Effects of Extraction Processes on the Oxidative Stability, Bioactive Phytochemicals, and Antioxidant Activity of Crude Rice Bran Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
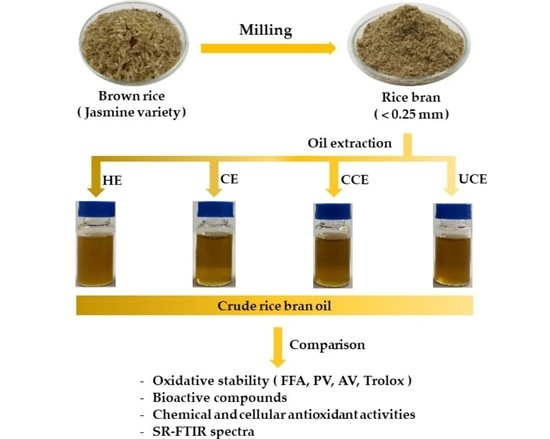
2.2. Extraction of Crude Rice Bran Oil (CRBO)
2.3. Physicochemical Analysis
2.4. Determination of Total Phenolic Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. Determination of γ-Oryzanol Content
2.7. Determination of α-Tocopherol Content
2.8. Gas Chromatography–Mass Spectrometry (GC–MS)
2.9. Determination of Chemical Antioxidant Capacity
2.10. Determination of Cellular Antioxidant Capacity
2.10.1. Cell Viability Assessment
2.10.2. Intracellular Reactive Oxygen Species (ROS) Scavenging Capacity
2.11. Synchrotron-Radiation-Based Fourier Transform Infrared (SR-FTIR)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of CRBO
3.2. Bioactive Compounds and Chemical Antioxidant Activity of CRBO
3.3. Cellular Antioxidant Activity of CRBO
3.3.1. Cell Viability Assessment
3.3.2. Intracellular Reactive Oxygen Species (ROS) Scavenging Capacity
3.4. Squalene, Sterols, and Triterpenoid in CRBO
3.5. SR-FTIR Analysis of CRBO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, D.; Hao, J.; Wang, Z.; Liang, D.; Wang, J.; Ma, Y.; Zhang, M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. LWT Food Sci. Technol. 2021, 149, 111817. [Google Scholar] [CrossRef]
- Liu, R.; Liu, R.; Shi, L.; Zhang, Z.; Zhang, T.; Lu, M.; Chang, M.; Jin, Q.; Wang, X. Effect of refining process on physicochemical parameters, chemical compositions and in vitro antioxidant activities of rice bran oil. LWT Food Sci. Technol. 2019, 109, 26–32. [Google Scholar] [CrossRef]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoed, V.; Vila Ayala, J.; Czarnowska, M.; De Greyt, W.; Verhé, R. Optimization of physical refining to produce rice bran oil with light color and high oryzanol content. J. Am. Oil Chem. Soc. 2010, 87, 1227–1234. [Google Scholar] [CrossRef]
- Mingyai, S.; Kettawan, A.; Srikaeo, K.; Singanusong, R. Physicochemical and antioxidant properties of rice bran oils produced from colored rice using different extraction methods. J. Oleo Sci. 2017, 66, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.A.; Islam, M.A.; Othman, N.H.; Noor, A.M.; Hossen, J.; Ibrahim, M. Effect of heating on compositional characteristics and oxidative stability of crude and refined rice bran oil. J. Oleo Sci. 2019, 68, 1085–1097. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Zhang, T.; Jiang, Z.; Guo, Y.; Qiu, F.; Liu, R.; Zhang, L.; Chang, M.; Liu, R.; Jin, Q.; et al. Physical properties and cellular antioxidant activity of vegetable oil emulsions with different chain lengths and saturation of triglycerides. LWT Food Sci. Technol. 2020, 121, 108948. [Google Scholar] [CrossRef]
- Oh, S.; Yi, B.; Ka, H.J.; Song, J.; Park, J.; Jung, J.; Kim, M.J.; Park, K.W.; Lee, J. Evaluation of in vitro antioxidant properties of roasted hulled barley (Hordeum vulgare L.). Food Sci. Biotechnol. 2014, 23, 1073–1079. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Hamzeh, A.; Noisa, P.; Yongsawatdigul, J. Characterization of the antioxidant and ACE-inhibitory activities of Thai fish sauce at different stages of fermentation. J. Funct. Foods 2020, 64, 103699. [Google Scholar] [CrossRef]
- Zhang, X.; Noisa, P.; Yongsawatdigul, J. Chemical and cellular antioxidant activities of in vitro digesta of tilapia protein and its hydrolysates. Foods 2020, 9, 833. [Google Scholar] [CrossRef] [PubMed]
- Yuwang, P.; Sulaeva, I.; Hell, J.; Henniges, U.; Böhmdorfer, S.; Rosenau, T.; Chitsomboon, B.; Tongta, S. Phenolic compounds and antioxidant properties of arabinoxylan hydrolysates from defatted rice bran. J. Sci. Food Agric. 2018, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative stability of selected edible oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, A.S.; Gopala Krishna, A.G. Lipid classes and subclasses of cold-pressed and solvent-extracted oils from commercial indian niger (Guizotia abyssinica (L.f.) Cass.) seed. J. Am. Oil Chem. Soc. 2014, 91, 1205–1216. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B. Application of FTIR spectroscopy for monitoring the stabilities of selected vegetable oils during thermal oxidation. Int. J. Food Prop. 2013, 16, 1594–1603. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zhang, H.; Ma, J.; Tuchiya, T.; Miao, Y. Determination of the degree of degradation of frying rapeseed oil using Fourier-transform infrared spectroscopy combined with partial least-squares regression. Int. J. Anal. Chem. 2015, 2015, 185367. [Google Scholar] [CrossRef] [Green Version]
- Matwijczuk, A.; Oniszczuk, T.; Matwijczuk, A.; Chruściel, E.; Kocira, A.; Niemczynowicz, A.; Wójtowicz, A.; Combrzyński, M.; Wiącek, D. Use of FTIR spectroscopy and chemometrics with respect to storage conditions of Moldavian dragonhead oil. Sustainability 2019, 11, 6414. [Google Scholar] [CrossRef] [Green Version]
- Javed, F.; Ahmad, S.W.; Rehman, A.; Zafar, S.; Malik, S.R. Recovery of rice bran oil using solid-liquid extraction technique. J. Food Process Eng. 2015, 38, 357–362. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, J.; Miao, Y.; Tuchiya, T.; Chen, J.Y. Analysis of carbonyl value of frying oil by Fourier transform infrared spectroscopy. J. Oleo Sci. 2015, 64, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573, 459–465. [Google Scholar] [CrossRef]
- Yu, D.; Wang, T.; Chen, J.; Tang, H.; Li, D.; Zhang, X.; Geng, H.; Wang, L.; Elfalleh, W.; Jiang, L. Enzymatic esterification of rice bran oil and phytosterol in supercritical CO2. J. Food Process. Preserv. 2019, 43, e14066. [Google Scholar] [CrossRef]
- Trevisani Juchen, P.; Araujo, M.N.; Hamerski, F.; Corazza, M.L.; Voll, F.A.P. Extraction of parboiled rice bran oil with supercritical CO2 and ethanol as co-solvent: Kinetics and characterization. Ind. Crops Prod. 2019, 139, 111506. [Google Scholar] [CrossRef]
- Pandey, R.; Shrivastava, S.L. Comparative evaluation of rice bran oil obtained with two-step microwave assisted extraction and conventional solvent extraction. J. Food Eng. 2018, 218, 106–114. [Google Scholar] [CrossRef]
- Phan, V.M.; Junyusen, T.; Liplap, P.; Junyusen, P. Effects of ultrasonication and thermal cooking pretreatments on the extractability and quality of cold press extracted rice bran oil. J. Food Process Eng. 2018, 42, e12975. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Wongyai, S.; McClements, D.J.; Decker, E.A. Effect of stabilization of rice bran by domestic heating on mechanical extraction yield, quality, and antioxidant properties of cold-pressed rice bran oil (Oryza saltiva L.). LWT Food Sci. Technol. 2012, 48, 231–236. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Siroha, A.K.; Purewal, S.S. Rice bran oil: Emerging trends in extraction, health benefit, and its industrial application. Rice Sci. 2021, 28, 217–232. [Google Scholar] [CrossRef]
- Phan, V.M.; Junyusen, T.; Liplap, P.; Junyusen, P. Optimization of chemical neutralization parameters for enhanced rice bran oil recovery and quality. Suranaree J. Sci. Technol. 2019, 26, 198–211. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS: Champaign, IL, USA, 1997. [Google Scholar]
- Jun, H.I.; Song, G.S.; Yang, E.I.; Youn, Y.; Kim, Y.S. Antioxidant activities and phenolic compounds of pigmented rice bran extracts. J. Food Sci. 2012, 77, C759–C764. [Google Scholar] [CrossRef]
- Phan, V.M.; Junyusen, T.; Liplap, P.; Junyusen, P. Isolation and characterization of gamma oryzanol from rice bran oil soapstock. Suranaree J. Sci. Technol. 2020, 27, 1–12. [Google Scholar]
- Chatchavanthatri, N.; Junyusen, T.; Arjharn, W.; Treeamnuk, T.; Junyusen, P.; Pakawanit, P. Effects of parboiling and infrared radiation drying on the quality of germinated brown rice. J. Food Process. Preserv. 2021, 45, e15892. [Google Scholar] [CrossRef]
- Kongmon, E.; Jitvisate, M.; Panchaisri, B.; Techarang, J.; Thumanu, K.; Rimjaem, S. Classification of ion-beam-induced traits in Thai jasmine rice mutants using synchrotron radiation FTIR microspectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B 2020, 465, 37–41. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M.; Nowrouzieh, S.; Alishah, O. The influence of cooking process on the microwave-assisted extraction of cottonseed oil. J. Food Sci. Technol. 2015, 52, 1138–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.S.; Wang, L.J.; Li, D.; Jiao, S.S.; Chen, X.D.; Mao, Z.H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008, 62, 192–198. [Google Scholar] [CrossRef]
- Aksoylu Özbek, Z.; Günç Ergönül, P. Determination of physicochemical properties, fatty acid, tocopherol, sterol, and phenolic profiles of expeller–pressed poppy seed oils from turkey. J. Am. Oil Chem. Soc. 2020, 97, 591–602. [Google Scholar] [CrossRef]
- Alfaro, L.; Zhang, J.; Chouljenko, A.; Scott, R.; Xu, Z.; Bankston, D.; Bechtel, P.J.; Sathivel, S. Development and characterization of emulsions containing purple rice bran and brown rice oils. J. Food Process. Preserv. 2017, 41, e13149. [Google Scholar] [CrossRef]
- Rajam, L.; Soban Kumar, D.R.; Sundaresan, A.; Arumughan, C. A novel process for physically refining rice bran oil through simultaneous degumming and dewaxing. J. Am. Oil Chem. Soc. 2005, 82, 213–220. [Google Scholar] [CrossRef]
- Charoonratana, T. Cold pressed rice (Oryza sativa) bran oil. In Cold Pressed Oil, 1st ed.; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 391–403. [Google Scholar]
- Lavanya, M.N.; Saikiran, K.C.H.S.; Venkatachalapathy, N. Stabilization of rice bran milling fractions using microwave heating and its effect on storage. J. Food Sci. Technol. 2019, 56, 889–895. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, W.; Chu, F.; Pei, D. Improvement of the flavor and oxidative stability of walnut oil by microwave pretreatment. J. Am. Oil Chem. Soc. 2016, 93, 1563–1572. [Google Scholar] [CrossRef]
- Rekha, B.; Lokesh, B.R.; Gopala Krishna, A.G. Chemistry of color fixation in crude, physically refined and chemically refined rice bran oils upon heating. J. Am. Oil Chem. Soc. 2014, 91, 1665–1676. [Google Scholar] [CrossRef]
- Fengxia, S.; Dishun, Z.; Zhanming, Z. Determination of oil color by image analysis. J. Am. Oil Chem. Soc. 2001, 78, 749–752. [Google Scholar] [CrossRef]
- Zimare, S.B.; Mankar, G.D.; Barmukh, R.B. Optimization of ultrasound-assisted extraction of total phenolics and flavonoids from the leaves of Lobelia nicotianifolia and their radical scavenging potential. Curr. Res. Green Sustain. Chem. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Mingyai, S.; Srikaeo, K.; Kettawan, A.; Singanusong, R.; Nakagawa, K.; Kimura, F.; Ito, J. Effects of extraction methods on phytochemicals of rice bran oils produced from colored rice. J. Oleo Sci. 2018, 67, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codex Alimentarius Commission. Standard for Named Vegetable Oils. Codex Stan 210; Codex Alimentarius Commission: Rome, Italy, 1999. [Google Scholar]
- Bakota, E.L.; Winkler-Moser, J.K.; Hwang, H.S. Properties of rice bran oil-derived functional ingredients. Lipid Technol. 2014, 26, 179–182. [Google Scholar] [CrossRef]
- Ali, M.A.; Islam, M.A.; Hossen, J.; Ibrahim, M. Antioxidative evaluation of solvent extracts and fractions of oil refining steps from rice bran. Int. Food Res. J. 2019, 26, 791–799. [Google Scholar]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gerardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Wu, G.; Shen, Y.; Nie, R.; Li, P.; Jin, Q.; Zhang, H.; Wang, X. The bioactive compounds and cellular antioxidant activity of Herbaceous peony (Paeonia lactiflora Pall) seed oil from China. J. Food Sci. 2020, 85, 3815–3822. [Google Scholar] [CrossRef]
- Shimizu, N.; Ito, J.; Kato, S.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Significance of squalene in rice bran oil and perspectives on squalene oxidation. J. Nutr. Sci. Vitaminol. 2019, 65, S62–S66. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Liu, R.; Chang, M.; Jin, Q.; Wang, X. Composition of rice bran stearin from various refineries across China. J. Am. Oil Chem. Soc. 2016, 93, 869–877. [Google Scholar] [CrossRef]







| Extraction Method | Yield (g Oil/100 g RB) | FFA (% Oleic acid) | PV (Meq O2/kg Oil) | ρ-AV | TOTOX | Lovibond Color Value | ||
|---|---|---|---|---|---|---|---|---|
| Red (R) | Yellow (Y) | 5R + Y | ||||||
| HE | 18.41 ± 0.50 a | 4.94 ± 0.11 a | 8.01 ± 0.10 a | 0.30 ± 0.01 a | 16.32 ± 0.05 a | 1.50 ± 0.02 a | 7.50 ± 0.01 a | 15.00 ± 0.05 a |
| CE | 14.27 ± 0.56 c | 3.31 ± 0.11 b | 5.09 ± 0.17 b | 0.20 ± 0.01 b | 10.38 ± 0.07 b | 1.00 ± 0.02 c | 5.40 ± 0.01 c | 10.40 ± 0.05 d |
| CCE | 17.31 ± 0.23 b | 3.53 ± 0.14 b | 5.11 ± 0.12 b | 0.19 ± 0.01 b | 10.41 ± 0.05 b | 1.00 ± 0.01 c | 5.50 ± 0.02 c | 11.00 ± 0.06 c |
| UCE | 16.68 ± 0.71 b | 4.97 ± 0.15 a | 8.33 ± 0.11 a | 0.31 ± 0.01 a | 16.97 ± 0.05 a | 1.25 ± 0.02 b | 6.50 ± 0.02 b | 12.75 ± 0.05 b |
| Extraction Method | γ-Oryzanol (g/100 g) | α-Tocopherol (mg/g) | TPC (mg GAE/g) | TFC (mg CE/g) | DPPH (mg TEAC/g) | ABTS (mg TEAC/g) | FRAP (mg TEAC/g) |
|---|---|---|---|---|---|---|---|
| HE | 1.83 ± 0.04 a | 0.51 ± 0.03 a | 6.51 ± 0.25 a | 1.95 ± 0.02 a | 5.94 ± 0.28 a | 9.38 ± 0.15 b | 3.55 ± 0.15 ab |
| CE | 1.80 ± 0.05 ab | 0.48 ± 0.03 ab | 6.57 ± 0.21 a | 1.94 ± 0.02 a | 6.21 ± 0.28 a | 10.35 ± 0.15 a | 3.75 ± 0.11 a |
| CCE | 1.82 ± 0.05 a | 0.50 ± 0.02 a | 6.43 ± 0.12 ab | 1.94 ± 0.05 a | 5.97 ± 0.25 a | 9.93 ± 0.17 ab | 3.50 ± 0.10 ab |
| UCE | 0.99 ± 0.02 c | 0.23 ± 0.01 c | 3.79 ± 0.15 c | 0.35 ± 0.01 b | 2.95 ± 0.14 b | 4.97 ± 0.19 c | 2.40 ± 0.09 b |
| Correlation | γ-Oryzanol | α-Tocopherol | TPC | TFC | DPPH | ABTS |
|---|---|---|---|---|---|---|
| α-Tocopherol | 0.994 * | |||||
| TPC | 0.996 * | 0.992 * | ||||
| TFC | 0.998 * | 0.986 * | 0.994 * | |||
| DPPH | 0.992 * | 0.986 * | 0.998 * | 0.991 * | ||
| ABTS | 0.981 * | 0.963 * | 0.986 * | 0.986 * | 0.991 * | |
| FRAP | 0.975 * | 0.967 * | 0.989 * | 0.973 * | 0.994 * | 0.984 * |
| Extraction Method | Squalene (% in CRBO) | Phytosterols (% in CRBO, % Total Phytosterols) | 24-Methylenecycloartanol (% in CRBO) | ||
|---|---|---|---|---|---|
| Campesterol | Stigmasterol | β-Sitosterol | |||
| HE | 0.14 | 0.25, 20.31 | 0.25, 20.47 | 0.73, 59.22 | 0.57 |
| CE | 0.15 | 0.28, 20.83 | 0.28, 21.36 | 0.76, 57.80 | 0.63 |
| CCE | 0.18 | 0.36, 25.69 | 0.33, 23.52 | 0.70, 50.79 | 0.63 |
| UCE | 0.15 | 0.32, 25.54 | 0.28, 22.44 | 0.66, 52.02 | 0.54 |
| Wavenumber (cm−1) | Type of Vibration and Functional Group Assignment |
|---|---|
| 3008 | C–H stretching of the cis-double bond (C=CH) |
| 2918, 2921 | C–H asymmetric stretching of the aliphatic CH2 group in triglycerides |
| 2850, 2852 | C–H symmetric stretching of the aliphatic CH2 group in triglycerides |
| 1743 | C=O stretching of the carbonyl group in ester groups of triglycerides |
| 1711 | C=O stretching of the carbonyl group in acidic groups of free fatty acids |
| 1462 | C–H bending (scissoring) of the aliphatic CH2 and CH3 groups |
| 1377 | C–H symmetric bending of the CH3 group |
| 1235, 1237 | C–O stretching of the ester group |
| 1158, 1160 | C–O stretching of the ester group |
| 1096 | C–O stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junyusen, T.; Chatchavanthatri, N.; Liplap, P.; Junyusen, P.; Phan, V.M.; Nawong, S. Effects of Extraction Processes on the Oxidative Stability, Bioactive Phytochemicals, and Antioxidant Activity of Crude Rice Bran Oil. Foods 2022, 11, 1143. https://doi.org/10.3390/foods11081143
Junyusen T, Chatchavanthatri N, Liplap P, Junyusen P, Phan VM, Nawong S. Effects of Extraction Processes on the Oxidative Stability, Bioactive Phytochemicals, and Antioxidant Activity of Crude Rice Bran Oil. Foods. 2022; 11(8):1143. https://doi.org/10.3390/foods11081143
Chicago/Turabian StyleJunyusen, Tiraporn, Natthaporn Chatchavanthatri, Pansa Liplap, Payungsak Junyusen, Van Man Phan, and Siriwan Nawong. 2022. "Effects of Extraction Processes on the Oxidative Stability, Bioactive Phytochemicals, and Antioxidant Activity of Crude Rice Bran Oil" Foods 11, no. 8: 1143. https://doi.org/10.3390/foods11081143