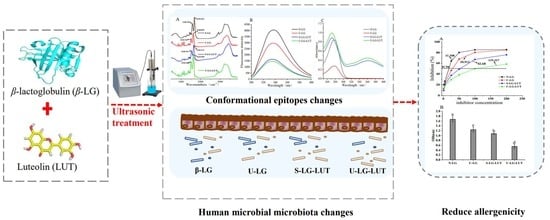
Ultrasound Improved the Non-Covalent Interaction of β-Lactoglobulin with Luteolin: Regulating Human Intestinal Microbiota and Conformational Epitopes Reduced Allergy Risks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Far-UV Circular Dichroism Spectra
2.4. Fourier Transform Infrared Measurement
2.5. Intrinsic Fluorescence Intensity and UV Spectrometry Measurement
2.6. Surface Hydrophobicity Determination
2.7. Molecular Docking
2.8. Determination of IgG/IgE Binding Ability
2.9. Degranulation of Human Basophil (KU812) Cells
2.10. Determination of Human Intestinal Microbiota
2.11. High-Throughput Sequencing and Microbial Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Infrared Spectral Analysis
3.2. Circular Dichroism Analysis
3.3. Analysis of Intrinsic Fluorescence and UV Absorption Spectra
3.4. Surface Hydrophobicity Analysis
3.5. Molecular Docking
3.6. IgG/IgE Binding Ability and Degranulation Analysis of KU812 Cells
3.7. Human Intestinal Microbiota Analysis
3.8. Ultrasound Improved the Non-Covalent Interaction of β-Lactoglobulin with Luteolin: Effect on the Structure, Allergenicity and Human Intestinal Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, X.; Yang, H.; Sun, J.; Cheng, J.; Luo, X.; Wang, Z.; Yang, M.; Bing Tao, D.; Yue, X.; Zheng, Y. Effects of enzymatic treatments on the hydrolysis and antigenicity reduction of natural cow milk. Food Sci. Nutr. 2021, 9, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yang, H.; Luo, X.; Chen, N.; Ai, Z.; Xing, Y.; Huang, W.; Wang, Z.; Zheng, Y.; Yue, X. Assessment of the allergenicity and antigenicity potential of enzymatically hydrolyzed cow milk. Food Biosci. 2022, 45, 101453. [Google Scholar] [CrossRef]
- Chen, Y.; Tu, Z.; Wang, H.; Zhang, Q.; Zhang, L.; Sha, X.; Huang, T.; Ma, D.; Pang, J.; Yang, P. The reduction in the IgE-binding ability of β-lactoglobulin by dynamic high-pressure microfluidization coupled with glycation treatment revealed by high-resolution mass spectrometry. J. Agric. Food Chem. 2017, 65, 6179–6187. [Google Scholar] [CrossRef] [PubMed]
- Exl, B.; Fritsché, R. Cow’s milk protein allergy and possible means for its prevention. Nutrition 2001, 17, 642–651. [Google Scholar] [CrossRef]
- Chanphai, P.; Bourassa, P.; Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Review on the loading efficacy of dietary tea polyphenols with milk proteins. Food Hydrocoll. 2018, 77, 322–328. [Google Scholar] [CrossRef]
- Pomés, A. Relevant B cell epitopes in allergic disease. Int. Arch. Allergy Immunol. 2010, 152, 1–11. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Li, X.; Gao, J.; Tong, P.; Lu, J.; Chen, H. Preparation, immunological characterization and polyclonal antibody development for recombinant epitope tandem derived from bovine β-lactoglobulin. Taylor Fr. 2016, 27, 806–819. [Google Scholar]
- Yhsa, B.; Yao, Z.; Mfza, B.; Jla, B.; Zctab, C. Glycation of β-lactoglobulin combined by sonication pretreatment reduce its allergenic potential. Int. J. Biol. Macromol. 2020, 164, 1527–1535. [Google Scholar]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review. Foods 2022, 11, 122. [Google Scholar] [CrossRef]
- Vukelić, I.; Detel, D.; Batičić, L.; Potočnjak, I.; Domitrović, R. Luteolin ameliorates experimental colitis in mice through ERK-mediated suppression of inflammation, apoptosis and autophagy. Food Chem. Toxicol. 2020, 145, 111680. [Google Scholar] [CrossRef]
- Pu, P.; Zheng, X.; Jiao, L.; Chen, L.; Yang, H.; Zhang, Y.; Liang, G. Six flavonoids inhibit the antigenicity of β-lactoglobulin by noncovalent interactions: A spectroscopic and molecular docking study. Food Chem. 2021, 339, 128106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Cen, C.; Zhang, J.; Wang, S.; Wang, Y.; Fu, L. Ultrasonic pre-treatment modifies the pH-dependent molecular interactions between β-lactoglobulin and dietary phenolics: Conformational structures and interfacial properties. Ultrason. Sonochem. 2021, 75, 105612. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Robert, Z.; Zeiger, R.; et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 2018, 73, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Chagoyan, O.C.; Fallani, M.; Maldonado, J.; Vieites, J.M.; Khanna, S.; Edwards, C.; Doré, J.; Gil, A. Faecal microbiota and short-chain fatty acid levels in faeces from infants with cow’s milk protein allergy. Int. Arch. Allergy Immunol. 2011, 156, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microb. 2014, 80, 2546–2554. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.H.; Zhang, Y.; Liu, J.; Tu, Z.C. Investigation into predominant peptide and potential allergenicity of ultrasonicated β-lactoglobulin digestion products. Food Chem. 2021, 361, 130099. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Hu, X.; Pan, J.; Gong, D.; Zhang, G. New insights into the binding mechanism between osthole and β-lactoglobulin: Spectroscopic, chemometrics and docking studies. Food Res. Int. 2019, 120, 226–234. [Google Scholar] [CrossRef]
- Li, X.; Dai, T.; Hu, P.; Zhang, C.; Chen, J.; Liu, C.; Li, T. Characterization the non-covalent interactions between beta lactoglobulin and selected phenolic acids. Food Hydrocoll. 2020, 105, 105761. [Google Scholar] [CrossRef]
- Ma, S.; Yang, X.; Zhao, C.; Guo, M. Ultrasound-induced changes in structural and physicochemical properties of β-lactoglobulin. Food Sci. Nutr. 2018, 6, 1053–1064. [Google Scholar] [CrossRef]
- Liu, J.; Tu, Z.; Liu, G.; Niu, C.; Yao, H.; Wang, H.; Sha, X.; Shao, Y.; Kaltashov, I.A. Ultrasonic pretreatment combined with dry-state glycation reduced the immunoglobulin E/immunoglobulin G-binding ability of α-lactalbumin revealed by high-resolution mass spectrometry. J. Agric. Food Chem. 2018, 66, 5691–5698. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ramesh, P.T.; Li, Z.X.; Hong, L. Optimisation of an extraction technique of fish allergens suitable for detection and diagnosis. Czech J. Food Sci. 2017, 35, 24–31. [Google Scholar]
- Li, M.; Ma, Y.; Ngadi, M.O. Binding of curcumin to β-lactoglobulin and its effect on antioxidant characteristics of curcumin. Food Chem. 2013, 141, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.; Qin, W. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, W.M.; Shao, Y.H.; Liu, Y.P.; Tu, Z.C. Improved antitumor activity and IgE/IgG–binding ability of α-Lactalbumin/β-lactoglobulin induced by ultrasonication prior to binding with oleic acid. J. Food Biochem. 2020, 44, e13502. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Fu, S.; Yu, H.; Zhou, L.; Liu, W.; Liu, C.; Prakash, S. Antigenicity of β-lactoglobulin reduced by combining with oleic acid during dynamic high-pressure microfluidization: Multi-spectroscopy and molecule dynamics simulation analysis. J. Dairy Sci. 2019, 102, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Cai, X.; Li, S.; Luo, L.; Wang, J.; Wang, M.; Zeng, L. Studies on the interactions of theaflavin-3, 3′-digallate with bovine serum albumin: Multi-spectroscopic analysis and molecular docking. Food Chem. 2022, 366, 130422. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Wang, J.; Zou, M.; Wang, S.; Li, Y.; Kong, Y.; Xia, L. Spectrometry researches on interaction and sonodynamic damage of riboflavin (RF) to bovine serum albumin (BSA). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 87, 1–10. [Google Scholar] [CrossRef]
- Feng, G.; Chen, C.; Liu, D.; Han, B.; Xiong, X.; Zhao, S. Study on the interaction between theasinesin and human serum albumin by fluorescence spectroscopy. J. Lumin. 2010, 130, 168–173. [Google Scholar]
- Cao, Y.; Xiong, Y.L.; Cao, Y.; True, A.D. Interfacial properties of whey protein foams as influenced by preheating and phenolic binding at neutral pH. Food Hydrocoll. 2018, 82, 379–387. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein-misfolding diseases: Getting out of shape. Nat. Int. Wkly. J. Sci. 2002, 418, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, P.P.; Andriani, R.D.; Maligan, J.M. Molecular docking studies and physicochemical properties on the interaction of xanthone with whey protein (β-lactoglobulin and α-lactalbumin). IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 12011–12019. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Du, J.; Yao, X.; Lei, R.; Zheng, X.; Liu, J.; Hu, H.; Hong, L. Interaction of curcumin with intravenous immunoglobulin: A fluorescence quenching and Fourier transformation infrared spectroscopy study. Immunobiology 2008, 213, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, H. Pathophysiologic role of histamine: Evidence clarified by histidine decarboxylase gene knockout mice. Int. Arch. Allergy Immunol. 2012, 158, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, K.J.; Kong, M.S.; Chang, H.J.; Huang, J.L. Alterations in the gut microbiotas of children with food sensitization in early life. Pediat. Allerg. Immunol. 2016, 27, 254–262. [Google Scholar] [CrossRef]
- Salgao, M.K.; Perina, N.P.; Tomé, T.M.; Mosquera, E.; Sivieri, K. Probiotic infant cereal improves children’s gut microbiota: Insights using the Simulator of Human Intestinal Microbial Ecosystem (SHIME). Food Res. Int. 2021, 143, 110292. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Tu, Z.; Chen, W.; Yuan, T. Bovine β-Lactoglobulin Covalent Modification by Flavonoids: Effect on the Allergenicity and Human Intestinal Microbiota. J. Agric. Food Chem. 2021, 69, 6820–6828. [Google Scholar] [CrossRef]









| Sample | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random (%) |
|---|---|---|---|---|
| N-LG | 13.24 ± 0.38 b | 29.14 ± 0.74 c | 22.96 ± 0.17 a | 34.64 ± 0.13 a |
| U-LG | 14.54 ± 0.20 a | 33.13 ± 0.31 b | 21.95 ± 0.23 a,b | 30.26 ± 0.36 c |
| S-LG–LUT | 2.39 ± 0.23 c | 42.37 ± 0.48 a | 22.33 ± 0.38 a,b | 32.90 ± 0.52 b |
| U-LG–LUT | 0.61 ± 0.12 d | 43.66 ± 0.76 a | 21.77 ± 0.71 b | 33.84 ± 0.81 a,b |
| Samples | Shannon | Simpson | Chao | Coverages |
|---|---|---|---|---|
| Control | 3.21 ± 0.12 a | 0.09 ± 0.01 c | 252.03 ± 10.98 a | 0.99 ± 0.00 a |
| N-LG | 1.68 ± 0.17 c | 0.45 ± 0.06 a | 227.84 ± 13.17 b | 0.99 ± 0.00 a |
| U-LG | 1.96 ± 0.33 b,c | 0.38 ± 0.09 a,b | 252.26 ± 9.04 a | 0.99 ± 0.00 a |
| S-LG–LUT | 2.39 ± 0.02 b | 0.29 ± 0.01 b | 251.65 ± 3.20 a | 0.99 ± 0.00 a |
| U-LG–LUT | 2.32 ± 0.18 b | 0.29 ± 0.06 b | 237.41 ± 2.30 a,b | 0.99 ± 0.00 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Chen, W.; Shao, Y.; Liu, J.; Tu, Z. Ultrasound Improved the Non-Covalent Interaction of β-Lactoglobulin with Luteolin: Regulating Human Intestinal Microbiota and Conformational Epitopes Reduced Allergy Risks. Foods 2022, 11, 988. https://doi.org/10.3390/foods11070988
Wang T, Chen W, Shao Y, Liu J, Tu Z. Ultrasound Improved the Non-Covalent Interaction of β-Lactoglobulin with Luteolin: Regulating Human Intestinal Microbiota and Conformational Epitopes Reduced Allergy Risks. Foods. 2022; 11(7):988. https://doi.org/10.3390/foods11070988
Chicago/Turabian StyleWang, Titi, Wenmei Chen, Yanhong Shao, Jun Liu, and Zongcai Tu. 2022. "Ultrasound Improved the Non-Covalent Interaction of β-Lactoglobulin with Luteolin: Regulating Human Intestinal Microbiota and Conformational Epitopes Reduced Allergy Risks" Foods 11, no. 7: 988. https://doi.org/10.3390/foods11070988