Otolith Microchemistry Assessment: Evidence of Migratory Coilia nasus of Yangtze River Living in the Shengsi Sea Area
Abstract
:1. Introduction
2. Materials and Methods
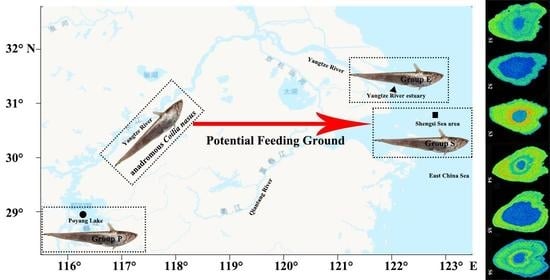
2.1. Samples Collection
2.2. Otolith Treatment and Microchemical Analysis
2.3. Data Analysis
3. Results
3.1. Age and Sexual Maturity
3.2. The Quantitative Line Analysis of Sr and Ca Contents (Sr/Ca Ratios)
3.3. The Area Distribution Analysis of Sr Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, T.; Liu, H.; Shen, X.; Shimasaki, Y.; Oshima, Y.; Yang, J. Life history variations among different populations of Coilia nasus along the chinese coast inferred from otolith microchemistry. J. Fac. Agric. Kyushu Univ. 2014, 59, 383–389. [Google Scholar] [CrossRef]
- Yuan, C.; Qin, A.; Liu, R.; Lin, J. Discussion on the subspecies classification of Coilia in the middle and lower reaches of the Yangtze River and southeast coastal provinces. J. Nanjing Univ. 1980, 3, 67–82, (In Chinese with English Abstract). [Google Scholar]
- Duan, J.; Zhang, H.; Liu, K.; Xu, D.; Zhang, M.; Shi, W. An Overview of Coilia ectenes in Jiangsu Section of the Yangtze River. Agric. Sci. Technol. 2012, 13, 1950–1954, (In Chinese with English Abstract). [Google Scholar]
- Ge, K.K.; Zhong, J.S. Daily-age structure and growth characteristics of Coilia nasus larvae and juveniles in the surf zone of Yangtze River estuary. Acta Hydrobiol. Sin. 2010, 34, 716–721, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Xuan, Z.Y.; Chen, X.B.; Yang, J. Classification of ecomorphotypes of Coilia nasus from the middle and lower reaches of the Yangtze River Basin. J. Lake Sci. 2020, 32, 518–527, (In Chinese with English Abstract). [Google Scholar]
- Sokta, L.; Jiang, T.; Liu, H.; Xuan, Z.; Qiu, C.; Che, X.; Yang, J. Loss of Coilia nasus habitats in Chinese freshwater lakes: An otolith microchemistry assessment. Heliyon 2020, 6, e04571. [Google Scholar] [CrossRef] [PubMed]
- Crossin, G.T.; Hinch, S.G.; Cooke, S.J.; Welch, D.W.; Batten, S.D.; Patterson, D.A.; Kraak, G.; Shrimpton, J.M.; Farrell, A.P. Behaviour and physiology of sockeye salmon homing through coastal waters to a natal river. Mar. Biol. 2007, 152, 905–918. [Google Scholar] [CrossRef]
- Li, Y.X.; Xie, S.G.; Li, Z.J.; Gong, W.B.; He, W.P. Gonad development of an anadromous fish Coilia ectenes (Engraulidae) in lower reach of Yangtze River, China. Fisheries. Sci. 2007, 73, 1224–1230. [Google Scholar]
- Zhong, L.; Guo, H.; Shen, H.; Li, X.; Tang, W.; Liu, J.; Jin, J.; Mi, Y. Preliminary results of Sr:Ca ratios of Coilia nasus in otoliths by micro–PIXE. Nucl. Instrum. Meth. A 2007, 260, 349–352. [Google Scholar] [CrossRef]
- Yuan, C.M. Spawning migration of Coilia nasus. Bull. Biol. 1987, 12, 1–3. (In Chinese) [Google Scholar]
- Zhu, D.L. Observations and studies on embryonic development and natural reproduction of Coilia nasus in Yangtze River estuary. Fish. Sci. Technol. Inf. 1992, 19, 49–51. (In Chinese) [Google Scholar]
- Jiang, T. Study on Migratory Ecology of Coilia Fish in Chinese Water Inferred from Otolith Morphometric and Microchemiscal Characteristics. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2014. (In Chinese with English Abstract). [Google Scholar]
- Gillanders, B.M. Otolith chemistry to determine movements of diadromous and freshwater fish. Aquat. Living. Resour. 2005, 18, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Jiang, T.; Liu, H. Are there habitat salinity markers of the Sr:Ca ratio in the otolith of wild diadromous fishes? A literature survey. Ichthyol. Res. 2011, 58, 291–294. [Google Scholar] [CrossRef]
- Yang, J.; Arai, H.; Liu, B.; Miyazaki, N.; Tsukamoto, K. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. J. Fish. Biol. 2006, 69, 1120–1135. [Google Scholar] [CrossRef]
- Secor, D.H.; Rooker, J.R. Is otolith strontium a useful scalar of life-cycles in estuarine fishes? Fish. Res. 2000, 46, 359–371. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Arai, T.; Kotake, A.; Aoyama, T.; Hayano, H.; Miyazaki, N. Identifying sea-run brown trout, Salmo trutta, using Sr:Ca ratios of otolith. Ichthyol. Res. 2002, 49, 380–383. [Google Scholar] [CrossRef]
- Arai, T.; Goto, A.; Miyazaki, N. Migratory history of the threespine stickleback Gasterosteus aculeatus. Ichthyol. Res. 2003, 50, 9–14. [Google Scholar] [CrossRef]
- Limburg, K.E.; Olson, C.; Walther, Y.; Dale, D.; Slomp, C.P.; Hoie, H. Tracking baltic hypoxia and cod migration over millennia with natural tags. Proc. Natl. Acad. Sci. USA 2011, 108, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, K.; Chow, S.; Otake, T.; Kurogi, H.; Mochioka, N.; Miller, M.J.; Aoyama, J.; Kimura, S.; Watanabe, S.; Yoshinaga, T.; et al. Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat. Commun. 2011, 2, 179. [Google Scholar] [CrossRef] [Green Version]
- Dou, S.Z.; Yokouchi, K.; Yu, X.; Cao, L.; Kuroki, M.; Otake, T.; Tsukamoto, K. The migratory history of anadromous and non–anadromous tapertail anchovy Coilia nasus in the Yangtze River Estuary revealed by the otolith Sr:Ca ratio. Environ. Biol. Fish. 2012, 95, 481–490. [Google Scholar] [CrossRef]
- Arai, T.; Hirata, T. Differences in the trace element deposition in otoliths between marine– and freshwater–resident Japanese eels, Anguilla japonica, as determined by laser ablation ICPMS. Environ. Biol. Fish. 2006, 75, 173–182. [Google Scholar] [CrossRef]
- Brown, R.J.; Severin, K.P. Otolith chemistry analyses indicate that water Sr:Ca is the primary factor influencing otolith Sr:Ca for freshwater and diadromous fish but not for marine fish. Can. J. Agric. Sci. 2009, 66, 1790–1808. [Google Scholar] [CrossRef] [Green Version]
- Kotake, A.; Arai, T.; Ozawa, T.; Nojima, S.; Miller, M.J.; Tsukamoto, K. Variation in migratory history of Japanese eels, Anguilla japonica, collected in coastal waters of the Amakusa Islands, Japan, inferred from otolith Sr/Ca ratios. Mar. Biol. 2003, 142, 849–854. [Google Scholar] [CrossRef]
- Limburg, K.; Waldman, R. Dramatic declines in North Atlantic diadromous fishes. Bio. Sci. 2009, 59, 955–965. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Arai, T. Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Mar. Ecol. Prog. Ser. 2001, 220, 265–276. [Google Scholar] [CrossRef]
- Wood, C.M.; Farrell, A.P.; Brauner, C.J. Homeostasis and toxicology of essential metals. Fish. Physiol. 2012, 31, 3. [Google Scholar]
- Kraus, R.T.; Secor, D.H. Incorporation of strontium into otoliths of an estuarine fish. J. Exp. Mar. Biol. Ecol. 2004, 302, 85–106. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Liu, H.; Shen, X.Q. Life history of Coilia nasus from the Yellow Sea inferred from otolith Sr:Ca ratios. Environ. Biol. Fish. 2012, 95, 503–508. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, X.Q.; Liu, H.B.; Liu, H.Z.; Yang, J. Two microchemistry patterns in otoliths of Coilia nasus from Poyang Lake, China. J. Fish. China 2013, 37, 239–244, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Qiu, C.; Chen, B.X.; Yang, J. Whether there is anadromous Coilia nasus in Dongting Lake. Acta Hydrobiol. Sin. 2020, 44, 838–843, (In Chinese with English Abstract). [Google Scholar]
- Chen, W.Y.; Li, J.L.; Lian, Q.P. Histological studies of gonadal development of Coilia ectenes in the Changjiang River. J. Fish. China 2006, 6, 773–777, (In Chinese with English Abstract). [Google Scholar]
- Jiang, T.; Liu, H.B.; Lu, M.J. The comparison of different pre-treatment methods for acquiring otolith annuli of Coilia nasus. Prog. Fish. Sci. 2016, 37, 62–67, (In Chinese with English Abstract). [Google Scholar]
- Khumbanyiwa, D.; Li, M.; Jiang, T.; Liu, H.; Yang, J. Unraveling habitat use of Coilia nasus from Qiantang River of China by otolith microchemistry. Reg. Stud. Mar. Sci. 2018, 18, 122–128. [Google Scholar] [CrossRef]
- Cai, D.; Ji, D.; Zhou, C. An ecological investigation and fishery of the anchovy (Coilia ectenes Jordan et seale) in the lower yellow river. J. Shandong. Univ. 1980, 2, 102–112, (In Chinese with English Abstract). [Google Scholar]
- Zhu, D. Observation on natural reproduction and embryonic development of Yangtze River Coilia nasus. Fish. Sci. Technol. Inf. 1992, 2, 49–51, (In Chinese with English Abstract). [Google Scholar]
- Administration of Fisheries and Fisheries of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. China Fisheries Statistical Yearbook; China Agricultural Publishing House: Beijing, China, 2018; (In Chinese with English Abstract).
- Xue, X. A Preliminary Study on the Spatial and Temporal Dynamics of Early Fish Resources and the Spawning Grounds of Coilia nasus in Sutong Section of the Changjiang River. Master Thesis, Shanghai Ocean University, Shanghai, China, 2021. (In Chinese with English Abstract). [Google Scholar]
- Jian, T.; Luo, M.; Zhang, H.; Wang, Y. Community ecology of trawl fishery resources in Shengsi sea area. Mar. Fish. 2020, 42, 687–698, (In Chinese with English Abstract). [Google Scholar]
- Liu, Q.; Zhu, Y.; Wang, F.S.; Gu, J. Assessment and analysis environment quality of Zhoushan fishing ground sea area. Guangzhou Chem. Ind. 2013, 41, 153–154+190, (In Chinese with English Abstract). [Google Scholar]
- Arthington, A.; Dulvy, N.; Gladstone, W.; Winfield, I.J. Fish conservation in freshwater and marine realms: Status, threats and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef] [Green Version]
- Shotyk, W.; Bicalho, B.; Cuss, C.; Grant-Weaver, I.; Nagel, A.; Noernberg, T.; Poesch, M.; Sinnatamby, N.R. Bioaccumulation of Tl in otoliths of Trout-perch (Percopsis omiscomaycus) from the Athabasca River, upstream and downstream of bitumen mining and upgrading. Sci. Total Environ. 2019, 650, 2559–2566. [Google Scholar] [CrossRef]
- Teichert, N.; Tabouret, H.; Lagarde, R.; Grondin, H.; Ponton, D.; Pécheyran, C.; Bareille, G. Site fidelity and movements of an amphidromous goby revealed by otolith multi-elemental signatures along a tropical watershed. Ecol. Freshw. Fish. 2018, 27, 834–846. [Google Scholar] [CrossRef]








| Analysis Item | Accelerating Voltage/kV | Beam Current/A | Direct Beam Spot/μm | Residence Time/s | Interval/Pixel/μm |
|---|---|---|---|---|---|
| Life history transect analysis | 15 | 2 × 10−8 | 5 | 15 | 10 |
| X-ray intensity mapping analysis | 15 | 5 × 10−7 | 5 | 0.03 | 7 × 7 |
| Sample Code | Weight/g | Total Length/mm | Jaw Length/Head Length | Sexual Maturity | Age |
|---|---|---|---|---|---|
| S1 | 66.50 | 275.90 | 1.34 | ♂Ⅰ | 2 |
| S2 | 84.50 | 304.74 | 1.31 | ♀Ⅱ | 2 |
| S3 | 83.50 | 306.93 | 1.14 | ♂Ⅰ | 2 |
| S4 | 77.30 | 303.86 | 1.30 | ♀Ⅰ | 2 |
| S5 | 103.20 | 347.46 | 1.43 | ♀Ⅰ | 2 |
| S6 | 63.20 | 267.56 | 1.25 | ♀Ⅰ | 2 |
| E1 | 129.74 | 337.42 | 1.25 | ♂Ⅱ | 2 |
| E2 | 135.20 | 339.66 | 1.29 | ♀Ⅱ | 2 |
| E3 | 106.96 | 329.81 | 1.36 | ♂Ⅱ | 2 |
| E4 | 110.99 | 335.19 | 1.20 | ♀Ⅱ | 2 |
| E5 | 167.58 | 380.38 | 1.32 | ♀Ⅱ | 2 |
| E6 | 129.79 | 360.70 | 1.36 | ♀Ⅲ | 2 |
| E7 | 121.34 | 355.46 | 1.28 | ♂Ⅱ | 2 |
| E8 | 143.33 | 334.76 | 1.49 | ♀Ⅱ | 2 |
| P1 | 91.60 | 271.71 | 1.39 | ♀Ⅳ | 2 |
| P2 | 66.60 | 248.19 | 1.28 | ♀Ⅳ | 2 |
| P3 | 69.40 | 243.75 | 1.34 | ♂Ⅳ | 2 |
| P4 | 94.00 | 282.90 | 1.25 | ♀Ⅳ | 2 |
| P5 | 91.80 | 269.00 | 1.39 | ♂Ⅳ | 2 |
| P6 | 112.00 | 298.54 | 1.31 | ♂Ⅲ | 2 |
| P7 | 103.80 | 290.28 | 1.16 | ♂Ⅳ | 2 |
| Water Area | Sample Number | Otolith Sr/Ca Value Change Stage | Length from Otolith Core Radius/μm | Number of Element Points (N) | Sr/Ca Value (Mean ± SD) * |
|---|---|---|---|---|---|
| Shengsi Sea area | S1 | 1 | 0–310 | 31 | 2.28 ± 0.70 a |
| 2 | 310–1830 | 152 | 4.62 ± 0.90 b | ||
| S2 | 1 | 0–1040 | 104 | 1.47 ± 0.76 a | |
| 2 | 1040–1710 | 67 | 4.00 ± 0.68 b | ||
| S3 | 1 | 0–710 | 71 | 2.08 ± 0.67 a | |
| 2 | 710–2150 | 144 | 4.70 ± 1.27 b | ||
| S4 | 1 | 0–270 | 27 | 2.38 ± 0.63 a | |
| 2 | 270–1850 | 158 | 5.25 ± 1.41 b | ||
| S5 | 1 | 0–830 | 83 | 1.41 ± 0.62 a | |
| 2 | 830–1860 | 103 | 3.60 ± 0.99 b | ||
| S6 | 1 | 0–770 | 77 | 2.13 ± 0.80 a | |
| 2 | 770–2140 | 137 | 4.93 ± 1.68 b | ||
| Yangtze River estuary | E1 | 1 | 0–830 | 83 | 1.50 ± 0.80 a |
| 2 | 830–1830 | 100 | 3.20 ± 1.07 b | ||
| 3 | 1830–2030 | 20 | 1.55 ± 0.93 a | ||
| E2 | 1 | 0–810 | 81 | 1.67 ± 0.68 a | |
| 2 | 810–1730 | 92 | 3.90 ± 0.99 b | ||
| 3 | 1730–2000 | 27 | 1.42 ± 0.90 a | ||
| E3 | 1 | 0–770 | 77 | 1.52 ± 0.77 a | |
| 2 | 770–1900 | 113 | 5.54 ± 1.17 b | ||
| 3 | 1900–2520 | 62 | 3.33 ± 0.75 b | ||
| E4 | 1 | 0–980 | 98 | 1.32 ± 0.66 a | |
| 2 | 980–1900 | 92 | 4.35 ± 1.30 b | ||
| 3 | 1900–2120 | 22 | 3.35 ± 0.74 b | ||
| E5 | 1 | 0–370 | 37 | 2.35 ± 0.84 a | |
| 2 | 370–1730 | 136 | 5.06 ± 1.35 b | ||
| E6 | 1 | 0–1250 | 125 | 1.60 ± 0.80 a | |
| 2 | 1250–1890 | 64 | 4.47 ± 1.14 b | ||
| 3 | 1890–1910 | 2 | 2.80 ± 0.06 a | ||
| E7 | 1 | 0–700 | 70 | 3.37 ± 0.77 b | |
| 2 | 700–2180 | 148 | 4.57 ± 1.36 a | ||
| 3 | 2180–2200 | 2 | 2.22 ± 0.44 b | ||
| E8 | 1 | 0–860 | 86 | 1.93 ± 0.81 a | |
| 2 | 860–1900 | 104 | 5.75 ± 1.16 b | ||
| 3 | 1900–2220 | 32 | 1.90 ± 1.00 a | ||
| Poyang Lake | P1 | 1 | 0–860 | 86 | 1.68 ± 0.69 a |
| 2 | 860–1910 | 105 | 4.26 ± 0.72 b | ||
| 3 | 1910–2190 | 28 | 1.16 ± 0.51 a | ||
| P2 | 1 | 0–570 | 57 | 2.72 ± 0.89 a | |
| 2 | 570–1700 | 113 | 4.21 ± 1.33 b | ||
| 3 | 1700–2300 | 60 | 2.06 ± 1.02 b | ||
| P3 | 1 | 0–940 | 94 | 1.80 ± 0.76 a | |
| 2 | 940–1510 | 57 | 4.99 ± 1.16 b | ||
| 3 | 1510–1600 | 9 | 1.73 ± 0.77 a | ||
| P4 | 1 | 0–810 | 81 | 1.57 ± 0.59 a | |
| 2 | 810–1720 | 91 | 3.34 ± 0.90 b | ||
| 3 | 1720–1850 | 13 | 1.23 ± 0.41 a | ||
| P5 | 1 | 0–730 | 73 | 1.45 ± 0.61 a | |
| 2 | 730–1670 | 94 | 4.76 ± 1.01 b | ||
| 3 | 1670–1800 | 13 | 1.14 ± 0.70 a | ||
| P6 | 1 | 0–850 | 85 | 1.82 ± 0.68 a | |
| 2 | 850–1840 | 99 | 4.38 ± 1.07 b | ||
| 3 | 1840–1920 | 8 | 1.43 ± 0.86 a | ||
| P7 | 1 | 0–840 | 84 | 1.46 ± 0.59 a | |
| 2 | 840–1880 | 104 | 4.27 ± 0.97 b | ||
| 3 | 1880–2060 | 18 | 1.38 ± 0.54 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, J.; Feng, G.; Yang, J.; Zhao, F.; Shen, C.; Song, C.; Jiang, T. Otolith Microchemistry Assessment: Evidence of Migratory Coilia nasus of Yangtze River Living in the Shengsi Sea Area. Fishes 2022, 7, 172. https://doi.org/10.3390/fishes7040172
Li Y, Chen J, Feng G, Yang J, Zhao F, Shen C, Song C, Jiang T. Otolith Microchemistry Assessment: Evidence of Migratory Coilia nasus of Yangtze River Living in the Shengsi Sea Area. Fishes. 2022; 7(4):172. https://doi.org/10.3390/fishes7040172
Chicago/Turabian StyleLi, Yu, Jianhua Chen, Guangpeng Feng, Jian Yang, Feng Zhao, Chenchen Shen, Chao Song, and Tao Jiang. 2022. "Otolith Microchemistry Assessment: Evidence of Migratory Coilia nasus of Yangtze River Living in the Shengsi Sea Area" Fishes 7, no. 4: 172. https://doi.org/10.3390/fishes7040172