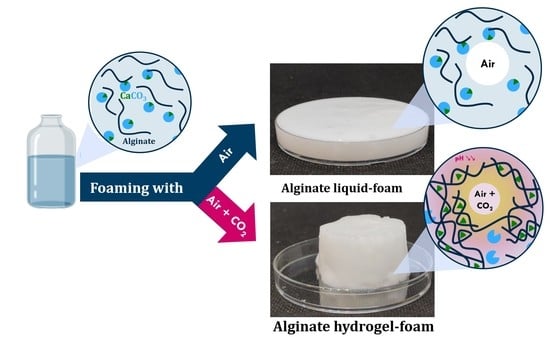
One-Step Generation of Alginate-Based Hydrogel Foams Using CO2 for Simultaneous Foaming and Gelation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Foam-Scale Investigation of CO2 Effect on Alginate Foam Gelation
2.2. Interfacial Investigation of CO2 Effect on Alginate Gelation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Foaming and Foam Ageing Analysis
4.2.2. Interfacial Shear Rheology
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baxter, J.; Lima, T.A.; Huneke, R.; Kanach, C.J.; Johal, P.; Reimold, E.; Alvarez, N.J.; Laub, G.W. The Efficacy of Hydrogel Foams in Talc Pleurodesis. J. Cardiothorac. Surg. 2020, 15, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley Wake, M.; Mikos, A.G.; Sarakinos, G.; Vacanti, J.P.; Langer, R. Dynamics of Fibrovascular Tissue Ingrowth in Hydrogel Foams. Cell Transplant. 1995, 4, 275–279. [Google Scholar] [CrossRef]
- Andersen, T.; Markussen, C.; Dornish, M.; Heier-Baardson, H.; Melvik, J.E.; Alsberg, E.; Christensen, B.E. In Situ Gelation for Cell Immobilization and Culture in Alginate Foam Scaffolds. Tissue Eng. Part A 2014, 20, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raya, B.; Caroline, C.; Christophe, T.; Benjamin, D.; Philippe, B.; Daniel, C.; Angelo, P.; Brigitte, S.; Sophie, G.F. Design of Biopolymer-Based 3D Scaffolds for Cardiac Mesenchymal Stem Cell Therapy. Front. Bioeng. Biotechnol. 2016. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Deshmukh, A.S. Polymeric Hydrogels as Smart Biomaterials; Springer: Berlin, Germany, 2016; ISBN 978-3-319-25320-6. [Google Scholar]
- Arzhavitina, A.; Steckel, H. Foams for Pharmaceutical and Cosmetic Application. Int. J. Pharm. 2010, 394, 1–17. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Du, Z.; Su, E.; Liu, X.; Wan, Z.; Yang, X. A Natural Supramolecular Saponin Hydrogelator for Creation of Ultrastable and Thermostimulable Food-Grade Foams. Adv. Mater. Interfaces 2019, 6, 1900417. [Google Scholar] [CrossRef]
- Rizwan, M.; Rubina Gilani, S.; Iqbal Durani, A.; Naseem, S. Materials Diversity of Hydrogel: Synthesis, Polymerization Process and Soil Conditioning Properties in Agricultural Field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Menner, A.; Bismarck, A.; Drenckhan, W. Emulsion and Foam Templating-Promising Routes to Tailor-Made Porous Polymers. Angew. Chemie Int. Ed. 2018, 57, 10024–10032. [Google Scholar] [CrossRef]
- Andrieux, S.; Quell, A.; Stubenrauch, C.; Drenckhan, W. Liquid Foam Templating – A Route to Tailor-Made Polymer Foams. Adv. Colloid Interface Sci. 2018, 256, 276–290. [Google Scholar] [CrossRef]
- BenDjemaa, I.; Auguste, S.; Drenckhan-Andreatta, W.; Andrieux, S. Hydrogel Foams from Liquid Foam Templates: Properties and Optimisation. Adv. Colloid Interface Sci. 2021, 294, 102478. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BeMiller, J. Carbohydrate Chemistry for Food Scientists; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological Interactions between Polysaccharides and Divalent Cations: The Egg-Box Model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Catanzano, O.; Soriente, A.; La Gatta, A.; Cammarota, M.; Ricci, G.; Fasolino, I.; Schiraldi, C.; Ambrosio, L.; Malinconico, M.; Laurienzo, P.; et al. Macroporous Alginate Foams Crosslinked with Strontium for Bone Tissue Engineering. Carbohydr. Polym. 2018, 202, 72–83. [Google Scholar] [CrossRef]
- Cole, S.M.; Garbe, J.E.; Woodson, L.P. Water-Insoluble Polysaccharide Hydrogel Foam for Medical Applications U.S. Patent 5,089,606, 18 February 1992.
- Andersen, T.; Melvik, J.E.; Gåserød, O.; Alsberg, E.; Christensen, B.E. Ionically Gelled Alginate Foams: Physical Properties Controlled by Type, Amount and Source of Gelling Ions. Carbohydr. Polym. 2014, 99, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ceccaldi, C.; Bushkalova, R.; Cussac, D.; Duployer, B.; Tenailleau, C.; Bourin, P.; Parini, A.; Sallerin, B.; Girod Fullana, S.; Fullana, G.; et al. Elaboration and Evaluation of Alginate Foam Scaffolds for Soft Tissue Engineering. Int. J. Pharm. 2017, 524, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Melvik, J.E.; Gåserød, O.; Alsberg, E.; Christensen, B.E. Ionically Gelled Alginate Foams: Physical Properties Controlled by Operational and Macromolecular Parameters. Biomacromolecules 2012, 13, 3703–3710. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Mishra, N.C.; Wang, C.; Lin, F.; Lin, K. Fabricating Scaffolds by Microfluidics. Biomicrofluidics 2009, 3, 22403. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-C.; Yang, K.-C.; Lin, K.-H.; Liu, H.-C.; Lin, F.-H. A Highly Organized Three-Dimensional Alginate Scaffold for Cartilage Tissue Engineering Prepared by Microfluidic Technology. Biomaterials 2011, 32, 7118–7126. [Google Scholar] [CrossRef]
- Ahmad, B.; Stride, E.; Edirisinghe, M. Calcium Alginate Foams Prepared by a Microfluidic T-Junction System: Stability and Food Applications. Food Bioprocess Technol. 2012, 5, 2848–2857. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically Crosslinked Alginate Hydrogels as Scaffolds for Tissue Engineering: Part 1. Structure, Gelation Rate and Mechanical Properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Wang, B.; Prinsen, P.; Wang, H.; Bai, Z.; Wang, H.; Luque, R.; Xuan, J. Macroporous Materials: Microfluidic Fabrication, Functionalization and Applications. Chem. Soc. Rev. 2017, 46, 855–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draget, K.I.; Østgaard, K.; Smidsrød, O. Homogeneous Alginate Gels: A Technical Approach. Carbohydr. Polym. 1990, 14, 159–178. [Google Scholar] [CrossRef]
- Liu, X.; Qian, L.; Shu, T.; Tong, Z. Rheology Characterization of Sol–Gel Transition in Aqueous Alginate Solutions Induced by Calcium Cations through in Situ Release. Polymer 2003, 44, 407–412. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Hattrem, M.N.; Draget, K.I. Propylene Glycol Alginate (PGA) Gelled Foams: A Systematic Study of Surface Activity and Gelling Properties as a Function of Degree of Esterification. Food Hydrocoll. 2016, 57, 80–91. [Google Scholar] [CrossRef]
- Valerón Bergh, V.J.; Johannessen, E.; Andersen, T.; Tønnesen, H.H. Evaluation of Porphyrin Loaded Dry Alginate Foams Containing Poloxamer 407 and β-Cyclodextrin-Derivatives Intended for Wound Treatment. Pharm. Dev. Technol. 2018, 23, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.; Ic Dumazert, L.; Dufourg, L.; Cucherat, C.; Sonnier, R.; Guibal, E. New Alginate Foams: Box-Behnken Design of Their Manufacturing; Fire Retardant and Thermal Insulating Properties. J. Appl. Polym. Sci 2017, 135, 45868. [Google Scholar] [CrossRef]
- Vincent, T.; Vincent, C.; Dumazert, L.; Otazaghine, B.; Sonnier, R.; Guibal, E. Fire Behavior of Innovative Alginate Foams. Carbohydr. Polym. 2020, 250, 116910. [Google Scholar] [CrossRef] [PubMed]
- Ginot, G.; Höhler, R.; Mariot, S.; Kraynik, A.; Drenckhan-Andreatta, W. Juggling bubbles in square capillaries: An experimental proof of non-pairwise bubble interactions. Soft Matter 2019, 15, 4570–4582. [Google Scholar]
- Meyssami, B.; Balaban, M.O.; Teixeira, A.A. Prediction of PH in Model Systems Pressurized with Carbon Dioxide. Biotechnol. Prog. 1992, 8, 149–154. [Google Scholar] [CrossRef]
- Puschett, J.B.; Rao, B.S.; Karandikar, B.M.; Matyjaszewski, K. Indicator Characteristics of Bromothymol Blue Derivatives. Talanta 1991, 38, 335–338. [Google Scholar] [CrossRef]
- Siemens, A.O.N.; Van Hecke, M. Jamming: A Simple Introduction. Phys. A Stat. Mech. its Appl. 2010, 389, 4255–4264. [Google Scholar] [CrossRef]
- Cantat, I.; Cohen-Addad, S.; Elias, F.; Graner, F.F.F.; Höhler, R.; Pitois, O.; Rouyer, F.; Saint-Jalmes, A.; HÖhler, R.; Pitois, O.; et al. Foams: Structure and Dynamics; Cox, S.J., Ed.; Oxford University Press: Oxford, UK, 2013; ISBN 9780199662890. [Google Scholar]
- Bey, H.; Wintzenrieth, F.; Ronsin, O.; Höhler, R.; Cohen-Addad, S. Stabilization of Foams by the Combined Effects of an Insoluble Gas Species and Gelation. Soft Matter 2017, 13, 6816–6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandebril, S.; Franck, A.; Fuller, G.G.; Moldenaers, P.; Vermant, J. A Double Wall-Ring Geometry for Interfacial Shear Rheometry. Rheol. Acta 2010, 49, 131–144. [Google Scholar] [CrossRef]
- Krägel, J.; Derkatch, S.R. Interfacial Shear Rheology. Curr. Opin. Colloid Interface Sci. 2010, 15, 246–255. [Google Scholar] [CrossRef]
- Fuller, G.G.; Vermant, J. Complex Fluid-Fluid Interfaces: Rheology and Structure. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 519–543. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.M.; Dynes, P.J. Relationship between Viscoelastic Properties and Gelation in Thermosetting Systems. J. Appl. Polym. Sci. 1982, 27, 569–574. [Google Scholar] [CrossRef]
- Rehage, H.; Husmann, M.; Walter, A. From Two-Dimensional Model Networks to Microcapsules. Rheol. Acta 2002 414 2002, 41, 292–306. [Google Scholar] [CrossRef]
- Turco, G.; Donati, I.; Grassi, M.; Marchioli, G.; Lapasin, R.; Paoletti, S. Mechanical Spectroscopy and Relaxometry on Alginate Hydrogels: A Comparative Analysis for Structural Characterization and Network Mesh Size Determination. Biomacromolecules 2011, 12, 1272–1282. [Google Scholar] [CrossRef]
- Grassi, M.; Sandolo, C.; Perin, D.; Coviello, T.; Lapasin, R.; Grassi, G. Structural Characterization of Calcium Alginate Matrices by Means of Mechanical and Release Tests. Molecules 2009, 14, 3003–3017. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Suo, Z. Hydrogel Ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Peñas-López, P.; Van Elburg, B.; Parrales, M.A.; Rodríguez-Rodríguez, J. Diffusion of Dissolved CO 2 in Water Propagating from a Cylindrical Bubble in a Horizontal Hele-Shaw Cell. APS 2017, 2, 063602. [Google Scholar] [CrossRef] [Green Version]
- Montellano Duran, N.; Galante, M.; Spelzini, D.; Boeris, V. The Effect of Carrageenan on the Acid-Induced Aggregation and Gelation Conditions of Quinoa Proteins. Food Res. Int. 2018, 107, 683–690. [Google Scholar] [CrossRef] [PubMed]
- De Alcântara, M.G.; de Freitas Ortega, N.; Souza, C.J.F.; Garcia-Rojas, E.E. Electrostatic Hydrogels Formed by Gelatin and Carrageenan Induced by Acidification: Rheological and Structural Characterization. Food Struct. 2020, 24, 100137. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, P.; Nicolai, T. Effect of Kappa Carrageenan on Acid-Induced Gelation of Whey Protein Aggregates. Part I: Potentiometric Titration, Rheology and Turbidity. Food Hydrocoll. 2020, 102, 105589. [Google Scholar] [CrossRef]
- Agoub, A.A.; Giannouli, P.; Morris, E.R. Gelation of High Methoxy Pectin by Acidification with D-Glucono-δ-Lactone (GDL) at Room Temperature. Carbohydr. Polym. 2009, 75, 269–281. [Google Scholar] [CrossRef]
- Cardoso, A.Z.; Alvarez Alvarez, A.E.; Cattoz, B.N.; Griffiths, P.C.; King, S.M.; Frith, W.J.; Adams, D.J. The Influence of the Kinetics of Self-Assembly on the Properties of Dipeptide Hydrogels. Faraday Discuss. 2013, 166, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, T.; Honorez, C.; Jumeau, M.; Elias, F.; Drenckhan, W. A simple technique for the automation of bubble size measurements. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 68–74. [Google Scholar]
- Franck, A.; Vermant, J.; Fuller, G.G. System and Method for Interfacial Rheometry. U.S. Patent 7926326B2, 5 March 2009. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge Solid State Science Series; Cambridge University Press: Cambridge, UK, 1997; ISBN 0521499119,9780521499118. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Djemaa, I.; Andrieux, S.; Auguste, S.; Jacomine, L.; Tarnowska, M.; Drenckhan-Andreatta, W. One-Step Generation of Alginate-Based Hydrogel Foams Using CO2 for Simultaneous Foaming and Gelation. Gels 2022, 8, 444. https://doi.org/10.3390/gels8070444
Ben Djemaa I, Andrieux S, Auguste S, Jacomine L, Tarnowska M, Drenckhan-Andreatta W. One-Step Generation of Alginate-Based Hydrogel Foams Using CO2 for Simultaneous Foaming and Gelation. Gels. 2022; 8(7):444. https://doi.org/10.3390/gels8070444
Chicago/Turabian StyleBen Djemaa, Imene, Sébastien Andrieux, Stéphane Auguste, Leandro Jacomine, Malgorzata Tarnowska, and Wiebke Drenckhan-Andreatta. 2022. "One-Step Generation of Alginate-Based Hydrogel Foams Using CO2 for Simultaneous Foaming and Gelation" Gels 8, no. 7: 444. https://doi.org/10.3390/gels8070444