, an in-depth review of the findings is required to estimate the improvement in the extent of injection.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | Description |
| CI | Compression ignition |
| BTE | Brake thermal efficiency |
| HRR | Heat release rate |
| HC | Hydrocarbon |
| CO | Carbon monoxide |
| CSOME | Cottonseed oil methyl ester |
| NOx | Nitrogen oxides |
| PM | Particulate matter |
| SOx | Sulphur oxides |
| HC | Hydrocarbons |
| CO2 | Carbon dioxide |
| CR | Compression ratio |
| COME | Coconut oil methyl ester |
| BSFC | Brake specific fuel consumption |
| HLB | Hydrophilic-lipophilic balance |
| W/O | Water in oil |
| O/W | Oil in water |
| Gf | Gibb free energy |
| EMA | Engine manufacturer association |
| GHG | Greenhouse gas |
| IEA | International energy agency |
| EGT | Exhaust gas temperature |
| MRPR | Maximum rate of pressure rise |
| IC | Internal combustion |
References
- Subramaniam, M.; Solomon, J.M.; Nadanakumar, V.; Anaimuthu, S.; Sathyamurthy, R. Experimental Investigation on Performance, Combustion and Emission Characteristics of DI Diesel Engine Using Algae as a Biodiesel. Energy Rep. 2020, 6, 1382–1392. [Google Scholar] [CrossRef]
- Reğitoʇlu, I.A.; Altinişik, K.; Keskin, A. The Pollutant Emissions from Diesel-Engine Vehicles and Exhaust Aftertreatment Systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Che Mat, S.; Idroas, M.Y.; Hamid, M.F.; Zainal, Z.A. Performance and Emissions of Straight Vegetable Oils and Its Blends as a Fuel in Diesel Engine: A Review. Renew. Sustain. Energy Rev. 2018, 82, 808–823. [Google Scholar] [CrossRef]
- Ashraful, A.M.; Masjuki, H.H.; Kalam, M.A.; Rizwanul Fattah, I.M.; Imtenan, S.; Shahir, S.A.; Mobarak, H.M. Production and Comparison of Fuel Properties, Engine Performance, and Emission Characteristics of Biodiesel from Various Non-Edible Vegetable Oils: A Review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Vafakish, B. Biodiesel Production by Transesterification of Tallow Fat Using Heterogeneous Catalysis. Kemija u Industriji/J. Chem. Chem. Eng. 2017, 66, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Chozhavendhan, S.; Vijay Pradhap Singh, M.; Fransila, B.; Praveen Kumar, R.; Karthiga Devi, G. A Review on Influencing Parameters of Biodiesel Production and Purification Processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Melo-Espinosa, E.A.; Piloto-Rodríguez, R.; Goyos-Pérez, L.; Sierens, R.; Verhelst, S. Emulsification of Animal Fats and Vegetable Oils for Their Use as a Diesel Engine Fuel: An Overview. Renew. Sustain. Energy Rev. 2015, 47, 623–633. [Google Scholar] [CrossRef]
- Maher, K.D.; Bressler, D.C. Pyrolysis of Triglyceride Materials for the Production of Renewable Fuels and Chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef]
- Duan, X.; Lai, M.C.; Jansons, M.; Guo, G.; Liu, J. A Review of Controlling Strategies of the Ignition Timing and Combustion Phase in Homogeneous Charge Compression Ignition (HCCI) Engine. Fuel 2021, 285, 119142. [Google Scholar] [CrossRef]
- Duan, X.; Xu, Z.; Sun, X.; Deng, B.; Liu, J. Effects of Injection Timing and EGR on Combustion and Emissions Characteristics of the Diesel Engine Fuelled with Acetone–Butanol–Ethanol/Diesel Blend Fuels. Energy 2021, 231, 121069. [Google Scholar] [CrossRef]
- Sathish, T.; Singaravelu, D.K. Combustion Analysis Using Third Generation Biofuels in Diesel Engine. J. Sci. Ind. Res. 2020, 79, 449–452. [Google Scholar]
- Dong, S.; Wang, Z.; Yang, C.; Ou, B.; Lu, H.; Xu, H.; Cheng, X. Investigations on the Effects of Fuel Stratification on Auto-Ignition and Combustion Process of an Ethanol/Diesel Dual-Fuel Engine. Appl. Energy 2018, 230, 19–30. [Google Scholar] [CrossRef]
- Kumar, M.S.; Jaikumar, M. A Comprehensive Study on Performance, Emission and Combustion Behavior of a Compression Ignition Engine Fuelled with WCO (Waste Cooking Oil) Emulsion as Fuel. J. Energy Inst. 2014, 87, 263–271. [Google Scholar] [CrossRef]
- Ashok, B.; Gopal, K.N.; Rajagopal, T.K.R.; Alagiasingam, S.; Appu, S.; Murugan, A. Design and Analysis of a Fuel Preheating Device for Evaluation of Ethanol Based Biofuel Blends in a Diesel Engine Application. SAE Int. J. Engines 2017, 10, 39–45. [Google Scholar] [CrossRef]
- Mekonen, M.W.; Sahoo, N. Effect of Fuel Preheating with Blended Fuels and Exhaust Gas Recirculation on Diesel Engine Operating Parameters. Renew. Energy Focus 2018, 26, 58–70. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Li, X.; Zhou, W.; Wang, C.; Wang, S. Explosion Characteristics of a Pyrolysis Biofuel Derived from Rice Husk. J. Hazard. Mater. 2019, 369, 324–333. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Hallajisani, A.; Alizadehdakhel, A.; Nouralishahi, A. Biofuel Production through Micro- and Macroalgae Pyrolysis—A Review of Pyrolysis Methods and Process Parameters. J. Anal. Appl. Pyrolysis 2019, 142, 104599. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current Biodiesel Production Technologies: A Comparative Review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A Review. Energies 2020, 13, 2204. [Google Scholar] [CrossRef]
- Atmanli, A.; Yüksel, B.; Ileri, E. Experimental Investigation of the Effect of Diesel-Cotton Oil-n-Butanol Ternary Blends on Phase Stability, Engine Performance and Exhaust Emission Parameters in a Diesel Engine. Fuel 2013, 109, 503–511. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Rajamanoharan, K. Experimental Investigations of Performance and Emissions of Karanja Oil and Its Blends in a Single Cylinder Agricultural Diesel Engine. Appl. Energy 2009, 86, 106–112. [Google Scholar] [CrossRef]
- Akubude, V.C.; Nwaigwe, K.N.; Dintwa, E. Production of Biodiesel from Microalgae via Nanocatalyzed Transesterification Process: A Review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Hamid, M.F.; Idroas, M.Y.; Sa’ad, S.; Saiful Bahri, A.J.; Sharzali, C.M.; Abdullah, M.K.; Zainal, Z.A. Numerical Investigation of In-Cylinder Air Flow Characteristic Improvement for Emulsified Biofuel (EB) Application. Renew. Energy 2018, 127, 84–93. [Google Scholar] [CrossRef]
- Hamid, M.F.; Mohamad Yusof, I.; Saiful Bahri, A.J.; Sa’ad, S.; Zainal, Z.A. Experimental Study of the Effect of Surfactant Agent Formulation to the Spray Characteristics of Emulsified Biofuel. ARPN J. Eng. Appl. Sci. 2016, 11, 7451–7456. [Google Scholar]
- Avulapati, M.M.; Megaritis, T.; Xia, J.; Ganippa, L. Experimental Understanding on the Dynamics of Micro-Explosion and Puffing in Ternary Emulsion Droplets. Fuel 2019, 239, 1284–1292. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Ganippa, L.C.; Xia, J.; Megaritis, A. Puffing and Micro-Explosion of Diesel-Biodiesel-Ethanol Blends. Fuel 2016, 166, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Sazhin, S.S.; Rybdylova, O.; Crua, C.; Heikal, M.; Ismael, M.A.; Nissar, Z.; Aziz, A.R.B.A. A Simple Model for Puffing/Micro-Explosions in Water-Fuel Emulsion Droplets. Int. J. Heat Mass Transf. 2019, 131, 815–821. [Google Scholar] [CrossRef]
- Shen, S.; Sun, K.; Che, Z.; Wang, T.; Jia, M.; Cai, J. Mechanism of Micro-Explosion of Water-in-Oil Emulsified Fuel Droplet and Its Effect on Soot Generation. Energy 2020, 191, 116488. [Google Scholar] [CrossRef]
- Huang, X.; Wang, J.; Wang, Y.; Qiao, X.; Ju, D.; Sun, C.; Zhang, Q. Experimental Study on Evaporation and Micro-Explosion Characteristics of Biodiesel/n-Propanol Blended Droplet. Energy 2020, 205, 118031. [Google Scholar] [CrossRef]
- Meng, K.; Fu, W.; Li, F.; Lei, Y.; Lin, Q.; Wang, G. Comparison of Ignition, Injection and Micro-Explosion Characteristics of RP-3/Ethanol and Biodiesel/Ethanol Mixed Drops. J. Energy Inst. 2020, 93, 152–164. [Google Scholar] [CrossRef]
- Ismael, M.A.; Heikal, M.R.; Aziz, A.R.A.; Crua, C.; El-Adawy, M.; Nissar, Z.; Baharom, M.B.; Zainal, E.Z.A. Firmansyah Investigation of Puffing and Micro-Explosion of Water-in-Diesel Emulsion Spray Using Shadow Imaging. Energies 2018, 11, 2281. [Google Scholar]
- Martins, J.; Brito, F.P. Alternative Fuels for Internal Combustion Engines. Energies 2020, 13, 4086. [Google Scholar] [CrossRef]
- IEA—International Energy Agency. World Energy Outlook; IEA: Paris, France, 2018. [Google Scholar]
- Mat, S.C.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. Physicochemical, Performance, Combustion and Emission Characteristics of Melaleuca Cajuputi Oil-Refined Palm Oil Hybrid Biofuel Blend. Energies 2018, 11, 3146. [Google Scholar] [CrossRef]
- Malaquias, A.; Netto, N.; Costa, R.; Teixeira, A.; Costa, S.; Baêta, J. An Evaluation of Combustion Aspects with Different Compression Ratios, Fuel Types and Injection Systems in a Single-Cylinder Research Engine. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 497. [Google Scholar] [CrossRef]
- Amaral, L.V.; Santos, N.D.S.A.; Roso, V.R.; de Oliveira Sebastião, R.D.C.; Pujatti, F.J.P. Effects of Gasoline Composition on Engine Performance, Exhaust Gases and Operational Costs. Renew. Sustain. Energy Rev. 2021, 135, 110196. [Google Scholar] [CrossRef]
- Serrano, J.R.; Novella, R.; Piqueras, P. Why the Development of Internal Combustion Engines Is Still Necessary to Fight against Global Climate Change from the Perspective of Transportation. Appl. Sci. 2019, 9, 4597. [Google Scholar] [CrossRef] [Green Version]
- IEA—International Energy Agency. Biomass-Based Diesel Biofuels Are Transportation Fuels. Available online: https://www.eia.gov/energyexplained/use-of-energy/transportation.php (accessed on 7 December 2022).
- Nabi, M.N.; Rahman, M.M.; Akhter, M.S. Biodiesel from Cotton Seed Oil and Its Effect on Engine Performance and Exhaust Emissions. Appl. Therm. Eng. 2009, 29, 2265–2270. [Google Scholar] [CrossRef]
- Altaie, M.A.H.; Janius, R.B.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R.; Zakaria, R.; Adam, N.M. Performance and Exhaust Emission Characteristics of Direct-Injection Diesel Engine Fueled with Enriched Biodiesel. Energy Convers. Manag. 2015, 106, 365–372. [Google Scholar] [CrossRef]
- Shaheed, A.; Swain, E. Performance and Exhaust Emission Evaluation of a Small Diesel Engine Fuelled with Coconut Oil Methyl Esters; Society of Automotive Engineers (SAE): Warrendale, PA, USA, 1998. [Google Scholar]
- Agarwal, D.; Agarwal, A.K. Performance and Emissions Characteristics of Jatropha Oil (Preheated and Blends) in a Direct Injection Compression Ignition Engine. Appl. Therm. Eng. 2007, 27, 2314–2323. [Google Scholar] [CrossRef]
- Jindal, S.; Nandwana, B.P.; Rathore, N.S.; Vashistha, V. Experimental Investigation of the Effect of Compression Ratio and Injection Pressure in a Direct Injection Diesel Engine Running on Jatropha Methyl Ester. Appl. Therm. Eng. 2010, 30, 442–448. [Google Scholar] [CrossRef]
- El-Kasaby, M.; Nemit-Allah, M.A. Experimental Investigations of Ignition Delay Period and Performance of a Diesel Engine Operated with Jatropha Oil Biodiesel. Alex. Eng. J. 2013, 52, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Dulger, Z.; Kaplan, C. Utilization of Sunflower Methyl Ester as a Diesel Engine Fuel; SAE Technical Papers; Society of Automotive Engineers (SAE): Warrendale, PA, USA, 2001. [Google Scholar]
- Kim, H.Y.; Ge, J.C.; Choi, N.J. Effects of Fuel Injection Pressure on Combustion and Emission Characteristics under Low Speed Conditions in a Diesel Engine Fueled with Palm Oil Biodiesel. Energies 2019, 12, 3264. [Google Scholar] [CrossRef] [Green Version]
- Devan, P.K.; Mahalakshmi, N.V. Study of the Performance, Emission and Combustion Characteristics of a Diesel Engine Using Poon Oil-Based Fuels. Fuel Process. Technol. 2009, 90, 513–519. [Google Scholar] [CrossRef]
- Usta, N. An Experimental Study on Performance and Exhaust Emissions of a Diesel Engine Fuelled with Tobacco Seed Oil Methyl Ester. Energy Convers. Manag. 2005, 46, 2373–2386. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Verma, M. Methyl Ester of Karanja Oil as an Alternative Renewable Source Energy. Fuel 2008, 87, 1673–1677. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Characterization and Effect of Using Rubber Seed Oil as Fuel in the Compression Ignition Engines. Renew. Energy 2005, 30, 795–803. [Google Scholar] [CrossRef]
- Agarwal, D.; Kumar, L.; Agarwal, A.K. Performance Evaluation of a Vegetable Oil Fuelled Compression Ignition Engine. Renew. Energy 2008, 33, 1147–1156. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Antonopoulos, K.A.; Rakopoulos, D.C.; Hountalas, D.T.; Giakoumis, E.G. Comparative Performance and Emissions Study of a Direct Injection Diesel Engine Using Blends of Diesel Fuel with Vegetable Oils or Bio-Diesels of Various Origins. Energy Convers. Manag. 2006, 47, 3272–3287. [Google Scholar] [CrossRef]
- Roy, M.M.; Wang, W.; Bujold, J. Biodiesel Production and Comparison of Emissions of a DI Diesel Engine Fueled by Biodiesel-Diesel and Canola Oil-Diesel Blends at High Idling Operations. Appl. Energy 2013, 106, 198–208. [Google Scholar] [CrossRef]
- Yusaf, T.F.; Yousif, B.F.; Elawad, M.M. Crude Palm Oil Fuel for Diesel-Engines: Experimental and ANN Simulation Approaches. Energy 2011, 36, 4871–4878. [Google Scholar] [CrossRef]
- Belkadi, A.; Tarlet, D.; Montillet, A.; Bellettre, J.; Massoli, P. Study of Two Impinging Flow Microsystems Arranged in Series. Application to Emulsified Biofuel Production. Fuel 2016, 170, 185–196. [Google Scholar] [CrossRef]
- Ochoterena, R.; Lif, A.; Nydén, M.; Andersson, S.; Denbratt, I. Optical Studies of Spray Development and Combustion of Water-in-Diesel Emulsion and Microemulsion Fuels. Fuel 2010, 89, 122–132. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-din, M.G.; Kemppi, T.; et al. Stabilization Mechanism and Chemical Demulsification of Water-in-Oil and Oil-in-Water Emulsions in Petroleum Industry: A Review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Eastoe, J. Colloid Science: Principles, Methods and Applications. In Colloid Science: Principles, Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 50–76. [Google Scholar]
- Kumar, M.S.; Bellettre, J.; Tazerout, M. The Use of Biofuel Emulsions as Fuel for Diesel Engines: A Review. Proc. Inst. Mech. Eng. Part A J. Power Energy 2009, 223, 729–742. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion Science and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Leung, R.; Shah, D.O. Solubilization and Phase Equilibria of Water-in-Oil Microemulsions: I. Effects of Spontaneous Curvature and Elasticity of Interfacial Films. J. Colloid Interface Sci. 1987, 120, 320–329. [Google Scholar] [CrossRef]
- Hou, M.; Shah, D. Effects of the Molecular Structure of the Interface and Continuous Phase on Solubilization of Water in Water/Oil Microemulsions. Langmuir 1987, 3, 1086–1096. [Google Scholar] [CrossRef]
- Anghel, D.; Balcan, M.; Miha, F.; Aricov, L.; Vasilescu, E. Microemulsion Systems Containing Diesel and Colza Oil as Alternative Fuels: Phase Studies, Interfacial Tension and Solubilization. Fuel 2014, 117, 251–258. [Google Scholar]
- Lin, Y.; Lin, H. Spray Characteristics of Emulsifird Castor Biodiesel on Engine Emissions and Deposit Formation. Renew. Energy 2011, 36, 3507–3516. [Google Scholar] [CrossRef]
- Hamid, M.F.; Idroas, M.Y.; Mohamed, M.; Sa, S.; Heng, T.Y.; Mat, S.C.; Miskam, M.A. Numerical Investigation of the Characteristics of the In-Cylinder Air Flow in a Compression-Ignition Engine for the Application of Emulsified Biofuels. Processes 2020, 8, 1517. [Google Scholar] [CrossRef]
- Mondal, P.K.; Mandal, B.K. A Comprehensive Review on the Feasibility of Using Water Emulsified Diesel as a CI Engine Fuel. Fuel 2019, 237, 937–960. [Google Scholar] [CrossRef]
- Tran, T.N.; Ghojel, J.I. Impact of Introducing Water into the Combustion Chamber of Diesel Engines on Emissions—An Overview. In Proceedings of the 5th Asia-Pacific Conference on Combustion, Adelaide, Australia, 17–20 July 2005. [Google Scholar]
- Crookes, R.J.; Kiannejad, F.; Nazha, M.A.A. Systematic Assessment of Combustion Characteristics of Biofuels and Emulsions with Water for Use as Diesel Engine Fuels. Energy Convers. Manag. 1997, 38, 1785–1795. [Google Scholar] [CrossRef]
- Ivanov, V.M.; Nefedov, P.I. Experimental Investigation of the Combustion Process of Natural and Emulsified Liquid Fuels [NASA TT F-258]; National Aeronautics and Space Administration: Washington, DC, USA, 1965.
- Selim, M.Y.E.; Ghannam, M.T. Combustion Study of Stabilized Water-in-Diesel Fuel Emulsion. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 256–274. [Google Scholar] [CrossRef]
- Mura, E.; Massoli, P.; Josset, C.; Loubar, K.; Bellettre, J. Study of the Micro-Explosion Temperature of Water in Oil Emulsion Droplets during the Leidenfrost Effect. Exp. Therm. Fluid Sci. 2012, 43, 63–70. [Google Scholar] [CrossRef]
- Jiao, J.; Burgess, D.J. Rheology and Stability of Water-in-Oil-in-Water Multiple Emulsions Containing Span 83 and Tween 80. AAPS PharmSci 2003, 5, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Alahmer, A.; Yamin, J.; Sakhrieh, A.; Hamdan, M.A. Engine Performance Using Emulsified Diesel Fuel. Energy Convers. Manag. 2010, 51, 1708–1713. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, X.; Ju, D.; Sun, C.; Wang, T. Bubble Nucleation, Micro-Explosion and Residue Formation in Superheated Jatropha Oil Droplet: The Phenomena of Vapor Plume and Vapor Cloud. Fuel 2020, 261, 116431. [Google Scholar] [CrossRef]
- Muhsin, A.; Noge, H.; Abdul, H. An Overview of Utilizing Water-in-Diesel Emulsion Fuel in Diesel Engine and Its Potential Research Study. J. Energy Inst. 2014, 87, 273–288. [Google Scholar]
- Attia, A.M.A.; Kulchitskiy, A.R. Influence of the Structure of Water-in-Fuel Emulsion on Diesel Engine Performance. Fuel 2014, 116, 703–708. [Google Scholar] [CrossRef]
- Leung, P.; Tsolakis, A.; Wyszynski, M.L. Performance, Emissions and Exhaust-Gas Reforming of an Emulsified Fuel: A Comparative Study with Conventional Diesel Fuel Performance, Emissions and Exhaust-Gas Reforming of an Emulsified Fuel: A Comparative Study with Conventional Diesel Fuel; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Lif, A.; Stark, M.; Nydén, M.; Holmberg, K. Colloids and Surfaces A: Physicochemical and Engineering Aspects Fuel Emulsions and Microemulsions Based on Fischer–Tropsch Diesel. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 91–98. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Shen, Y.; Shu, G.; Chen, G.; Xu, B. In Fl Uence of Water Emulsi Fi Ed Diesel & Oxygen-Enriched Air on Diesel Engine NO-Smoke Emissions and Combustion Characteristics. Energy 2013, 55, 369–377. [Google Scholar]
- Califano, V.; Calabria, R.; Massoli, P. Experimental Evaluation of the Effect of Emulsion Stability on Micro-Explosion Phenomena for Water-in-Oil Emulsions. Fuel 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Samec, N.; Kegl, B.; Dibble, R.W. Numerical and Experimental Study of Water/Oil Emulsified Fuel Combustion in a Diesel Engine. Fuel 2002, 81, 2035–2044. [Google Scholar] [CrossRef]
- Reyes, J.S.D.L.; Charcosset, C. Preparation of Water-in-Oil and Ethanol-in-Oil Emulsions by Membrane Emulsification. Fuel 2010, 89, 3482–3488. [Google Scholar] [CrossRef]
- Bibette, J.; Calderon, F.L.; Poulin, P. Emulsions: Basic Principles. Rep. Prog. Phys. 1999, 62, 969–1033. [Google Scholar] [CrossRef]
- Kerihuel, A.; Kumar, M.; Bellettre, J.; Tazerout, M. Investigations on a CI Engine Using Animal Fat and Its Emulsions with Water and Methanol as Fuel; SAE Technical Paper: Nantes, France, 2005. [Google Scholar]
- Marchitto, L.; Calabria, R.; Tornatore, C.; Bellettre, J.; Massoli, P.; Montillet, A.; Valentino, G. Optical Investigations in a CI Engine Fueled with Water in Diesel Emulsion Produced through Microchannels. Exp. Therm. Fluid Sci. 2018, 95, 96–103. [Google Scholar] [CrossRef]
- Kerihuel, A.; Kumar, M.S.; Bellettre, J.; Tazerout, M. Ethanol Animal Fat Emulsions as a Diesel Engine Fuel—Part 1: Formulations and Influential Parameters. Fuel 2006, 85, 2640–2645. [Google Scholar] [CrossRef]
- Necati, A.; Canakci, M.; Turkcan, A.; Sayin, C. Performance and Combustion Characteristics of a DI Diesel Engine Fueled with Waste Palm Oil and Canola Oil Methyl Esters. Fuel 2009, 88, 629–636. [Google Scholar]
- Debnath, B.K.; Sahoo, N.; Saha, U.K. Adjusting the Operating Characteristics to Improve the Performance of an Emulsified Palm Oil Methyl Ester Run Diesel Engine. Energy Convers. Manag. 2013, 69, 191–198. [Google Scholar] [CrossRef]
- Bora, P.; Jyoti, L.; Mausoom, M.; Deka, D. Microemulsion Based Hybrid Biofuels from Thevetia Peruviana Seed Oil: Structural and Dynamic Investigations. Fuel 2015, 157, 208–218. [Google Scholar] [CrossRef]
- Mollet, H.; Grubenmann, A. Formulation Technology: Emulsions, Suspensions, Solid Forms; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 59–104. [Google Scholar]
- Arang, B.; Estate, I. Properties of Palm Oil-in-Water Emulsions Stabilized by Nonionic Emulsifiers. J. Colloid Interface Sci. 1996, 181, 595–604. [Google Scholar]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Ashraful, A.M. Performance and Emission Assessment of Diesel–Biodiesel–Ethanol/Bioethanol Blend as a Fuel in Diesel Engines: A Review. Renew. Sustain. Energy Rev. 2015, 48, 62–78. [Google Scholar] [CrossRef]
- Dinesha, P.; Kumar, S.; Rosen, M.A. Combined Effects of Water Emulsion and Diethyl Ether Additive on Combustion Performance and Emissions of a Compression Ignition Engine Using Biodiesel Blends. Energy 2019, 179, 928–937. [Google Scholar] [CrossRef]
- Kerihuel, A.; Kumar, M.S.; Bellettre, J.; Tazerout, M. Use of Animal Fats as CI Engine Fuel by Making Stable Emulsions with Water and Methanol. Fuel 2005, 84, 1713–1716. [Google Scholar] [CrossRef]
- Bhimani, S.; Alvarado, J.L.; Annamalai, K.; Marsh, C. Emission Characteristics of Methanol-in-Canola Oil Emulsions in a Combustion Chamber. Fuel 2013, 113, 97–106. [Google Scholar] [CrossRef]
- Venu, H.; Raju, V.D.; Lingesan, S.; Elahi, M.; Soudagar, M. In Fl Uence of Al2O3 Nano Additives in Ternary Fuel (Diesel-Biodiesel- Ethanol) Blends Operated in a Single Cylinder Diesel Engine: Performance, Combustion and Emission Characteristics. Energy 2021, 215, 119091. [Google Scholar] [CrossRef]
- Janakiraman, S.; Lakshmanan, T.; Chandran, V.; Subramani, L. Comparative Behavior of Various Nano Additives in a DIESEL Engine Powered by Novel Garcinia Gummi-Gutta Biodiesel. J. Clean. Prod. 2020, 245, 118940. [Google Scholar] [CrossRef]
- Subramani, L.; Parthasarathy, M.; Balasubramanian, D. Novel Garcinia Gummi-Gutta Methyl Ester (GGME) as a Potential Alternative Feedstock for Existing Unmodi Fi Ed DI Diesel Engine. Renew. Energy 2018, 125, 568–577. [Google Scholar] [CrossRef]
- Nesterenko, A.; Drelich, A.; Lu, H.; Clausse, D.; Pezron, I. Colloids and Surfaces A: Physicochemical and Engineering Aspects Influence of a Mixed Particle/Surfactant Emulsifier System on Water-in-Oil Emulsion Stability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 49–57. [Google Scholar] [CrossRef]
- Perdih, T.S.; Zupanc, M.; Dular, M. Revision of the Mechanisms behind Oil-Water (O/W) Emulsion Preparation by Ultrasound and Cavitation. Ultrason. Sonochem. 2019, 51, 298–304. [Google Scholar] [CrossRef]
- Qi, D.H.; Chen, H.; Matthews, R.D.; Bian, Y.Z.H. Combustion and Emission Characteristics of Ethanol–Biodiesel–Water Micro-Emulsions Used in a Direct Injection Compression Ignition Engine. Fuel 2010, 89, 958–964. [Google Scholar] [CrossRef]
- Raheman, H.; Kumari, S. Combustion Characteristics and Emissions of a Compression Ignition Engine Using Emulsified Jatropha Biodiesel Blend. Biosyst. Eng. 2014, 123, 29–39. [Google Scholar] [CrossRef]
- Lin, C.; Lin, S. Effects of Emulsification Variables on Fuel Properties of Two- and Three-Phase Biodiesel Emulsions. Fuel 2007, 86, 210–217. [Google Scholar] [CrossRef]
- Sajith, V.; Sobhan, C.B.; Peterson, G.P. Experimental Investigations on the Effects of Cerium Oxide Nanoparticle Fuel Additives on Biodiesel. Adv. Mech. Eng. 2010, 2010, 581407. [Google Scholar] [CrossRef]
- Awang, R.; May, C.Y. Water-in-Oil Emulsion of Palm Biodiesel. J. Oil Palm Res. 2008, 20, 571–576. [Google Scholar]
- Chen, G.; Tao, D. An Experimental Study of Stability of Oil–Water Emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Moradi, M.; Kazempour, M.; French, J.T.; Alvarado, V. Dynamic Flow Response of Crude Oil-in-Water Emulsion during Flow through Porous Media. Fuel 2014, 135, 38–45. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N.; Benichou, A.; Aserin, A.; Garti, N. Polyols, High Pressure, and Refractive Indices Equalization for Improved Stability of W/O Emulsions for Food Applications. J. Dispers. Sci. Technol. 2001, 22, 269–280. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Bonini, M.; Fratini, E.; Tondi, G.; Gartner, K.; Bridgwater, A.V. Development of Emulsions from Biomass Pyrolysis Liquid and Diesel and Their Use in Engines—Part 1: Emulsion Production. Biomass Bioenergy 2003, 25, 85–99. [Google Scholar] [CrossRef]
- Velmurugan, R.; Mayakrishnan, J.; Induja, S.; Raja, S.; Sasikumar, N.; Sathyamurthy, R. Comprehensive Study on the Effect of CuO Nano Fluids Prepared Using One-Step Chemical Synthesis Method on the Behavior of Waste Cooking Oil Biodiesel in Compression Ignition Engine. J. Therm. Sci. Eng. Appl. 2019, 11, 041003. [Google Scholar] [CrossRef]
- Kannan, G.R. Experimental Studies of Diestrol-Micro Emulsion Fuel in a Direct Injection Compression Ignition Engine under Varying Injection Pressures and Timings. J. Inst. Eng. Ser. C 2018, 99, 19–32. [Google Scholar] [CrossRef]
- Basha, J.S. Performance, Emission and Combustion Characteristics of a Diesel Engine Using Carbon Nanotubes Blended Jatropha Methyl Ester Emulsions. Alex. Eng. J. 2014, 53, 259–273. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J. The Science and Applications of Emulsions—An Overview. Colloids Surf. A Physicochem. Eng. Asp. 1994, 91, 1–8. [Google Scholar] [CrossRef]
- Armas, O.; Ballesteros, R.; Martos, F.J.; Agudelo, J.R. Characterization of Light Duty Diesel Engine Pollutant Emissions Using Water-Emulsified Fuel. Fuel 2005, 84, 1011–1018. [Google Scholar] [CrossRef]
- Gad, M.S.; Jayaraj, S. A Comparative Study on the Effect of Nano-Additives on the Performance and Emissions of a Diesel Engine Run on Jatropha Biodiesel. Fuel 2020, 267, 117168. [Google Scholar] [CrossRef]
- Jin, C.; Geng, Z.; Liu, X.; Ampah, J.D.; Ji, J.; Wang, G.; Niu, K.; Hu, N.; Liu, H. Effects of Water Content on the Solubility between Isopropanol-Butanol-Ethanol (IBE) and Diesel Fuel under Various Ambient Temperatures. Fuel 2021, 286, 119492. [Google Scholar] [CrossRef]
- Kumar, N.; Khare, U. Use of Macro-Emulsion of Vegetable Oil in Compression Ignition Engine; SAE 28-0040. SAE Technical Paper; SAE International: Warrendale, PA, USA, 2004. [Google Scholar]
- Crookes, R.J.; Kiannejad, F.; Nazha, L. Seed-Oil Biofuel of Low Cetane Number: The Effect of Water Emulsification on Diesel-Engine Operation and Emissions. J. Inst. Energy 1995, 68, 142–151. [Google Scholar]
- Ogunkunle, O.; Ahmed, N.A. Exhaust Emissions and Engine Performance Analysis of a Marine Diesel Engine Fuelled with Parinari Polyandra Biodiesel–Diesel Blends. Energy Rep. 2020, 6, 2999–3007. [Google Scholar] [CrossRef]
- Moka, S.; Pande, M.; Rani, M.; Gakhar, R.; Sharma, M.; Rani, J.; Bhaskarwar, A.N. Alternative Fuels: An Overview of Current Trends and Scope for Future. Renew. Sustain. Energy Rev. 2014, 32, 697–712. [Google Scholar]
- Rao, N.D.; Premkumar, B.; Yohan, M. Performance and Emission Characteristics of Straight Vegetable Oil-Ethanol Emulsion in a Compression Ignition Engine. ARPN J. Eng. Appl. Sci. 2012, 7, 447–452. [Google Scholar]
- Krishnamoorthi, M.; Malayalamurthi, R.; He, Z.; Kandasamy, S. A Review on Low Temperature Combustion Engines: Performance, Combustion and Emission Characteristics. Renew. Sustain. Energy Rev. 2019, 116, 109404. [Google Scholar] [CrossRef]
- Wagner, D.; McLennan, J. Formulation of Canola-Diesel Microemulsion Fuels and Their Selective Diesel Engine Performance. J. Am. Oil Chem. Soc. 2012, 89, 1905–1912. [Google Scholar]
- Yusri, I.M.; Mamat, R.; Akasyah, M.K.; Jamlos, M.F.; Yusop, A.F. Evaluation of Engine Combustion and Exhaust Emissions Characteristics Using Diesel/Butanol Blended Fuel. Appl. Therm. Eng. 2019, 156, 209–219. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Shahabuddin, M.; Hazrat, M.A. Energy Procedia Palm Oil Methyl Ester and Its Emulsions Effect on Lubricant Performance and Engine Components Wear. Energy Procedia 2012, 14, 1748–1753. [Google Scholar] [CrossRef]
- Shashidhara, Y.M.Ã.; Jayaram, S.R. Tribology International Vegetable Oils as a Potential Cutting Fluid—An Evolution. Tribiol. Int. 2010, 43, 1073–1081. [Google Scholar] [CrossRef]
- Siniawski, M.T.; Saniei, N.; Adhikari, B.; Doezema, L.A. Influence of Fatty Acid Composition on the Tribological Performance of Two Vegetable-Based Lubricants. J. Synth. Lubr. 2007, 24, 101–110. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ramesh, A.; Nagalingam, B. An Experimental Comparison of Methods to Use Methanol and Jatropha Oil in a Compression Ignition Engine. Biomass Bioenergy 2003, 25, 309–318. [Google Scholar] [CrossRef]
- Schwab, A.W.; Bagby, M.O.; Freedman, B. Diesel Fuels from Vegetable Oils. In Proceedings of the American Chemical Society National Meeting, New York, NY, USA, 13 April 1986; Volume 31:1. [Google Scholar]
- Liaquat, A.M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.M.R. Impact of Biodiesel Blend on Injector Deposit Formation. Energy 2014, 72, 813–823. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. A Critical Review of Bio-Diesel as a Vehicular Fuel. Energy Convers. Manag. 2008, 49, 2727–2741. [Google Scholar] [CrossRef]
- Goering, C.E.; Fry, B. Engine Durability Screening Test of a Diesel Oil/Soy Oil/Alcohol Microemulsion Fuel. J. Am. Oil Chem. Soc. 1984, 61, 1627–1632. [Google Scholar] [CrossRef]








| Type of Alternative Fuel | Engine Type | Test Setup | Performance | Combustion | Emission | Reference |
|---|---|---|---|---|---|---|
| Cottonseed oil methyl ester (CSOME) blend | 4 strokes, 1 cylinder, CR: 16.5:1 RP: 4.48 kW | Constant Speed (850 rpm) Variable load | BSFC | - | PM NOx | [39] |
| Jatropha Oil | 1 cylinder, 4 Stroke, CR: 17.5:1 RP: 7.4 kW | Constant Speed (1500 rpm) | BTE | - | CO CO2 HC Smoke Opacity Close to diesel fuel PM | [42] |
| Jatropha methyl ester | 1 cylinder 4 strokes CR: 17.5:1 RP: 3.5 kW | Constant Speed (1500 rpm) | (after CR) BTE | - | NOx Smoke Opacity PM | [43] |
| Jatropha biodiesel | 1 cylinder, 4 strokes CR: 18:1 DI | Variable speeds (1000–2000 rpm) | NOx PM | [44] | ||
| Coconut | 1 cylinder, DI, AC | Full Throttle at variable speeds (1200–3600 rpm) | EGT | Almost similar | HC Soot and PM | [41] |
| Sunflower | 4 strokes 4 cylinders DI | Full load Variable speeds | BTE Power ( 5%) | Peak cylinder pressure | HC PM | [45] |
| Palm oil | Multi cylinder, IDI | Full load at different speeds | 2.5%) BSFC | Lower than diesel | Smoke | [46] |
| Poon | 1 cylinder, 4 strokes | Speed 1500 rpm | Power ( 23%) | - | [47] | |
| Tobacco | 4 cylinders, DI, NA | Speed (1500–3000 rpm) | - | NOx PM | [48] | |
| Karanja | 2 cylinders DI | Speed (1500 rpm) | Power ( 24.87%) | - | NOx PM | [49] |
| Rapeseed | 1 cylinder DI | Speed (1500 rpm) | BTE | - | NOx | [50] |
| Mahua Oil | 4 strokes 1 cylinder | Speed (1500 rpm) | BTE at low load | - | Smoke | [51] |
| Olive kernel | 4 strokes DI engine | Speed (2000 rpm) | - | NOx | [52] | |
| Canola oil | 2 cylinders 4 strokes Naturally aspirated DI engine | Speed (1200–1800) | BTE | - | HC at low rpm HC at high rpm NOx | [53] |
| Crude Palm Oil | 4 cylinders 4 strokes DI engine | Variety of load and rpm | - | HC↑ at high rpm NOx | [54] |
| Biofuel | Iodin Value | Cetane Number | Heating Value (kJ/kg) | Viscosity (mm2/s (C)) | CP | Density (kg/L) | Sulphur (%) | Carbon Residue (%) | Ash(%) |
|---|---|---|---|---|---|---|---|---|---|
| Mahua | - | 44.0 | 30,182 | 15.5 | - | - | 0.01 | - | - |
| Karanja | - | - | 37,200 | 122 | - | - | 0.01 | - | - |
| Neem Seeds | - | 46.8 | 38,279 | 29.0 | - | - | 0.01 | - | - |
| Jatropa | - | 41.8 | 38,998 | 48.8 | - | - | 0.01 | - | - |
| Soybean | 121–134 | 36.7 | 38,893 | 31.9 | −3.7 | 0.87 | 0.01 | 0.25 | 0.010 |
| Sesame | 114–130 | 39.6 | 38,829 | 34.7 | −3.7 | 0.81 | 0.01 | 0.24 | 0.010 |
| Sunflower | 120–153 | 36.3 | 37,983 | 36.8 | 6.9 | 0.88 | 0.01 | 0.22 | 0.010 |
| Cotton seed | 95–145 | 42.4 | 38,864 | 32.8 | 2.1 | 0.89 | 0.01 | 0.19 | 0.010 |
| Palm | 40–65 | 39.0 | 37,241 | 38.5 | 30.0 | 0.82 | 0.01 | - | - |
| Olive | 85–96 | - | - | - | - | - | 0.01 | - | - |
| Coconut | 8–10 | - | - | - | - | - | 0.01 | - | - |
| Sunflower | 134–161 | 40.8 | 38,915 | 30.8 | 17.2 | 0.89 | 0.01 | 0.24 | 0.006 |
| Castor | 79–89 | - | 38,920 | 289 | - | - | 0.01 | - | - |
| Corn | 113–130 | 37.6 | 38,920 | 33.8 | −1.2 | 0.87 | 0.01 | 0.23 | 0.011 |
| Peanut | 79–104 | 40.1 | 38,972 | 38.5 | 11.4 | 0.91 | 0.01 | 0.25 | 0.004 |
| Rapeseed | 92–132 | 36.8 | 38,909 | 36.4 | −3.8 | 0.89 | 0.01 | 0.28 | 0.063 |
| Additive | Quantities | References |
|---|---|---|
| Dimethyl Ether, | 12 | [100] |
| Diethyl Ether (%) | 12 | [101] |
| H2O2 (%) | 12 | [100] |
| Alumina | 25, 50, 100 | [102] |
| Carbon Nanotube (CNT) | 25, 50 | [103] |
| Aqueous Ammonia (%) | 5, 10 | [104,105] |
| Fuel | Surfactant | Type of Emulsion | HLB Value | Stability Time | References |
|---|---|---|---|---|---|
| Palm oil emulsion | Laboratory prepared surfactant | (W/O) | - | 1 week | [105] |
| Palm oil methyl ester + water 5% | Span 80 and Tween 80 | (W/O) | 4.3, 5, 6 | 3 h almost no deposition | [88] |
| Waste cooking oil (WCO) + 15% water | Span 80 + Ethanol | (W/O) | 4.3 | 2 weeks | [110] |
| Waste cooking palm oil (B70) + 0.5% water | Span80 + Ethanol | Microemulsion | 4.3 | 2 weeks | [111] |
| Soybean biodiesel + 0.5% water | Span 80 + Ethanol | Microemulsion | 4.3 | Stable | [101] |
| 88.4% Canola oil + 9.8% methanol + surfactant | Span 80 + Tween 80 | (W/O) (in this case methanol in oil) | 7 | 7 h | [95] |
| 93% Jatropha methyl ester + 2% surfactant + 5% water | Span 80 + Tween 80 | (W/O) | 10 | 5 days | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamid, M.F.; Teoh, Y.H.; Idroas, M.Y.; Mohamed, M.; Sa’ad, S.; Mat, S.C.; Abdullah, M.K.; Le, T.D.; How, H.G.; Nguyen, H.T. A Review of the Emulsification Method for Alternative Fuels Used in Diesel Engines. Energies 2022, 15, 9429. https://doi.org/10.3390/en15249429
Hamid MF, Teoh YH, Idroas MY, Mohamed M, Sa’ad S, Mat SC, Abdullah MK, Le TD, How HG, Nguyen HT. A Review of the Emulsification Method for Alternative Fuels Used in Diesel Engines. Energies. 2022; 15(24):9429. https://doi.org/10.3390/en15249429
Chicago/Turabian StyleHamid, Mohd Fadzli, Yew Heng Teoh, Mohamad Yusof Idroas, Mazlan Mohamed, Shukriwani Sa’ad, Sharzali Che Mat, Muhammad Khalil Abdullah, Thanh Danh Le, Heoy Geok How, and Huu Tho Nguyen. 2022. "A Review of the Emulsification Method for Alternative Fuels Used in Diesel Engines" Energies 15, no. 24: 9429. https://doi.org/10.3390/en15249429