Assessment of the Phytochemical and Nutrimental Composition of Dark Chia Seed (Salvia hispánica L.)
Abstract
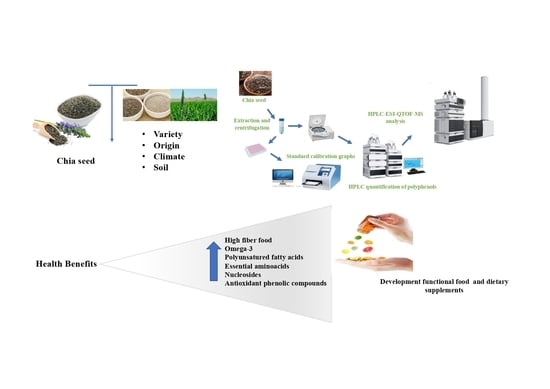
:1. Introduction
2. Materials and Methods
2.1. Proximal Analyses
2.2. Fatty Acid Profile Analysis
2.3. Phenolic Compounds Determination
Sample Preparation
2.4. HPLC–ESI–QTOF–MS Analysis
3. Results
Characterization of Chia Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A.; Hussain, J. Nutritional and therapeutic perspectives of Chia (Salvia hispanica L.): A review. J. Food Sci. Technol. 2016, 53, 1750–1758. [Google Scholar] [CrossRef] [Green Version]
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Chia seed (Salvia hispanica L.) as a source of proteins and bioactive peptides with health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 480–499. [Google Scholar] [CrossRef] [Green Version]
- European Parliament. Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients. Off. J. Eur. Communities 1997, 40, 1–7. [Google Scholar]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Alves, S.C.; Vendramini-Costa, D.B.; Cazarin, C.B.B.; Júnior, M.R.M.; Ferreira, J.P.B.; Silva, A.B.; Prado, M.A.; Bronze, M.R. Characterization of phenolic compounds in chia (Salvia hispanica L.) seeds, fiber flour and oil. Food Chem. 2017, 232, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Nadeem, M.; Imran, M. Omega-3 fatty acids and oxidative stability of ice cream supplemented with olein fraction of chia (Salvia hispanica L.) oil. Lipids Health Dis. 2017, 16, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, L.A.; Cobos, A.; Diaz, O.; Aguilera, J.M. Chia seed (Salvia hispanica): An ancient grain and a new functional food. Food Rev. Int. 2013, 29, 394–408. [Google Scholar] [CrossRef]
- Das, A. Advances in Chia Seed Research. Adv. Biotechnol. Microbiol. 2018, 5, 5–7. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Ivanovski, M.; Cör, D.; Knez, Ž. Chia Seeds (Salvia hispanica L.): An overview—phytochemical profile, isolation methods, and application. Molecules 2020, 25, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suri, S.; Passi, S.J.; Goyat, J. Chia seed (Salvia hispanica L.)—A new age functional food. In 4th International Conference on Recent Innovations in Science Engineering and Management; 2016; Volume 4, pp. 286–299. Available online: http://www.ijates.com/images/short_pdf/1459080855_695I.pdf (accessed on 11 November 2021).
- Din, Z.U.; Alam, M.; Ullah, H.; Shi, D.; Xu, B.; Li, H.; Xiao, C. Nutritional, phytochemical and therapeutic potential of chia seed (Salvia hispanica L.). A mini-review. Food Hydrocoll. Health 2021, 1, 100010. [Google Scholar] [CrossRef]
- Nitrayová, S.; Brestenský, M.; Heger, J.; Patráš, P.; Rafay, J.; Sirotkin, A. Amino acids and fatty acids profile of chia (Salvia hispanica L.) and flax (Linum usitatissimum L.) seed. Slovak J. Food Sci. 2014, 8, 72–76. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Mohamed, R.S.; Fouda, K. Anti-inflammatory potential of chia seeds oil and mucilage against adjuvant-induced arthritis in obese and non-obese rats. J. Basic Clin. Physiol. Pharmacol. 2020, 31. Available online: https://www.researchgate.net/publication/339724715_Anti-inflammatory_potential_of_chia_seeds_oil_and_mucilage_against_adjuvant-induced_arthritis_in_obese_and_non-obese_rats (accessed on 11 November 2021). [CrossRef]
- Coelho, M.S.; Salas-Mellado, M.D.L.M. Chemical characterization of chia (Salvia hispanica L.) for use in food products. J. Food Nutr. Res. 2014, 2, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Teoh, S.L.; Lai, N.M.; Vanichkulpitak, P.; Vuksan, V.; Ho, H.; Chaiyakunapruk, N. Clinical evidence on dietary supplementation with chia seed (Salvia hispanica L.): A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Blanco-Metzler, A.; Tovar, J.; Fernández-Piedra, M. Caracterización nutricional de los carbohidratos y composición centesimal de raíces y tubérculos tropicales cocidos, cultivados en Costa Rica. Arch. Latinoam. Nutr. 2004, 54, 322–327. [Google Scholar] [PubMed]
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Johnson, M.A.C.B.; Bradford, C. Omega-3, omega-6 and omega-9 fatty acids: Implications for cardiovascular and other diseases. J. Glycom. Lipidom. 2014, 4, 2153-0637. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Cruz, O.; Paredes-López, O. Phytochemical profile and nutraceutical potential of chia seeds (Salvia hispanica L.) by ultra high performance liquid chromatography. J. Chromatogr. A 2014, 1346, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Ziemichód, A.; Wójcik, M.; Różyło, R. Ocimum tenuiflorum seeds and Salvia hispanica seeds: Mineral and amino acid composition, physical properties, and use in gluten-free bread. CyTA—J. Food 2019, 17, 804–813. [Google Scholar] [CrossRef] [Green Version]
- Olivos-Lugo, B.L.; Valdivia-López, M.A.; Tecante, A. Thermal and physicochemical properties and nutritional value of the protein fraction of Mexican chia seed (Salvia hispanica L.). Food Sci. Technol. Int. 2010, 16, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pastell, H.; Putkonen, T.; Rita, H. Dietary fibre in legumes, seeds, vegetables, fruits and mushrooms: Comparing traditional and semi-automated filtration techniques. J. Food Compos. Anal. 2019, 75, 1–7. [Google Scholar] [CrossRef]
- De Falco, B.; Incerti, G.; Bochicchio, R.; Phillips, T.D.; Amato, M.; Lanzotti, V. Metabolomic analysis of Salvia hispanica seeds using NMR spectroscopy and multivariate data analysis. Ind. Crop. Prod. 2017, 99, 86–96. [Google Scholar] [CrossRef]
- De Falco, B.; Grauso, L.; Fiore, A.; Bochicchio, R.; Amato, M.; Lanzotti, V. Metabolomic analysis and antioxidant activity of wild type and mutant chia (Salvia hispanica L.) stem and flower grown under different irrigation regimes. Ind. Crop. Prod. 2018, 112, 584–592. [Google Scholar] [CrossRef] [Green Version]
- Xiang, X.; Sha, X.; Su, S.; Zhu, Z.; Guo, S.; Yan, H.; Qian, D.; Duan, J. Simultaneous determination of polysaccharides and 21 nucleosides and amino acids in different tissues of Salvia miltiorrhiza from different areas by UV-visible spectrophotometry and UHPLC with triple quadrupole MS/MS. J. Sep. Sci. 2018, 41, 996–1008. [Google Scholar] [CrossRef]
- Coelho, M.S.; Salas-Mellado, M.M. How extraction method affects the physicochemical and functional properties of chia proteins. LWT 2018, 96, 26–33. [Google Scholar] [CrossRef]
- Gómez-Favela, M.A.; Gutiérrez-Dorado, R.; Cuevas-Rodríguez, E.O.; Canizalez-Román, V.A.; Del Rosario León-Sicairos, C.; Milán-Carrillo, J.; Reyes-Moreno, C. Improvement of Chia Seeds with Antioxidant Activity, GABA, Essential Amino Acids, and Dietary Fiber by Controlled Germination Bioprocess. Plant Foods Hum. Nutr. 2017, 72, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Caruso, M.C.; Guzzo, F.; Galgano, F.; Commisso, M.; Bochicchio, R.; Labella, R.; Favati, F. Nutritional quality of seeds and leaf metabolites of Chia (Salvia hispanica L.) from Southern Italy. Eur. Food Res. Technol. 2015, 241, 615–625. [Google Scholar] [CrossRef]
- De Falco, B.; Fiore, A.; Rossi, R.; Amato, M.; Lanzotti, V. Metabolomics driven analysis by UAEGC-MS and antioxidant activity of chia (Salvia hispanica L.) commercial and mutant seeds. Food Chem. 2018, 254, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamrouni-Sellami, I.; Rahali, F.Z.; Rebey, I.B.; Bourgou, S.; Limam, F.; Marzouk, B. Total Phenolics, Flavonoids, and Antioxidant Activity of Sage (Salvia officinalis L.) Plants as Affected by Different Drying Methods. Food Bioprocess Technol. 2013, 6, 806–817. [Google Scholar] [CrossRef]
- Shen, Y.; Feng, Z.; Yang, M.; Zhou, Z.; Han, S.; Hou, J.; Li, Z.; Wu, W.; Guo, D.A. Rapid profiling of polymeric phenolic acids in Salvia miltiorrhiza by hybrid data-dependent/targeted multistage mass spectrometry acquisition based on expected compounds prediction and fragment ion searching. J. Sep. Sci. 2018, 41, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Q.; Wang, X.; Yang, J.; Wang, Q. Qualitative analysis and simultaneous quantification of phenolic compounds in the aerial parts of Salvia miltiorrhiza by HPLC-DAD and ESI/MS(n). Phytochem. Anal. 2011, 22, 247–257. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Al-Juhaimi, F.Y.; Mohamed Ahmed, I.A.; Osman, M.A.; Gassem, M.A. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chem. 2019, 278, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gahfoor, K.; Aljuhaimi, F.; Oezcan, M.M.; Uslu, N.; Hussain, S.; Babiker, E.E.; Fadimu, G. Effects of roasting on bioactive compounds, fatty acid, and mineral composition of chia seed and oil. J. Food Process. Preserv. 2018, 42, 1–7. [Google Scholar] [CrossRef]
- Beltrán-Orozco, M.C.; Martínez-Olguín, A.; Robles-Ramírez, M.C. Changes in the nutritional composition and antioxidant capacity of chia seeds (Salvia hispanica L.) during germination process. Food Sci. Biotechnol. 2020, 29, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; He, J.; Jiang, Y.; Yang, B. Quality analysis of Polygala tenuifolia root by ultrahigh performance liquid chromatography–tandem mass spectrometry and gas chromatography–mass spectrometry. J. Food Drug Anal. 2015, 23, 144–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, M.; Ye, K.; Jiang, Q.; Wang, M.; Wen, X.; Yang, J. Chemical profile of Xian-He-Cao-Chang-Yan formula and its effects on ulcerative colitis. J. Ethnopharmacol. 2021, 267, 113517. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martinez, E.J.; Zengin, G.; Ortega-Barrales, P.; Zakariyyah Aumeeruddy, M.; Locatelli, M.; Mollica, A.; Mahomoodally, M.F. Characterization of the phytochemical profiles and biological activities of Ajuga chamaepitys subsp. chia var. chia and Ajuga bombycina by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry (HPLC-ESI-MSn). Anal. Lett. 2019, 52, 852–868. [Google Scholar] [CrossRef]
- Pigni, N.B.; Aranibar, C.; Lucini Mas, A.; Aguirre, A.; Borneo, R.; Wunderlin, D.; Baroni, M.V. Chemical profile and bioaccessibility of polyphenols from wheat pasta supplemented with partially-deoiled chia flour. LWT 2020, 124, 109134. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Gerez, C.L.; Mohtar Mohtar, L.G.; Zanini, P., VI; Nazareno, M.A.; Taranto, M.P.; Iturriaga, L.B. Lactic Acid Fermentation Improved Textural Behaviour, Phenolic Compounds and Antioxidant Activity of Chia (Salvia hispanica L.) Dough. Food Technol. Biotechnol. 2017, 55, 381–389. [Google Scholar] [CrossRef]
- Samani, M.R.; D’Urso, G.; Montoro, P.; Pirbalouti, A.G.; Piacente, S. Effects of bio-fertilizers on the production of specialized metabolites in Salvia officinalis L. leaves: An analytical approach based on LC-ESI/LTQ-Orbitrap/MS and multivariate data analysis. J. Pharm. Biomed. Anal. 2021, 197, 113951. [Google Scholar] [CrossRef]
- Fan, M.; Wang, T.; Peng, L.Y.; Huang, J.S.; Wu, X.D.; Wang, H.Y.; Zhao, Q.S. Neo-clerodane Diterpenoids with Hypoglycemic Effects in Vivo from the Aerial Parts of Salvia hispanica L. Chem. Biodivers. 2021, 18, 2100517. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, G.; Choi, F.F.; Ding, L.S.; Han, Q.B.; Song, J.Z.; Qiao, C.F.; Zhao, Q.S.; Xu, H.X. Qualitative and quantitative analysis of diterpenoids in Salvia species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 4847–4858. [Google Scholar] [CrossRef] [PubMed]
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Stanoeva, J.P.; Karapandzova, M.; Stefova, M.; Dimitrovska, A.; Kulevanova, S. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr. A 2013, 1282, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zhang, Y.T.; Ye, M.; Wang, B.R.; Han, J.; Guo, D.A. Analysis of chemical constituents and taxonomic similarity of Salvia species in China using LC/MS. Planta Med. 2009, 75, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Zettel, V.; Hitzmann, B. Applications of chia (Salvia hispanica L.) in food products. Trends Food Sci. Technol. 2018, 80, 43–50. [Google Scholar] [CrossRef]
- Jin, F.; Nieman, D.C.; Sha, W.; Xie, G.; Qiu, Y.; Jia, W. Supplementation of milled chia seeds increases plasma ALA and EPA in postmenopausal women. Plant Foods Hum. Nutr. 2012, 67, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The chemical composition and nutritional value of chia seeds—Current state of knowledge. Nutrients 2019, 11, 1242. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Caudillo, E.; Tecante, A.; Valdivia-López, M.A. Dietary fibre content and antioxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107, 656–663. [Google Scholar] [CrossRef]
- Coates, W. Protein content, oil content and fatty acid profiles as potential criteria to determine the origin of commercially grown chia (Salvia hispanica L.). Ind. Crop. Prod. 2011, 34, 1366–1371. [Google Scholar]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud (OMS); FAO. Analisis de Riesgos Relativos a la Inocuidad de los Alimentos: Guía para las Autoridades Nacionales de Inocuidad de los Alimentos. 2007. Available online: https://www.fao.org/3/a0822s/a0822s00.htm (accessed on 11 November 2021).
- Stephen, A.M.; Champ, M.M.J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.E.; Ruivo, T.D.; da Silva Scapim, M.R.; Madrona, G.S.; Bergamasco, R.D.C. Optimization of the mucilage extraction process from chia seeds and application in ice cream as a stabilizer and emulsifier. LWT—Food Sci. Technol. 2016, 65, 874–883. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.G.; Pozo, A.S. Metabolismo de los nucleótidos. In Tratado de Nutrición; Editorial Médica Panamericana: Madrid, Spain, 2010; pp. 379–404. [Google Scholar]
- Muñoz-González, I.; Merino-Álvarez, E.; Salvador, M.; Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Herrero, A.M. Chia (Salvia hispanica L.) a promising alternative for conventional and gelled emulsions: Technological and lipid structural characteristics. Gels 2019, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciftci, O.N.; Przybylski, R.; Rudzińska, M. Lipid components of flax, perilla, and chia seeds. Eur. J. Lipid Sci. Technol. 2012, 114, 794–800. [Google Scholar] [CrossRef]
- Muhammad, I.; Muhammad, N.; Manzoor, M.F.; Amna, J.; Zafar, A.; Akhtar, M.N.; Muhammad, A.; Yasir, H. Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 2016, 15, 1–13. [Google Scholar]
- Ayerza, R.; Coates, W. Ground chia seed and chia oil effects on plasma lipids and fatty acids in the rat. Nutr. Res. 2005, 25, 995–1003. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Gai, F. Fatty acid and nutritive quality of chia (Salvia hispanica L.) seeds and plant during growth. Anim. Feed Sci. Technol. 2009, 148, 267–275. [Google Scholar] [CrossRef]
- Noshe, A.S.; Al-Bayyar, A.H. Effect of extraction method of Chia seeds Oil on its content of fatty acids and antioxidants. Int. Res. J. Eng. Technol. 2017, 234, 1–9. [Google Scholar]
- Singh, J.P.; Kaur, A.; Singh, B.; Singh, N.; Singh, B. Physicochemical evaluation of corn extrudates containing varying buckwheat flour levels prepared at various extrusion temperatures. J. Food Sci. Technol. 2019, 56, 2205–2212. [Google Scholar] [CrossRef]
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Zachwieja, Z. Análisis de ácidos fenólicos y flavonoides seleccionados en semillas y brotes de quinua Amaranthus cruentus y Chenopodium por HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Martirosyan, D.M.; Miroshnichenko, L.A.; Kulakova, S.N.; Pogojeva, A.V.; Zoloedov, V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The promising future of chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012, 2012. Available online: https://pubmed.ncbi.nlm.nih.gov/23251075/ (accessed on 11 November 2021). [CrossRef] [PubMed] [Green Version]
- Segura-Campos, M.R.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Salvia hispanica: Nutritional and functional potential. In Functional Properties of Traditional Foods; Springer: Boston, MA, USA, 2016; pp. 115–118. [Google Scholar]
- Ayerza, R.; Coates, W.; Lauria, M. Chia seed (Salvia hispanica L.) as an omega-3 fatty acid source for broilers: Influence on fatty acid composition, cholesterol and fat content of white and dark meats, growth performance, and sensory characteristics. Poult. Sci. 2002, 81, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Sierra, L.; Roco, J.; Alarcon, G.; Medina, M.; van Nieuwenhove, C.; de Bruno, M.P.; Jerez, S. Dietary intervention with Salvia hispanica (Chia) oil improves vascular function in rabbits under hypercholesterolaemic conditions. J. Funct. Foods 2015, 14, 641–649. [Google Scholar] [CrossRef]
- Creus, A.; Benmelej, A.; Villafañe, N.; Lombardo, Y.B. Dietary Salba (Salvia hispanica L) improves the altered metabolic fate of glucose and reduces increased collagen deposition in the heart of insulin-resistant rats. Prostaglandins Leukot. Essent. Fat. Acids 2017, 121, 30–39. [Google Scholar] [CrossRef]
- Rossi, A.S.; Oliva, M.E.; Ferreira, M.R.; Chicco, A.; Lombardo, Y.B. Dietary chia seed induced changes in hepatic transcription factors and their target lipogenic and oxidative enzyme activities in dyslipidaemic insulin-resistant rats. Br. J. Nutr. 2013, 109, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castejón, N.; Señoráns, F.J. Enzymatic modification to produce health-promoting lipids from fish oil, algae and other new omega-3 sources: A review. New Biotechnol. 2020, 57, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ayerza, R. The seed’s protein and oil content, fatty acid composition, and growing cycle length of a single genotype of chia (Salvia hispanica L.) as affected by environmental factors. J. Oleo Sci. 2009, 58, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, M.E.; Ferreira, M.R.; Chicco, A.; Lombardo, Y.B. Dietary Salba (Salvia hispanica L.) seed rich in α-linolenic acid improves adipose tissue dysfunction and the altered skeletal muscle glucose and lipid metabolism in dyslipidemic insulin-resistant rats. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ovando, J.A.; Rosado-Rubio, J.G.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Procesamiento en seco de harina de chía (Salvia hispanica L.): Caracterización química de fibra y proteína Dry processing of chía (Salvia hispanica L.) flour: Chemical characterization of fiber and protein. CyTA—J. Food 2010, 8, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Monroy-Torres, R.; Mancilla-Escobar, M.L.; Gallaga-Solórzano, J.C.; Santiago-García, E.J. Protein digestibility of chia seed Salvia hispanica L. Rev. Salud Pública Nutr. 2008, 9, 1–9. [Google Scholar]
- Capitani, M.I.; Spotorno, V.; Nolasco, S.M.; Tomás, M.C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT—Food Sci. Technol. 2012, 45, 94–102. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2014, 145, 372–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval-Oliveros, M.R.; Paredes-López, O. Isolation and characterization of proteins from chia seeds (Salvia hispanica L.). J. Agric. Food Chem. 2013, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Plimmer, R.H.A. The chemical composition of the proteins. In Monographs on Biochemistry. Part I. Analysis, 2nd ed.; Longmans, Green and Co.: London, UK, 1908; p. 82. [Google Scholar]
- FAO (Organizacion de las Naciones Unidas para la Agricultura y la Alimentacion, IT). Analítico: Contenido de Aminoacidos de los Alimentos y Datos Biologicos sobre las Proteina; FAO: Rome, Italy, 1970. [Google Scholar]
- Enriquez, C.; Sofia, G. Aporte de Aminoácidos Esenciales en Mezclas Alimenticias de Origen Vegetal por Métodos Computacionales. 2020. Available online: https://repositorio.upeu.edu.pe/handle/20.500.12840/3387 (accessed on 11 November 2021).
- Segura-Campos, M.R.; Salazar-Vega, I.M.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Biological potential of chia (Salvia hispanica L.) protein hydrolysates and their incorporation into functional foods. LWT—Food Sci. Technol. 2013, 50, 723–731. [Google Scholar] [CrossRef]
- Niers, L.; Stasse-Wolthuis, M.; Rombouts, F.M.; Rijkers, G.T. Nutritional support for the infant’s immune system. Nutr. Rev. 2007, 65, 347–360. [Google Scholar] [CrossRef]
- Di Caro, S.; Fragkos, K.C.; Keetarut, K.; Koo, H.F.; Sebepos-Rogers, G.; Saravanapavan, H.; Barragry, J.; Rogers, J.; Mehta, S.J.; Rahman, F. Enteral nutrition in adult Crohn’s disease: Toward a paradigm shift. Nutrients 2019, 11, 2222. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, G.; Lidestri, M.; Casetta, P.; Jelinek, J.; Negretti, F.; Stahl, B.; Marini, A. Supplementation of a bovine milk formula with an oligosaccharide mixture increases counts of faecal bifidobacteria in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 86, F178–F181. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Raanan, S. Nucleotides in infant nutrition: A must or an option. Isr. Med. Assoc. J. 2000, 2, 772–774. [Google Scholar] [PubMed]
- Fontana, L.; Martínez-Augustin, O.; Gil, Á. Dietary Nucleotides and Immunity. In Nutrition, Immunity, and Infection, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 387–404. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to fructooligosaccharides (FOS) from sucrose and decreasing potentially pathogenic gastro intestinal microorganisms (ID 774), changes in short chain fatty acid (SCFA) production and pH in the gastro-intestinal tract (ID 775), changes in bowel function (ID 775, 778), reduction of gastro intestinal discomfort (ID 775, 778), increase in calcium and/or magnesium absorption leading to an increase in magnesium and/or calcium retention (ID 776…). EFSA J. 2011, 9, 2023. [Google Scholar]
- Krzyzanowska, J.; Czubacka, A.; Oleszek, W. Dietary phytochemicals and human health. In Bio-Farms Nutraceuticals; Springer: Boston, MA, USA, 2012; pp. 74–98. [Google Scholar]
- Dimitrić-Marković, J. Flavonoids, the role and the importance in modern investigations. Acta Agric. Serbica 2007, 12, 25–36. [Google Scholar]
- Oleszek, W. Dietary phytochemicals and human health. Phytochem. Rev. 2002, 1, 163–166. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Ai, C.B.; Li, L.N. Salvianolic acids D and E: Two new depsides from Salvia miltiorrhiza. Planta Med. 1992, 58, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, D.M.; Burrows, G.E.; Prenzler, P.D.; Hill, R.A. Potential role of phenolic extracts of mentha in managing oxidative stress and Alzheimer’s disease. Antioxidants 2020, 9, 631. [Google Scholar] [CrossRef]
- Chen, F.Y.; Bi, L.; Qian, L.; Gao, J.; Jiang, Y.C.; Chen, W.P. Identification of multidrug resistance gene MDR1 associated microRNA of salvianolic acid A reversal in lung cancer. China J. Chin. Mater. Med. 2016, 41, 3279–3284. [Google Scholar] [CrossRef]
- Du, G.; Sun, L.; Zhao, R.; Du, L.; Song, J.; Zhang, L.; He, G.; Zhang, Y.; Zhang, J. Polyphenols: Potential source of drugs for the treatment of ischaemic heart disease. Pharmacol. Ther. 2016, 162, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hao, Y.; Ji, H.; Fang, Y.; Guo, Y.; Sha, W.; Zhou, Y.; Pang, X.; Southerland, W.M.; Califano, J.A.; et al. Combination effects of salvianolic acid B with low-dose celecoxib on inhibition of head and neck squamous cell carcinoma growth in vitro and in vivo. Cancer Prev. Res. 2010, 3, 787–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, Y.Y.; Jiang, Q.; Li, K.R.; Zhao, Y.X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Katunzi-Kilewela, A.; Kaale, L.D.; Kibazohi, O.; Rweyemamu, L.M. Nutritional, health benefits and usage of chia seeds (Salvia hispanica): A review. Afr. J. Food Sci. 2021, 15, 48–59. [Google Scholar]
- Rajput, R.; Bhullar, H.K.; Kaur, A.; Kaur, J. Chia Seeds: Composition, health benefits, and potential applications. In Cereals and Cereal-Based Foods: Functional Benefits and Technological Advances for Nutrition and Healthcare; CRC Press: Boca Raton, FL, USA, 2021; pp. 123–132. [Google Scholar]
- Melo, D.; Machado, T.B.; Oliveira, M.B.P. Chia seeds: An ancient grain trending in modern human diets. Food Funct. 2019, 10, 3068–3089. [Google Scholar] [CrossRef] [PubMed]
- Menga, V.; Amato, M.; Phillips, T.D.; Angelino, D.; Morreale, F.; Fares, C. Gluten-free pasta incorporating chia (Salvia hispanica L.) as thickening agent: An approach to naturally improve the nutritional profile and the in vitro carbohydrate digestibility. Food Chem. 2017, 221, 1954–1961. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Calibration Equation | Calibration Range (μg/mL) | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Sucrose | y = 0.1602x + 1.0751 | 10–50 | 0.9752 | 0.05 ± 0.01 | 0.18 ± 0.05 |
| Citric acid | y = 0.012x + 0.194 | 10–50 | 0.9701 | 0.9 ± 0.3 | 3.0 ± 0.9 |
| Tyrosine | y = 0.1248x + 0.0364 | 1–10 | 0.9906 | 0.071 ± 0.005 | 0.24 ± 0.02 |
| Phenylalanine | y = 0.0634x + 0.0062 | 1–10 | 0.9886 | 0.10 ± 0.01 | 0.32 ± 0.05 |
| Protocatechuic acid | y = 0.0264x − 0.0201 | 1–10 | 0.9699 | 0.31 ± 0.06 | 1.0 ± 0.2 |
| Gentisic acid | y = 0.0390x − 0.0050 | 1–10 | 0.9843 | 0.19 ± 0.03 | 0.6 ± 0.1 |
| Vanillic acid | y = 0.0293x + 0.0081 | 1–10 | 0.9877 | 0.18 ± 0.03 | 0.59 ± 0.08 |
| Caffeic acid | y = 0.2119x − 0.0094 | 0.25–5 | 0.9864 | 0.027 ± 0.004 | 0.09 ± 0.01 |
| Ferulic acid | y = 0.1207x + 0.0135 | 0.5–5 | 0.9759 | 0.041 ± 0.004 | 0.14 ± 0.01 |
| Rosmarinic acid | y = 0.1067x + 0.0398 | 0.5–20 | 0.9827 | 0.028 ± 0.005 | 0.09 ± 0.02 |
| Luteolin | y = 2.0691x + 0.0586 | 0.05–1 | 0.9933 | 0.0019 ± 0.0004 | 0.006 ± 0.001 |
| Component | Composition |
|---|---|
| Carbohydrates | 21.8% |
| Sugars | <0.1% |
| Fiber | 35.1% |
| Fat | 18.3% |
| Saturated fat | 1.2% |
| Insaturated fats | 17.1% |
| Protein | 18.8% |
| Ash | 4.7% |
| Moisture | 5.8% |
| Energy (kcal) | 388.3 |
| Energy (kJ) | 1612 |
| Fatty Acids | Composition (%) |
|---|---|
| Miristic C14:0 | 0.41 |
| Palmític C16:0 | 7.49 |
| Palmitoleic C16:1 cis-9 | 0.5 |
| Stearic C18:0 | 4.0 |
| Oleic C18:1 cis-9 | 8.34 |
| Linoleic C18:2 cis-9,12 (ω-6) | 19.5 |
| α-Linolenic C18:3 cis-6,9,15 (ω-3) | 59.2 |
| ƴ-Linolenic C18:3 cis-6,9,12 (ω-6) | 0.52 |
| W6:w3 | 3.02 |
| Atherogenic index | 0.045 |
| Thrombogenic index | 0.007 |
| Peak | RT (min) | Formula | Species | m/z | Error (ppm) | Score | MS/MS Spectra Peaks (Relative Intensity) | Proposed Compound | Chemical Nature | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.701 | - | - | 272.9574 | - | - | 158.9765 (100), 114.9868 (16.55) | Unknown | - | - |
| 2 | 3.019 | C4H8N2O3 | (M-H)− | 131.0463 | −0.52 | 99.95 | - | L-Asparagine | Amino acid | [21] |
| 3 | 3.091 | C4H7NO4 | (M-H)− | 132.0298 | 2.98 | 98.97 | 115.0025 (100) | L-Aspartic acid | Amino acid | [22,23] |
| 4 | 3.186 | C24H42O21 | (M-H) (M+Cl) (M+COOH)− | 665.2153 701.1920 711.2192 | −0.67 | 98.99 | 179.0543 (100), 665.2119 (41.13), 101.0231 (35.57), 119.0332 (25.79), 221.0643 (23.52), 161.0437 (21.31) | Stachyose | Sugar | [24] |
| 5 | 3.281 | C18H32O16 | (M-H) (M+Cl) (M+COOH)− | 503.1642 539.1411 549.1699 | −4.51 | 89.54 | 503.1593 (100), 539.1364 (50.51), 179.0544 (14.76), 119.0335 (4.15) | Raffinose isomer 1 | Sugar | [23,24] |
| 6 | 3.351 | C25H38O22 | (M-H)− | 689.1802 | −2.5 | 91.35 | 185.0206 (100) | Unknown | - | - |
| 7 | 3.511 | C12H22O11 | (M-H)− (M+COOH)- | 341.1086 387.1140 | 1.29 | 98.13 | - | Sucrose * | Sugar | [23,25] |
| 8 | 3.565 | C18H32O16 | (M-H)− (M+Cl)− (M+COOH)- | 503.1628 539.1383 549.1685 | −0.18 | 99.46 | 209.0672 (100), 503.1656 (85.25), 179.0566 (19.31) | Raffinose isomer 2 | Sugar | [23,24] |
| 9 | 4.22 | C4H6O5 | (M-H)− | 133.0145 | −1.98 | 99.52 | 115.0026 (100), 107.0357 (43.12), 133.0109 (24.5) | Malic acid | Organic acid | [25] |
| 10 | 6.623 | C6H8O7 | (M-H)− | 191.0196 | 0.71 | 99.52 | 111.0062 (100), 191.0153 (28.29), 129.0161 (8.26) | Citric acid * | Organic acid | [5,23,24,25] |
| 11 | 7.458 | C9H12N2O6 | (M-H)− (M+Cl)− (M+COOH)- | 243.0622 279.0383 289.0672 | 0.41 | 99.87 | 110.0246 (100), 200.0572 (57.92), 243.0623 (55.74), 140.0357 (23.12), 152.0348 (21.31) | Uridine | Pyrimidine nucleoside | [26] |
| 12 | 7.677 | C23H32O19 | (M-H)− | 611.1443 | 4.98 | 73.97 | 306.0776 (100), 611.1493 (65.81), 272.0894 (26.19), 338.0497 (12.97) | Unknown | - | - |
| 13 | 8.06 | C9H11NO3 | (M-H)− | 180.0667 | −0.35 | 99.65 | 119.0507 (100), 163.0405 (30.56) | L-Tyrosine * | Amino acid | [22,27,28] |
| 14 | 9.307 | C10H13N5O4 | (M-H)− (M+Cl)− (M+COOH)− | 266.0890 302.0656 312.0949 | 0.2 | 99.5 | 134.0473 (100), 266.0915 (13.86) | Adenosine | Pyrimidine nucleoside | [26] |
| 15 | 10.279 | C10H13N5O5 | (M-H)− | 282.0849 | −1.86 | 97.24 | 133.016 (100), 108.0206 (93.79), 150.0424 (32.97) | Guanosine | Pyrimidine nucleoside | [26] |
| 16 | 12.063 | C42H51NO17 | (M-H)− | 840.3096 | −0.65 | 90.76 | 840.3184 (100), 241.004 (5.54) | Unknown | - | - |
| 17 | 12.7 | C9H11NO2 | (M-H)− | 164.0716 | 0.9 | 99.68 | 103.0551 (100), 147.0452 (34.09) | L-Phenylalanine * | Amino acid | [22,27,29,30] |
| 18 | 13.549 | C8H8O4 | (M-H)− | 167.0346 | 2.6 | 98.72 | 123.0448 (100), 121.0294 (73.95), 109.0291 (33.23), 137.0243 (23.82) | Dihydroxyphenylacetic acid | Phenolic acid | [31] |
| 19 | 13.937 | C13H16O9 | (M-H)− | 315.0714 | 2.59 | 97.69 | 152.0116 (100), 108.0215 (70.99), 153.0191 (52.14), 109.0294 (37.98) | Dihydroxybenzoyl glucoside | Phenolic acid | [32] |
| 20 | 14.407 | C9H10O5 | (M-H)− | 197.0448 | 3.87 | 96.9 | 179.0359 (100), 135.0456 (57.62), 123.0456 (55.32) | Salvianic acid A | Phenolic acid | [5,30] |
| 21 | 14.829 | C14H18O9 | (M-H)− (M+Cl)− | 329.0888 365.0649 | −2.65 | 96.17 | 167.0355 (100), 123.0455 (5.58) | Vanillic acid glucoside | Phenolic acid | [32] |
| 22 | 16.284 | C10H12O5 | (M-H)− | 211.0614 | −0.9 | 99.34 | 181.0509 (100), 163.0397 (57.5) | Oresbiusin A | Catechol | [33] |
| 23 | 16.887 | C57H59N5O21 | (M-H)− | 1148.363 | 0.33 | 98.46 | 822.305 (100), 1048.3322 (90.14), 241.0041 (4.03) | Unknown | - | - |
| 24 | 16.902 | C13H12O9 | (M-H)− | 311.0393 | 5.38 | 90.4 | 149.0086 (100), 179.0345 (98.75), 135.0442 (10.77) | Caftaric acid | Phenolic acid | [34,35] |
| 25 | 17.082 | C18H24O13 | (M-H)− | 447.1142 | 0.63 | 99.01 | 152.0115 (100), 108.0214 (38.37), 109.0292 (16.72) | Dihydroxybenzoic acid apiosyl glucoside | Phenolic acid | - |
| 26 | 18.051 | C11H12N2O2 | (M-H)− | 203.0828 | −0.77 | 99.54 | 116.0502 (100), 142.0657 (26.79) | L-Tryptophan | Amino acid | [28,29,36] |
| 27 | 18.46 | C19H26O13 | (M-H)− | 461.1291 | 2.21 | 97.26 | 137.0248 (100), 461.133 (57.96), 239.0571 (22.15) | Sibiricose A3 | Phenolic acid | [37] |
| 28 | 18.911 | C7H6O3 | (M-H)− | 137.0247 | −1.61 | 99.53 | 108.0213 (100) | Dihydroxybenzaldehyde isomer 1 | Phenol | [38] |
| 29 | 19.74 | C17H22O11 | (M-H)− | 401.1081 | 2.18 | 97.34 | 267.0734 (100), 401.1126 (95.86), 249.0633 (69.13), 151.0407 (65.01), 113.0246 (46.11) | Unknown | - | - |
| 30 | 20.713 | C21H28O14 | (M-H)− (M+Cl)− | 503.1404 539.1169 | 0.56 | 99.53 | 503.1465 (100), 161.0255 (33.26), 323.08 (13.1), 281.068 (11.53) | Caffeoyl gentiobiose | Phenolic acid | [39] |
| 31 | 21.13 | C20H34O11 | (M-H)− | 449.202 | 1.98 | 98.03 | 112.9853 (100), 449.2049 (39.63), 248.9621 (46.98), 167.1072 (37.07), 180.9744 (29.26) | Unknown | - | - |
| 32 | 21.279 | C15H18O9 | (M-H)− | 341.087 | 2.46 | 97.68 | 179.0355 (100), 135.0451 (24.28) | Caffeoyl glucoside | Phenolic acid | [33] |
| 33 | 21.353 | C14H14O9 | (M-H)− | 325.0545 | 6.48 | 86.2 | - | Fertaric acid | Phenolic acid | [40] |
| 34 | 21.458 | C7H6O4 | (M-H)− | 153.0192 | 1.15 | 99.74 | 109.0294 (100), 135.0087 (21.35) | Dihydroxybenzoic acid | Phenolic acid | [41] |
| 35 | 22.244 | C18H28O9 | (M-H)− | 387.1677 | −4.14 | 92.9 | 101.0244 (100), 113.0246 (39.64), 163.1133 (20.55), 119.0346 (17), 207.1031 (11.35) | Tuberonic acid glucoside | Iridoid | [42] |
| 36 | 22.347 | C48H65N3O28 | (M-H)− | 1130.368 | 0.96 | 95.82 | 1130.3801 (100), 164.9867 (8.73) | Unknown | - | - |
| 37 | 22.509 | C48H65N3O28 | (M-H)− | 1130.3675 | 0.83 | 97.95 | - | Unknown | - | - |
| 38 | 22.623 | C36H30O16 | (M-H)− | 717.1457 | 0.84 | 98.31 | 519.0981 (100); 475.1072 (62.91), 339.0535 (36.96) | Salvianolic acid B/E | Phenolic acid | [40] |
| 39 | 22.84 | C36H27NO12 | (M-H)− | 664.1444 | 3.15 | 81.38 | 272.09 (100), 664.1502 (98.4), 502.1162 (83.26), 391.0527 (61.95), 229.0183 (29.74) | Unknown | - | - |
| 40 | 23.075 | C24H26O13 | (M-H)− | 521.1293 | 3.33 | 84.3 | 359.0653 (100), 323.0659 (91.9), 161.0182 (80.26), 197.0381 (22.23), 179.0282 (20.33) | Rosmarinic acid glucoside | Phenolic acid | [5] |
| 41 | 23.394 | C18H16O8 | (M-H)− | 359.0772 | 1.84 | 89.16 | 161.0189 (100), 197.039 (37.86), 179.0287 (15.9) | Rosmarinic acid * | Phenolic acid | [5,23,34] |
| 42 | 23.545 | C20H20O7 | (M-H)− | 371.1131 | 1.69 | 98.6 | 177.0562 (100), 162.033 (31.34), 193.0513 (22.61) | Salviandulin B/Tehuanin G/C/F /E | Iridoid | [43] |
| 43 | 23.732 | C19H18O8 | (M-H)− | 373.092 | 2.49 | 97.29 | 197.0464 (100), 135.0456 (96.83), 175.0402 (95.03), 179.0355 (33.23) | Methyl rosmarinate | Phenolic acid | [5,40] |
| 44 | 23.757 | C20H18O5 | (M-H)− | 337.1074 | 2.34 | 97.93 | 307.0632 (100), 322.0868 (47.61), 279.0662 (26.64) | Methyltanshinonate | Other compounds | [44] |
| 45 | 24.252 | C7H6O3 | (M-H)− | 137.0243 | 1.02 | 99.6 | 137.0243 (100), 109.0634 (1.37) | Dihydroxybenzaldehyde isomer 2 | Phenol | [38] |
| 46 | 24.411 | C17H14O6 | (M-H)− | 313.0706 | 3.92 | 94.39 | 161.0245 (100), 295.2296 (28.77), 313.0733 (9.18), 151.04 (7.05) | Salvianolic acid F | Phenolic acid | [45] |
| 47 | 24.74 | C17H14O7 | (M-H)− | 329.0652 | 4.74 | 92.22 | 299.02 (100), 314.0432 (74.91) | Jaceosidin | Flavonoid | [46] |
| 48 | 25.718 | C21H32O7 | (M-H)− | 395.2091 | −3.16 | 88.6 | - | Unknown | - | - |
| Peak | Compound | Calibration Curve Used | Concentration Mean ± SD (µg/g Seeds) |
|---|---|---|---|
| 7 | Sucrose | Sucrose | 5.1 × 103 ± 0.8 × 103 |
| 10 | Citric acid | Citric acid | 503.9 ± 82.1 |
| 13 | L-Tyrosine | Tyrosine | 33.8 ± 1.7 |
| 17 | L-Phenylalanine | Phenylalanine | 51.0 ± 11.9 |
| 18 | Dihydroxyphenylacetic acid | Protocatechuic acid | 22.8 ± 0.004.7 |
| 19 | Dihydroxybenzoyl glucoside | Gentisic acid | 189.6 ± 32.4 |
| 20 | Salvianic acid A | Protocatechuic acid | 40.1 ± 6.7 |
| 21 | Vanillic acid glucoside | Vanillic acid | 165.7 ± 29.1 |
| 22 | Oresbiusin A | Protocatechuic acid | 15.0 ± 2.5 |
| 24 | Caftaric acid | Caffeic acid | 7.5 ± 2.4 |
| 25 | Dihydroxybenzoic acidapiosyl glucoside | Gentisic acid | 80.5 ± 6.1 |
| 28 | Dihydroxybenzaldehyde | Gentisic acid | 56.3 ± 2.1 |
| 30 | Caffeoyl gentiobiose | Caffeic acid | 4.0 ± 0.4 |
| 32 | Caffeoyl glucoside | Caffeic acid | 9.5 ± 1.7 |
| 33 | Fertaric acid | Ferulic acid | 15.8 ± 3.7 |
| 34 | Dihydroxybenzoic acid | Gentisic acid | <LOQ * |
| 38 | Salvianolic acid B/E | Rosmarinic acid | 61.6 ± 13.5 |
| 40 | Rosmarinic acid glucoside | Rosmarinic acid | 3.9 × 103 ± 0.7 × 103 |
| 41 | Rosmarinic acid | Rosmarinic acid | 1.2 × 103 ± 0.1 × 103 |
| 43 | Methyl rosmarinate | Rosmarinic acid | 13.5 ± 0.5 |
| 47 | Jaceosidin | Luteolin | 0.7 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez Lara, A.; Mesa-García, M.D.; Medina, K.A.D.; Quirantes Piné, R.; Casuso, R.A.; Segura Carretero, A.; Huertas, J.R. Assessment of the Phytochemical and Nutrimental Composition of Dark Chia Seed (Salvia hispánica L.). Foods 2021, 10, 3001. https://doi.org/10.3390/foods10123001
Rodríguez Lara A, Mesa-García MD, Medina KAD, Quirantes Piné R, Casuso RA, Segura Carretero A, Huertas JR. Assessment of the Phytochemical and Nutrimental Composition of Dark Chia Seed (Salvia hispánica L.). Foods. 2021; 10(12):3001. https://doi.org/10.3390/foods10123001
Chicago/Turabian StyleRodríguez Lara, Avilene, María Dolores Mesa-García, Karla Alejandra Damián Medina, Rosa Quirantes Piné, Rafael A. Casuso, Antonio Segura Carretero, and Jesús Rodríguez Huertas. 2021. "Assessment of the Phytochemical and Nutrimental Composition of Dark Chia Seed (Salvia hispánica L.)" Foods 10, no. 12: 3001. https://doi.org/10.3390/foods10123001