Liquid Hot Water Pretreatment and Enzymatic Hydrolysis as a Valorization Route of Italian Green Pepper Waste to Delivery Free Sugars
Abstract
:1. Introduction
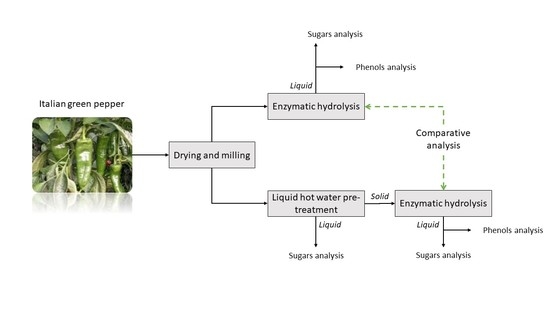
2. Materials and Methods
2.1. Raw Material: Italian Green Pepper Waste
2.2. Characterization of Italian Green Pepper Residue Samples
2.3. Liquid Hot Water Pretreatment
2.4. Enzymatic Hydrolysis (Saccharification Step)
2.5. Analytical Methods
2.6. Parameters Definition
- Solid yield (SY, %)
- Component concentration in the pretreated material
- Recovery of components after pretreatment
- Saccharification efficiency (SE, %)
- Global efficiency (GE, %)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Green Pepper Waste Characterization
3.2. Performance of Hydrothermal Pretreatment
3.2.1. Solid Yield
3.2.2. Recovery of Sugars
3.2.3. Content of Structural Components in Residual Solids
3.3. Performance of the Enzymatic Hydrolysis
3.4. Global Efficiency of a Two-step Process of Pretreatment and Consecutive Enzymatic Hydrolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Albergamo, A.; Costa, R.; Bartolomeo, G.; Rando, R.; Vadalà, R.; Nava, V.; Gervasi, T.; Toscano, G.; Germanò, M.P.; D’Angelo, V.; et al. Grape water: Reclaim and valorization of a by-product from the industrial cryoconcentration of grape (Vitis vinifera) must. J. Sci. Food Agric. 2020, 100, 2971–2981. [Google Scholar] [CrossRef]
- Albergamo, A.; Salvo, A.; Carabetta, S.; Arrigo, S.; Di Sanzo, R.; Costa, R.; Dugo, G.; Russo, M. Development of an antioxidant formula based on peanut by-products and effects on sensory properties and aroma stability of fortified peanut snacks during storage. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Eurostat. The Fruit and Vegetable Sector in the EU—A Statistical Overview; Eurostat: Luxembourg, 2015. [Google Scholar]
- Krauß, S.; Vetter, W. Stable carbon and nitrogen isotope ratios of red bell pepper samples from Germany, The Netherlands, and Spain. J. Agric. Food Chem. 2019, 67, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Statistica. Available online: https://es.statista.com/estadisticas/529468/producciones-de-pimientos-frescos-en-el-mundo (accessed on 12 August 2020).
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 12 August 2020).
- Statistics from Ministry of Agriculture, Fisheries and Food (Spain). Available online: https://www.mapa.gob.es/es/estadistica/temas/default.aspx (accessed on 12 August 2020).
- National Institute of Statistics—Agriculture and the Environment (Spain). Available online: http://www.ine.es/dyngs/INEbase/es/categoria.htm?c=Estadistica_P&cid=1254735727106 (accessed on 12 August 2020).
- FEPEX, Spanish Federation of Associations of Exporting Producers of Fruits, Vegetables, Flowers and Live Plants. Available online: https://www.fepex.es/inicio.aspx (accessed on 12 August 2020).
- EUROSTAT. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 12 August 2020).
- FRUITLOGISTICA. European Statistics Handbook. Available online: https://www.fruitlogistica.de/media/fl/fl_dl_all/auf_einen_blick/European_Statistics_Handbook_2019.pdf (accessed on 12 August 2020).
- Díaz, A.I.; Laca, A.; Laca, A.; Díaz, M. Treatment of supermarket vegetable wastes to be used as alternative substrates in bioprocesses. Waste Manag. 2017, 67, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, E.; Muñoz, M.J.; Martín, R.; Caro, I.; Curbelo, C.; Díaz, A.B. Alkaline and alkaline peroxide pretreatments at mild temperature to enhance enzymatic hydrolysis of rice hulls and straw. Bioresour. Technol. 2014, 167, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Intanakul, P.; Krairiksh, M.; Kitchaiya, P. Enhancement of enzymatic hydrolysis of lignocellulosic wastes by microwave pretreatment under atmospheric pressure. J. Wood Chem. Technol. 2003, 23, 217–225. [Google Scholar] [CrossRef]
- Loow, Y.L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential use of deep eutectic solvents to facilitate lignocellulosic biomass utilization and conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, Y.; Li, Y. Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour. Technol. 2011, 102, 6254–6259. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhuang, X.; Yuan, Z.; Wang, Q.; Qi, W.; Wang, W.; Zhang, Y.; Xu, J.; Xu, H. Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2010, 101, 4895–4899. [Google Scholar] [CrossRef] [PubMed]
- Panahi, H.K.S.; Dehhaghi, M.; Aghbashlo, M.; Karimi, K.; Tabatabaei, M. Conversion of residues from agro-food industry into bioethanol in Iran: An under-valued biofuel additive to phase out MTBE in gasoline. Renew. Energy 2020, 145, 699–710. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pretreatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef] [Green Version]
- Del Campo, I.; Alegría, I.; Zazpe, M.; Echeverría, M.; Echeverría, I. Diluted acid hydrolysis pretreatment of agri-food wastes for bioethanol production. Ind. Crops Prod. 2006, 24, 214–221. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Qiamuddin, K.; Majrashi, A.; Dalorima, T.; Hailmi, M.S.; Sharif Hossain, A.B.M. Bio-ethanol production from fruit and vegetable waste by using saccharomyces cerevisiae. Biosci. Res. 2018, 15, 1703–1711. [Google Scholar]
- Morais, A.R.C.; Mata, A.C.; Bogel-Lukasik, R. Integrated conversion of agroindustrial residue with high pressure CO2 within the biorefinery concept. Green Chem. 2014, 16, 4312–4322. [Google Scholar] [CrossRef] [Green Version]
- Morais, A.R.C.; Da Costa Lopes, A.M.; Bogel-Łukasik, R. Carbon dioxide in biomass processing: Contributions to the green biorefinery concept. Chem. Rev. 2015, 115, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Polanowska, K.; Nowak, J.; Łukasik, R.M. Efficient extraction of vicine from faba beans using reactive system of high-pressure CO2/water. J. CO2 Util. 2019, 33, 473–477. [Google Scholar] [CrossRef]
- Özbek, H.N.; Fockink, D.H.; Yanık, D.K.; Göğüş, F.; Lukasik, R. The green biorefinery concept for the valorisation of pistachio shell by high-pressure CO2/H2O system. J. Clean. Prod. 2018, 196, 842–851. [Google Scholar] [CrossRef] [Green Version]
- Ao, T.; Luo, Y.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. Towards zero waste: A valorization route of washing separation and liquid hot water consecutive pretreatment to achieve solid vinasse based biorefinery. J. Clean. Prod. 2020, 248, 119253. [Google Scholar] [CrossRef]
- Ballesteros, I.; Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, M. Ethanol production from olive oil extraction residue pretreated with hot water. In Applied Biochemistry and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2002; Volume 98–100, pp. 717–732. [Google Scholar]
- Chu, Q.; Song, K.; Hu, J.; Bu, Q.; Zhang, X.; Chen, X. Integrated process for the coproduction of fermentable sugars and lignin adsorbents from hardwood. Bioresour. Technol. 2019, 289, 121659. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Liquid hot water pretreatment of cellulosic biomass. Methods Mol. Biol. 2009, 581, 93–102. [Google Scholar]
- Li, M.; Cao, S.; Meng, X.; Studer, M.; Wyman, C.E.; Ragauskas, A.J.; Pu, Y. The effect of liquid hot water pretreatment on the chemical-structural alteration and the reduced recalcitrance in poplar. Biotechnol. Biofuels 2017, 10, 237. [Google Scholar] [CrossRef]
- Manzanares, P.; Ballesteros, I.; Negro, M.J.; González, A.; Oliva, J.M.; Ballesteros, M. Processing of extracted olive oil pomace residue by hydrothermal or dilute acid pretreatment and enzymatic hydrolysis in a biorefinery context. Renew. Energy 2020, 145, 1235–1245. [Google Scholar] [CrossRef]
- Pérez, J.A.; Ballesteros, I.; Ballesteros, M.; Sáez, F.; Negro, M.J.; Manzanares, P. Optimizing Liquid Hot Water pretreatment conditions to enhance sugar recovery from wheat straw for fuel-ethanol production. Fuel 2008, 87, 3640–3647. [Google Scholar] [CrossRef]
- Tian, D.; Shen, F.; Yang, G.; Deng, S.; Long, L.; He, J.; Zhang, J.; Huang, C.; Luo, L. Liquid hot water extraction followed by mechanical extrusion as a chemical-free pretreatment approach for cellulosic ethanol production from rigid hardwood. Fuel 2019, 252, 589–597. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, W.; Yu, Q.; Qi, W.; Wang, Q.; Tan, X.; Zhou, G.; Yuan, Z. Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour. Technol. 2016, 199, 68–75. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.M.; Drielak, E.; Surendra, K.C.; Kumar Khanal, S. Hot water pretreatment of lignocellulosic biomass: Modeling the effects of temperature, enzyme and biomass loadings on sugar yield. Bioresour. Technol. 2020, 300, 122593. [Google Scholar] [CrossRef] [PubMed]
- ASTM D1106-96:2013. Standard Test Method for Acid-Insoluble Lignin in Wood; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Wise, L.E.; Murphy, M.; D’Addieco, A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade J. 1946, 122, 35–43. [Google Scholar]
- ASTM D1103-60:1977. Withdrawn Standard: Method of Test for Alpha-Cellulose in Wood (Withdrawn 1985); ASTM International: West Conshohocken, PA, USA, 1977. [Google Scholar]
- ASTM D1107-96:2013. Standard Test Method for Ethanol-Toluene Solubility of Wood; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ISO 18123:2015. Solid Biofuels—Determination of the Content of Volatile Matter; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ISO 18122:2015. Solid Biofuels—Determination of Ash Content; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ISO 18134-1:2015. Solid Biofuels—Determination of Moisture Content. Oven Dry Method. Total Moisture. Reference Method; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Jang, S.K.; Choi, J.H.; Kim, J.H.; Kim, H.; Jeong, H.; Choi, I.G. Statistical analysis of glucose production from Eucalyptus pellita with individual control of chemical constituents. Renew. Energy 2020, 148, 298–308. [Google Scholar] [CrossRef]
- Binod, P.; Janu, K.U.; Sindhu, R.; Pandey, A. Chapter 10—Hydrolysis of lignocellulosic biomass for bioethanol production. Biofuels. Altern. Feedstocks Convers. Process. 2011, 229–250. [Google Scholar] [CrossRef]
- Larnaudie, V.; Ferrari, M.D.; Lareo, C. Enzymatic hydrolysis of liquid hot water-pretreated switchgrass at high solid content. Energy Fuels 2019, 33, 4361–4368. [Google Scholar] [CrossRef]
- Greenberg, A.E.; Clesceri, L.S.; Eaton, A. Standard Methods for the Examination of Water and Wastewater, APHA/AWWA/WEF, 22nd ed.; Cabs: Washington, DC, USA, 2005. [Google Scholar]
- Quesada, L.; Pérez, A.; Calero, M.; Blázquez, G.; Martín-Lara, M.A. Kinetic study of thermal degradation of olive cake based on a scheme of fractionation and its behavior impregnated of metals. Bioresour. Technol. 2018, 261, 104–116. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/787811/nutrients (accessed on 12 August 2020).
- Callejón-Ferre, A.J.; Carreño-Sánchez, J.; Suárez-Medina, F.J.; Pérez-Alonso, J.; Velázquez-Martí, B. Prediction models for higher heating value based on the structural analysis of the biomass of plant remains from the greenhouses of Almería (Spain). Fuel 2014, 116, 377–387. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers (Basel) 2017, 9, 495. [Google Scholar] [CrossRef]
- Vinay, D. Nutritional evaluation of pea peel and pea peel extracted byproducts. Int. J. Food Sci. Nutr. 2018, 3, 65–67. [Google Scholar]
- Singh, A.; Kuila, A.; Adak, S.; Bishai, M.; Banerjee, R. Utilization of vegetable wastes for bioenergy generation. Agric. Res. 2012, 1, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Pinna-Hernández, M.G.; Martínez-Soler, I.; Díaz Villanueva, M.J.; Acien Fernández, F.G.; López, J.L.C. Selection of biomass supply for a gasification process in a solar thermal hybrid plant for the production of electricity. Ind. Crops Prod. 2019, 137, 339–346. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Martínez-Guirado, C.; Del Mar Rebolloso-Fuentes, M.; Carrique-Pérez, A. Nutrient composition and antioxidant activity of 10 pepper (Capsicum annuun) varieties. Eur. Food Res. Technol. 2006, 224, 1–9. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Ronda, A.; Zamora, M.C.; Calero, M. Torrefaction of olive tree pruning: Effect of operating conditions on solid product properties. Fuel 2017, 109–117. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Shah, A. Techno-Economic bottlenecks of the fungal pretreatment of lignocellulosic biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Gallina, G.; Cabeza, Á.; Grénman, H.; Biasi, P.; García-Serna, J.; Salmi, T. Hemicellulose extraction by hot pressurized water pretreatment at 160 °C for 19 different woods: Yield and molecular weight. J. Supercrit. Fluids 2018, 133, 716–725. [Google Scholar] [CrossRef] [Green Version]
- Mok, W.S.L.; Antal, M.J. Uncatalyzed solvolysis of whole biomass hemicellulose by hot compressed liquid water. Ind. Eng. Chem. Res. 1992, 31, 1157–1161. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, P.; Xue, S.; Kong, F.; Ren, H.; Zhai, H. Green biorefinery—The ultra-high hydrolysis rate and behavior of Populus tomentosa hemicellulose autohydrolysis under moderate subcritical water conditions. RSC Adv. 2020, 10, 18908–18917. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Horváth, I.S. Sustainable and efficient sugar production from wheat straw by pretreatment with biogas digestate. RSC Adv. 2019, 9, 27692–27701. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 112, 252–262. [Google Scholar] [CrossRef]
- Hongdan, Z.; Shaohua, X.; Shubin, W. Enhacement of enzymatic saccharification of sugarcane bagasse by liquid hot wáter pretreatment. Bioresour. Technol. 2013, 143, 391–396. [Google Scholar] [CrossRef]
- Cara, C.; Romero, I.; Oliva, J.M.; Sáez, F.; Castro, E. Liquid Hot Water Pretreatment of Olive Tree Pruning Residues. In Applied Biochemistry and Biotechnology. ABAB Symposium; Mielenz, J.R., Klasson, K.T., Adney, W.S., McMillan, J.D., Eds.; Humana Press: Totowa, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Wang, X.-J.; Hsiao, K.-C. Sugar degradation during autoclaving: Effects of duration and solution volume on breakdown of glucose. Physiol. Plant. 1995, 94. [Google Scholar] [CrossRef]
- Orozco, A.M.; Al-Muhtaseb, A.H.; Albadarin, A.B.; Rooney, D.; Walker, G.M.; Ahmad, M.N.M. Acid-catalyzed hydrolysis of cellulose and cellulosic waste using a microwave reactor system. RSC Adv. 2011, 1, 839–846. [Google Scholar] [CrossRef]
- Huang, J.; He, C.; Wu, L.; Tong, H. Thermal degradation reaction mechanism of xylose: A DFT study. Chem. Phys. Lett. 2016, 658, 114–124. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Gullón, P.; González-Muñoz, M.J.; Parajó, J.C. Manufacture and prebiotic potential of oligosaccharides derived from industrial solid wastes. Bioresour. Technol. 2011, 102, 6112–6119. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.M.; El-Wahab, Z.H.A.; Ibrahim, A.A.; Al-Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Ázar, R.I.S.; Bordignon-Junior, E.; Laufer, C.; Specht, J.; Ferrier, D.; Kim, D. Effect of lignin content on cellulolytic saccharification of liquid hot water pretreated sugarcane bagasse. Molecules 2020, 25, 623. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Chen, D.; Yong, Q.; Huang, C.; Huang, S. Improving enzymatic hydrolysis of acid-pretreated bamboo residues using amphiphilic surfactant derived from dehydroabietic acid. Bioresour. Technol. 2019, 293, 122055. [Google Scholar] [CrossRef]
- Aguilar, D.L.; Rodríguez-Jasso, R.M.; Zanuso, E.; de Rodríguez, D.J.; Amaya-Delgado, L.; Sanchez, A.; Ruiz, H.A. Scale-up and evaluation of hydrothermal pretreatment in isothermal and non-isothermal regimen for bioethanol production using agave bagasse. Bioresour. Technol. 2018, 263, 112–119. [Google Scholar] [CrossRef]
- Ra, C.H.; Choi, J.G.; Kang, C.-H.; Sunwoo, I.Y.; Jeong, G.-T.; Kim, S.-K. Thermal acid hydrolysis pretreatment, enzymatic saccharification and ethanol fermentation from red seaweed, Gracilaria Verrucosa. Microbiol. Biotechnol. Lett. 2015, 43, 9–15. [Google Scholar] [CrossRef]
- Sukwong, P.; Sunwoo, I.Y.; Lee, M.J.; Ra, C.H.; Jeong, G.T.; Kim, S.K. Application of the severity factor and HMF removal of red macroalgae Gracilaria Verrucosa to production of bioethanol by Pichia Stipitis and Kluyveromyces Marxianus with adaptive evolution. Appl. Biochem. Biotechnol. 2019, 187, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Chandra, R.; Chung, P.A.; Saddler, J. Can the same steam pretreatment conditions be used for most softwoods to achieve good, enzymatic hydrolysis and sugar yields? Bioresour. Technol. 2010, 101, 7827–7833. [Google Scholar] [CrossRef] [PubMed]
- Imman, S.; Laosiripojana, N.; Champreda, V. Effects of liquid hot water pretreatment on enzymatic hydrolysis and physicochemical changes of corncobs. Appl. Biochem. Biotechnol. 2017, 184, 1–12. [Google Scholar] [CrossRef]
- Güçlü-Üstündaǧ, Ö.; Mazza, G. Effects of pressurized low polarity water extraction parameters on antioxidant properties and composition of cow cockle seed extracts. Plant Foods Hum. Nutr. 2009, 64, 32–38. [Google Scholar] [CrossRef]
- Plaza, M.; Abrahamsson, V.; Turner, C. Extraction and neoformation of antioxidant compounds by pressurized hot water extraction from apple byproducts. J. Agric. Food Chem. 2013, 61, 5500–5510. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Rajha, H.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.; Maroun, R. Effect of the drying process on the intensification of phenolic compounds recovery from grape pomace using accelerated solvent extraction. Int. J. Mol. Sci. 2014, 15, 18640–18658. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wang, W.; Jiang, J.; Yuan, F.; Gao, Y. Subcritical water extraction and antioxidant activity evaluation with on-line HPLC-ABTS·+ assay of phenolic compounds from marigold (Tagetes erecta L.) flower residues. J. Food Sci. Technol. 2015, 52, 3803–3811. [Google Scholar] [CrossRef] [Green Version]
- Fosso-Kankeu, E.; Marx, S.; Meyer, A. Simulated inhibitory effects of typical byproducts of biomass pretreatment process on the viability of Saccharomyces cerevisiae and bioethanol production yield. Afr. J. Biotechnol. 2015, 14, 2383–2394. [Google Scholar] [CrossRef]
- Allard-Massicotte, R.; Chadjaa, H.; Marinova, M. Phenols removal from hemicelluloses pre-hydrolysate by laccase to improve butanol production. Fermentation 2017, 3, 31. [Google Scholar] [CrossRef] [Green Version]





| Analysis | Data | |
|---|---|---|
| Structural and nutritional analysis | Carbohydrates (data adapted from USDA) | 75.9 |
| Protein (data adapted from USDA) | 14.1 | |
| Total lipid (data adapted from USDA) | 2.8 | |
| Lignin | 8.7 ± 0.8 | |
| Cellulose | 24.8 ± 1.9 | |
| Hemicellulose | 10.7 ± 1.1 | |
| Extractives | 2.3 ± 0.1 | |
| Proximate analysis | Equilibrium moisture | 3.0 ± 0.1 |
| Fixed carbon | 14.7 ± 1.9 | |
| Volatile matter | 75.3 ± 3.6 | |
| Ashes | 7.0 ± 0.4 | |
| Elemental analysis | Carbon, C% | 43.9 ± 1.2 |
| Nitrogen, N% | 3.2 ± 0.2 | |
| Hydrogen, H% | 6.4 ± 0.4 | |
| Sulfur, S% | <0.1 | |
| Oxygen, O% | 39.4 ± 1.0 | |
| Potential sugars (experimental data) | Glucose | 33.7 ± 3.4 |
| Xylose | 11.4 ± 0.7 | |
| Fructose | 0.9 ± 0.1 | |
| Lactose | 3.4 ± 0.4 | |
| Sacarose | 1.1 ± 0.3 | |
| Process | |||||||
|---|---|---|---|---|---|---|---|
| No Pretreatment | 150 °C | 165 °C | 180 °C | ||||
| 10 min | 40 min | 10 min | 40 min | 10 min | 40 min | ||
| Log R0 | - | 2.5 | 2.9 | 3.4 | 3.1 | 3.5 | 4.0 |
| Glucose, % | 20.30 ± 1.08 B | 17.20 ± 1.68 A | 27.86 ± 0.76 D | 24.75 ± 2.56 C | 45.84 ± 0.90 F | 34.56 ± 0.52 E | 61.02 ± 1.75 G |
| Xylose, % | 8.58 ± 0.42 A | 8.60 ± 0.51 A | 13.94 ± 1.49 B | 12.89 ± 0.91 B | 19.03 ± 0.76 C | 13.64 ± 0.50 B | 26.12 ± 1.01 D |
| Phenolic compounds concentration, mg/L | 605.57 ± 29.40 E | 72.24 ± 6.51 AB | 80.36 ± 3.16 D | 79.81 ± 6.24 D | 77.44 ± 9.00 CD | 75.58 ± 2.05 BC | 69.12 ± 9.41 A |
| Dependent Variable | Source | Type III Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|---|
| Glucose, % | Main effects | |||||
| Temperature | 1914.2 | 1 | 1914.2 | 126.89 | 0.0000 | |
| Time | 1694.4 | 1 | 1694.4 | 112.32 | 0.0000 | |
| Blocks-replicates | 5.77951 | 2 | 2.88976 | 0.19 | 0.8280 | |
| Total error | 196.115 | 13 | 15.0858 | |||
| Total (corrected) | 3810.28 | 17 | ||||
| Xylose, % | Main effects | |||||
| Temperature | 222.396 | 1 | 222.396 | 57.92 | 0.0000 | |
| Time | 287.041 | 1 | 287.041 | 78.65 | 0.0000 | |
| Blocks-replicates | 1.77303 | 2 | 0.886517 | 0.23 | 0.7970 | |
| Total error | 49.9145 | 13 | 3.83957 | |||
| Total (corrected) | 561.125 | 17 | ||||
| Phenolic compounds concentration, mg/L | Main effects | |||||
| Temperature | 59.4075 | 1 | 59.4075 | 3.34 | 0.0905 | |
| Time | 1.46205 | 1 | 1.46205 | 0.08 | 0.7787 | |
| Blocks-replicates | 30.4436 | 2 | 15.2218 | 0.86 | 0.4471 | |
| Total error | 230.943 | 13 | 17.7649 | |||
| Total (corrected) | 327.256 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Lara, M.A.; Chica-Redecillas, L.; Pérez, A.; Blázquez, G.; Garcia-Garcia, G.; Calero, M. Liquid Hot Water Pretreatment and Enzymatic Hydrolysis as a Valorization Route of Italian Green Pepper Waste to Delivery Free Sugars. Foods 2020, 9, 1640. https://doi.org/10.3390/foods9111640
Martín-Lara MA, Chica-Redecillas L, Pérez A, Blázquez G, Garcia-Garcia G, Calero M. Liquid Hot Water Pretreatment and Enzymatic Hydrolysis as a Valorization Route of Italian Green Pepper Waste to Delivery Free Sugars. Foods. 2020; 9(11):1640. https://doi.org/10.3390/foods9111640
Chicago/Turabian StyleMartín-Lara, M.A., L. Chica-Redecillas, A. Pérez, G. Blázquez, G. Garcia-Garcia, and M. Calero. 2020. "Liquid Hot Water Pretreatment and Enzymatic Hydrolysis as a Valorization Route of Italian Green Pepper Waste to Delivery Free Sugars" Foods 9, no. 11: 1640. https://doi.org/10.3390/foods9111640