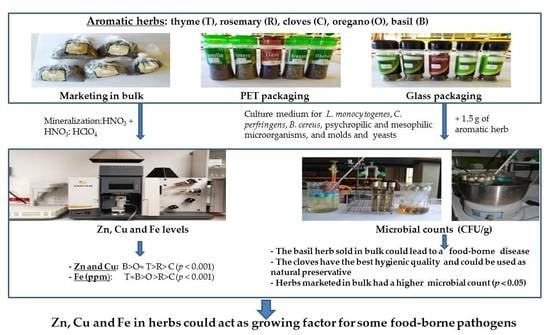
Zn, Cu, and Fe Concentrations in Dehydrated Herbs (Thyme, Rosemary, Cloves, Oregano, and Basil) and the Correlation with the Microbial Counts of Listeria monocytogenes and Other Foodborne Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Aromatic Herbs
2.2. Elemental Analysis in Samples of Aromatic Herbs
2.3. Microbiological Analysis Methods
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seddigi, Z.S.; Kandhro, G.A.; Shah, F.; Danish, E.; Soylak, M. Assessment of metal contents in spices and herbs from Saudi Arabia. Toxicol. Ind. Health 2016, 32, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Bukva, M.; Kapo, D.; Huseinbašić, N.; Gojak-Salimović, S.; Huremović, J. Iron content in fruits, vegetables, herbs and spices samples marketed in Sarajevo, Bosnia and Herzegovina. Kem. Ind. 2019, 68, 281–287. [Google Scholar] [CrossRef]
- Arantes, S.; Picarra, A.; Candeias, F.; Caldeira, A.T.; Martins, M.R.; Teixeira, D. Antioxidant activity and cholinesterase inhibition studies of four flavouring herbs from Alentejo. Nat. Prod. Res. 2017, 31, 2183–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farshchi, H.K.; Azizi, M.; Jaafari, M.R.; Nemati, S.H.; Fotovat, A. Green synthesis of iron nanoparticles by rosemary extract and cytotoxicity effect evaluation on cancer cell lines. Biocatal. Agric. Biotechnol. 2018, 16, 54–62. [Google Scholar] [CrossRef]
- Ferriccioni, N.; Mateuccic, R.; Zangrando, A.; Santana, S.; Campos, C.A. Effect of decontamination treatment on the quality of dehydrated thyme, coriander, and mustard. Food Sci.-Technol. Int. 2019, 25, 579–587. [Google Scholar] [CrossRef]
- Hinneburg, I.; Dorman, H.J.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2005, 97, 122–129. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants - a mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Bassanetti, I.; Carcelli, M.; Buschini, A.; Montalbano, S.; Leonardi, G.; Pelagatti, P.; Tosi, G.; Massi, P.; Fiorentini, L.; Rogolino, D. Investigation of antibacterial activity of new classes of essential oils derivatives. Food Control 2017, 73, 606–612. [Google Scholar] [CrossRef]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green alternatives to synthetic antioxidants, antimicrobials, nitrates, and nitrites in clean label Spanish chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Weisany, W.; Samadi, S.; Amini, J.; Hossaini, S.; Yousefi, S.; Maggi, F. Enhancement of the antifungal activity of thyme and dill essential oils against Colletotrichum nymphaeae by nano-encapsulation with copper NPs. Ind. Crops Prod. 2019, 132, 213–225. [Google Scholar] [CrossRef]
- Mohapatra, S.; Leelavathi, L.; Meignana, A.I.; Arumugham, I.; Pradeep, K.R.; Rajeshkumar, S. Assessment of antimicrobial efficacy of zinc oxide nanoparticles synthesized using clove and cinnamon formulation against oral pathogens–An in vitro study. J. Evol. Med. Dental Sci. 2020, 9, 2034–2039. [Google Scholar] [CrossRef]
- Andrade, M.; Ribeiro-Santos, R.; Costa Bonito, M.C.; Saraiva, M.; Sanchez-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT Food Sci. Technol. 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Srinivasan, K. Plant foods in the management of diabetes mellitus: Spices as beneficial antidiabetic food adjuncts. Int. J. Food Sci. Nutr. 2005, 56, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.; Botsoglou, N.; Govaris, A.; Giannenas, I.; Iliadis, S.; Botsoglou, E. Effect of dietary oregano oil and alpha-tocopheryl acetate supplementation on iron-induced lipid oxidation of turkey breast, thigh, liver and heart tissues. J. Anim. Physiol. Anim. Nutr. 2003, 87, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Hanif, M.A.; Ayub, M.A.; Ishtiaq, F.; Kanwal, N.; Rashid, N.; Saleem, M.; Ahmad, M. Raman spectroscopy for the evaluation of the effects of different concentrations of Copper on the chemical composition and biological activity of basil essential oil. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2017, 185, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.A.; Nawaza, H.; Ayub, M.A.; Tabassum, N.; Kanwal, N.; Rashid, N.; Saleem, M.; Ahmad, M. Evaluation of the effects of Zinc on the chemical composition and biological activity of basil essential oil by using Raman spectroscopy. Ind. Crops Prod. 2017, 96, 91–101. [Google Scholar] [CrossRef]
- Cantú-Váldez, J.A.; Gutiérrrez-Soto, G.; Hernández-Martínez, C.A.; Sinagawa-García, S.R.; Quintero-Ramos, A.; Hume, M.E.; Herrera-Balandrano, D.D.; Méndez-Zamora, G. Mexican oregano essential oils as alternatives to butylated hydroxytoluene to improve the shelf life of ground beef. Food Sci. Nutr. 2020, 8, 4555–4564. [Google Scholar] [CrossRef]
- Zhou, F.B.; Jongberg, S.; Zhao, M.M.; Sun, W.Z.; Skibsted, L.H. Antioxidant efficiency and mechanisms of green tea, rosemary or mate extracts in porcine Longissimus dorsi subjected to iron-induced oxidative stress. Food Chem. 2019, 298, 125030. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Pohl, P.; Dzimitrowicz, A.; Jedryczko, D.; Szymczycha-Madeja, A.; Welna, M.; Jamroz, P. The determination of elements in herbal teas and medicinal plant formulations and their tisanes. J. Pharm. Biomed. Anal. 2016, 130, 326–335. [Google Scholar] [CrossRef]
- Potorti, A.G.; Bua, G.D.; Lo Turco, V.; Ben Tekaya, A.B.; Beltifa, A.; Ben Mansour, H.; Dugo, G.; Di Bella, G. Major, minor and trance element concentrations in spices and aromatic herbs from Sicily (Italy) and Mahdia (Tunisia) by ICP-MS and multivariate analysis. Food Chem. 2020, 313, 126094. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Food and Nutrition Board. Dietary References Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silico, Vanadium and Zinc; The National Academies Press: Washington, DC, USA, 2002. [Google Scholar]
- Di Bella, G.; Potorti, A.G.; Lo Turco, V.; Bua, D.; Licata, P.; Cicero, N.; Dugo, G. Trace elements in Thunnus thynnus from Mediterranean sea and benefit-risk assessment for consumers. Food Add. Contam. Part B Surveill. 2015, 8, 175–181. [Google Scholar] [CrossRef]
- Sabina, R.O.; Santos, E.S.; Abreu, M.M. Accumulation of Mn and Fe in aromatic plant species from the abandoned Rosalgar mine and their potential risk to human health. Appl. Geochem. 2019, 104, 42–50. [Google Scholar] [CrossRef]
- Symeonidis, A.; Marangos, M. Iron and microbial growth. In Insight and Control of Infectious; Priti, R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 289–330. [Google Scholar]
- Tirawat, D.; Phongpaichit, S.; Benjakul, S.; Sumpavapol, P. Microbial load reduction of sweet basil using acidic electrolyzed water and lactic acid in combination with mild heat. Food Control 2016, 64, 29–36. [Google Scholar] [CrossRef]
- Sospedra, I.; Soriano, J.M.; Mañes, J. Assessment of the microbiological safety of dried spices and herbs commercialized in Spain. Plant Foods Hum. Nutr. 2010, 65, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Gekenidis, M.T.; Gossin, D.; Schmelcher, M.; Schoner, U.; Remus-Emsermann, M.N.P.; Drissne, D. Dynamics of culturable mesophilic bacterial communities of three fresh herbs and their production environment. J. Appl. Microbiol. 2017, 123, 916–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altay, K.; Dirim, S.N.; Hayaloglu, A.A. The effect of gamma irradiation on microbial load of purple basil (Ocimum bacilicum L.) leaves dried in different methods. J. Food Saf. 2018, 39, e12610. [Google Scholar]
- Melo, J.; Quevedo, C.; Graca, A.; Dia Quintas, C. Hygienic quality of dehydrated aromatic herbs marketed in Southern Portugal. Agric. Food 2019, 5, 46–53. [Google Scholar] [CrossRef]
- Dogu-Baykut, E.; Gunes, G. Ultraviolet (UV-C) radiation as a practical alternative to decontaminate thyme (Thymus vulgaris). J. Food Process. Preserv. 2018, 43, e13842. [Google Scholar] [CrossRef]
- Lechowicz, J.; Krawczyk-Balska, A. An update on the transport and metabolism of iron in Listeria monocytogenes: The role of proteins involved in pathogenicity. Biometals 2015, 28, 587–603. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Jazani, N.H.; Kouhkan, M.; Babaganjeh, L. Antibacterial effects of microbial synthesized silver-copper nanoalloys on Escherichia coli, Burkholderia cepacia, Listeria monocytogenes and Brucella abortus. Iran. J. Microbiol. 2018, 10, 171–179. [Google Scholar] [PubMed]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J.; Sánchez-González, C.; Rufián-Henares, J.A. Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO 4833:2003). Microbiology of Food and Animal Feeding Stuffs Horizontal Method for the Enumeration of Microorganisms-Colony Count Technique at 30°C; ISO Publishers: Geneve, Italy, 2003. [Google Scholar]
- International Organization for Standardization (ISO 21527-2:2008). Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0,95; ISO Publishers: Geneve, Italy, 2008. [Google Scholar]
- International Organization for Standardization (ISO 11290:2017). Microbiology of the Food Chain-Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and Listeria spp”. Part 1: Detection Method and Part 2: Enumeration Method; ISO Publishers: Geneve, Italy, 2017. [Google Scholar]
- International Organization for Standardization (ISO 7932:2005). Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Presumptive Bacillus Cereus-Colony-Count Technique at 30 °C; ISO Publishers: Geneve, Italy, 2005. [Google Scholar]
- International Organization for Standardization (ISO 7937:2005). Microbiology of Food and Animal Feeding Stuffs-Horizontal Method for the Enumeration of Clostridium Perfringens-Colony-Count Technique; ISO Publishers: Geneve, Italy, 2005. [Google Scholar]
- Özcan, M.M.; Akbulut, M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem. 2007, 106, 852–858. [Google Scholar] [CrossRef]
- Kara, D. Evaluation of trace metal concentrations in some herbs and herbal teas by principal component analysis. Food Chem. 2009, 114, 347–354. [Google Scholar] [CrossRef]
- Shim, J.; Cho, T.; Leem, D.; Cho, Y.; Lee, C. Heavy metals in spices commonly consumed in Republic of Korea. Food Addit. Contam. Part B-Surveill. 2019, 12, 52–58. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.K.; Abou Donia, M.A. Heavy metals in egyptian spices and medicinal plants and the effect of processing on their levels. J. Agric. Food Chem. 2000, 48, 2300–2304. [Google Scholar] [CrossRef]
- Bua, D.G.; Annuario, G.; Albergamo, A.; Cicero, N.; Dugo, G. Heavy metals in aromatic spices by inductively coupled plasma-mass spectrometry. Food Addit. Contam. Part B Surveil. 2016, 9, 210–216. [Google Scholar] [CrossRef]
- Ejaz, M.; Arfat, Y.A.; Mulla, M.; Ahmed, J. Zinc oxide nanorods/clove essential oil incorporated Type B gelatin composite films and its applicability for shrimp packaging. Food Packag. Shelf Life 2018, 15, 113–121. [Google Scholar] [CrossRef]
- Akthar, M.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Biol. Sci. Pharm. Res. 2014, 2, 1–7. [Google Scholar]
- Adeyinka, A.; Richard, F. Application of phytochemical extracts and essential oils in food products. Intern. J. Biotech. Food Sci. 2015, 3, 31–35. [Google Scholar]
- Ortega-Ramirez, L.A.; Rodriguez-Garcia, I.; Leyva, J.M.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Siddiqui, M.W.; Ayala-Zavala, J.F. Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: A hypothesis. J. Food Sci. 2014, 79, R129–R137. [Google Scholar] [CrossRef] [PubMed]
- Volpe, K.E.; Kathariou, S.; Edwards, J.S.; Wolf, L.A. Multiple locus variable-number tandem-repeat analysis as a tool for subtyping Listeria monocytogenes strain. J. Clin. Microbiol. 2008, 46, 1435–1450. [Google Scholar]
- Samapundo, S.; Heyndrickx, M.; Xhaferi, R.; Devlieghere, F. Incidence, diversity and toxin gene characteristics of Bacillus cereus group strains isolated from food products marketed in Belgium. Int. J. Food Microbiol. 2011, 150, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Dolea, D.; Rizo, A.; Fuentes, A.; Barat, J.M.; Fernandez-Segovia, I. Effect of thyme and oregano essential oils on the shelf life of salmon and seaweed burgers. Food Sci. Technol. Int. 2018, 24, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, M.; Da Silva, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; Mendonca Alves da Costa, L.A.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.C.C.; Barbosa, L.; Seito, L.N.; Fernandes, A. Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat. Prod. Res. 2012, 26, 1510–1514. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Antimicrobial properties of basil and its possible application in food packaging. J. Agric. Food Chem. 2003, 51, 3197–3207. [Google Scholar] [CrossRef]
- Sokovic, M.; Van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Biological Hazards (BIOHAZ) on Bacillus cereus and other Bacillus spp. in foodstuffs. EFSA J. 2005, 175, 1–48. [Google Scholar]
- FSAI. Bacillus Cereus Factsheet; Microbial Factsheet Series, Issue No. 2; Food Safety Authority of Ireland: Dublin, Ireland, 2016.
- Dgaim, R.; Khatib, S.A.; Rasool, H.; Khan, M.A. Determination of heavy metals concentrations in traditional herbs commonly consumed in the United Arab Emirates. J. Environ. Public Health 2015, 2015, 972878. [Google Scholar] [CrossRef] [Green Version]
- Miranda, G.; Berna, A.; Bon, J.; Mulet, A. Modeling of the process of moisture loss during the storage of dried apricots. Food Sci. Technol. Int. 2011, 17, 439–447. [Google Scholar] [CrossRef]
- Coles, R.; McDowell, D.; Kirwan, M.J. Food Packaging Technology; CRC Press: Boca Raton, FL, USA; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Miranda, G.; Berna, A.; Mulet, A. Dried-Fruit storage: An analysis of package headspace atmosphere changes. Foods 2019, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Meng, X.; Bhandari, B.; Fang, Z.; Chen, H. Recent application of modified atmosphere packaging.(map) in fresh and fresh-cut foods. Food Rev. Int. 2014, 31, 172–193. [Google Scholar] [CrossRef]
- Zengin, M.; Özcan, M.M.; Çetin, Ü.; Gezgin, S. Mineral contents of some aromatic plants, their growth soils and infusions. J. Sci. Food Agric. 2008, 88, 581–589. [Google Scholar] [CrossRef]
- Sekeroglu, N.; Ozkutlu, F.; Kara, S.M.; Ozguven, M. Determination of cadmium and selected micronutrients in commonly used and traded medicinal plants in Turkey. J. Sci. Food Agric. 2008, 88, 86–90. [Google Scholar] [CrossRef]
- Tercan, H.S.; Ayanoglu, F.; Bahadirli, N.P. Determination of heavy metal contents and some basic aspects of widely used herbal teas in Turkey. Rev. Chim. 2016, 67, 1019–1022. [Google Scholar]
- Obiajunwa, E.I.; Adebajo, A.C.; Omobuwajo, O.R. Essential and trace element of some Nigerian medicinal plants. J. Radioanal. Nucl. Chem. 2002, 252, 473–476. [Google Scholar] [CrossRef]




| Aromatic Herb | Zn ± SEM | Cu ± SEM | Fe ± SEM | Reference |
|---|---|---|---|---|
| Thyme | - | - | 857 ± 57.9 | [2] |
| Thyme | 330 ± 67 | 26 ± 4.1 | 41 ± 11 | [21] |
| Thyme | 17 ± 1.7 | 1.9 ± 0.64 | 112 ± 13 | [40] |
| Thyme | 22 ± 2.3 | 6.1 ± 1.9 | 440 ± 1.4 | [41] |
| Thyme | 5.4 ± 1.6 | 6.6 ± 0.32 | 203 ± 1.2 | [42] |
| Thyme | 13 ± 0.75 | 6.6 ± 0.75 | 135 ± 11 | Present study |
| Rosemary | - | - | 118 ± 16.6 | [2] |
| Rosemary | 41 ± 7.6 | 84 ± 14 | n.d.a | [21] |
| Rosemary | 31 ± 3.2 | 3.0 ± 0.02 | 735 ± 39 | [40] |
| Rosemary | 9.0 ± 0.30 | 5.0 ± 1.0 | 173 ± 3.2 | [43] |
| Rosemary | 52 | 8.5 | 432 | [44] |
| Rosemary | 10 ± 0.23 | 3.4 ± 0.16 | 63 ± 7.6 | Present study |
| Cloves | 6.3 ± 0.90 | 3.2 ± 0.10 | 90 ± 6.0 | [1] |
| Cloves | 14 ± 1.7 | 1.1 ± 0.02 | 65 ± 3.6 | [40] |
| Cloves | 4.5 ± 0.19 c | 2.2 ± 0.11 c | 25 ± 5.9 c | Present study |
| Oregano | - | - | 918 ± 44 | [2] |
| Oregano | 31 ± 8.5 | 85 ± 2.7 | n.d. | [21] |
| Oregano | 59 ± 6.8 | 8.4 ± 6.5 | 240 ± 217 | [24] |
| Oregano | 9.0 ± 0.40 | 3.0 ± 1.0 | 198 ± 2.4 | [43] |
| Oregano | 14 ± 0.46 | 7.7 ± 0.36 | 88 ± 6.4 | Present study |
| Basil | - | - | 112 ± 65.1 | [2] |
| Basil | 43 ± 1.4 | 7.6 ± 0.06 | 251 ± 38 | [40] |
| Basil | 46 ± 1.5 | 4.8 ± 0.15 | 448 ± 12 | [42] |
| Basil | 16 ± 0.20 | 11 ± 0.30 | 390 ± 14 | [43] |
| Basil | 35 ± 4.1 | 6.8 ± 1.5 | 671 ± 20 | [45] |
| Basil | 89 | 138 | 305 | [46] |
| Basil | 18 ± 0.48 | 14 ± 0.42 | 115 ± 7.6 | Present study |
| Marketing System | Zn ± SEM | Cu ± SEM | Fe ± SEM |
|---|---|---|---|
| PET | 12 ± 0.95 a | 5.6 ± 0.72 a | 53 ± 9.8 a |
| Glass | 12 ± 1.0 a | 5.5 ± 0.70 a | 90 ± 8.0 b |
| Bulk | 12 ± 0.72 a | 8.5 ± 0.69 a | 90 ± 8.7 b |
| Microorganisms (CFU/g) | Packaging in Glass (Mean ± SEM) | Packaging in PET (Mean ± SEM) | In Bulk (Mean ± SEM) |
|---|---|---|---|
| L. monocytogenes | 71 ± 17 a | 601 ± 223 ab | 5119 ± 921 b |
| C. perfringens | 142 ± 24 a | 172 ± 36 a | 274 ± 38 a |
| B. cereus | 198,936 ± 77,352 a | 55,286 ± 18,921 ab | 286,851 ± 79,455 b |
| Psychrophilic microorganisms | 287,361 ± 62,082 a | 1,245,643 ± 266,560 a | 2,488,507 ± 382,092 b |
| Mesophilic microorganisms | 14,403 ± 4537 a | 324,500 ± 104,357 ab | 184,792 ± 25,579 b |
| Molds and yeasts | 73,833 ± 14,560 a | 32,143 ± 13,416 b | 209,306 ± 24,368 c |
| Microorganisms | Zn | Cu | Fe | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| L. monocytogenes | 0.264 | 0.012 | 0.559 | 0.001 | 0.427 | 0.001 |
| C. perfringens | 0.632 | 0.001 | 0.775 | 0.001 | 0.655 | 0.001 |
| B. cereus | 0.253 | 0.017 | 0.428 | 0.001 | 0.356 | 0.001 |
| Psychrophilic microorganisms | 0.267 | 0.011 | 0.546 | 0.001 | 0.375 | 0.001 |
| Mesophilic microorganisms | 0.204 | 0.055 | 0.383 | 0.001 | 0.097 | 0.377 |
| Molds and yeasts | 0.125 | 0.242 | 0.435 | 0.001 | 0.201 | 0.065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Galdeano, J.M.; Villalón-Mir, M.; Medina-Martínez, J.; Vázquez-Foronda, L.M.; Zamora-Bustillos, J.G.; Agil, A.; Moor-Davie, S.M.F.; Navarro-Alarcón, M. Zn, Cu, and Fe Concentrations in Dehydrated Herbs (Thyme, Rosemary, Cloves, Oregano, and Basil) and the Correlation with the Microbial Counts of Listeria monocytogenes and Other Foodborne Pathogens. Foods 2020, 9, 1658. https://doi.org/10.3390/foods9111658
García-Galdeano JM, Villalón-Mir M, Medina-Martínez J, Vázquez-Foronda LM, Zamora-Bustillos JG, Agil A, Moor-Davie SMF, Navarro-Alarcón M. Zn, Cu, and Fe Concentrations in Dehydrated Herbs (Thyme, Rosemary, Cloves, Oregano, and Basil) and the Correlation with the Microbial Counts of Listeria monocytogenes and Other Foodborne Pathogens. Foods. 2020; 9(11):1658. https://doi.org/10.3390/foods9111658
Chicago/Turabian StyleGarcía-Galdeano, José María, Marina Villalón-Mir, José Medina-Martínez, Lydia María Vázquez-Foronda, Jessandra Gabriela Zamora-Bustillos, Ahmad Agil, Sofía María Fonseca Moor-Davie, and Miguel Navarro-Alarcón. 2020. "Zn, Cu, and Fe Concentrations in Dehydrated Herbs (Thyme, Rosemary, Cloves, Oregano, and Basil) and the Correlation with the Microbial Counts of Listeria monocytogenes and Other Foodborne Pathogens" Foods 9, no. 11: 1658. https://doi.org/10.3390/foods9111658