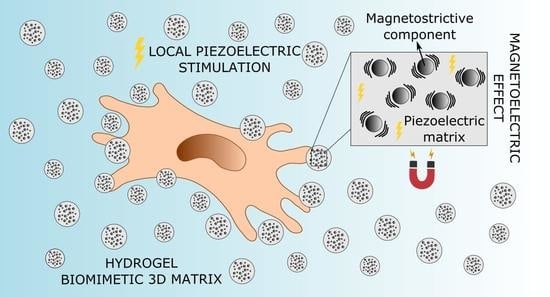
Magnetically Activated Piezoelectric 3D Platform Based on Poly(Vinylidene) Fluoride Microspheres for Osteogenic Differentiation of Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microsphere Characterisation
2.2. MSCs Viability and Distribution within the 3D Construct
2.3. Effect of Piezoelectric Stimulation on MSCs Proliferation and Osteogenic Differentiation
3. Conclusions
4. Materials and Methods
4.1. Microsphere Production by Electrospray Technique
4.2. Microsphere Characterisation
4.2.1. Field Emission Scanning Electron Microscopy
4.2.2. Vibrational Sample Magnetometer
4.2.3. Fourier Transform Infrared Spectroscopy
4.2.4. Differential Scanning Calorimetry
4.3. Microsphere Polarisation
4.4. Cell Response
4.4.1. Microsphere and MSCs Encapsulation in 3D Gelatin Hydrogels
4.4.2. Cell Viability Assessment
4.4.3. Cell Spreading and Microsphere Distribution
4.4.4. Piezoelectric Stimulation Influence on MSCs Proliferation
4.4.5. Influence of Piezoelectric Stimulation on MSCs Osteogenic Differentiation
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Peters, A.; Toben, D.; Lienau, J.; Schell, H.; Bail, H.J.; Matziolis, G.; Duda, G.N.; Kaspar, K. Locally Applied Osteogenic Predifferentiated Progenitor Cells Are More Effective than Undifferentiated Mesenchymal Stem Cells in the Treatment of Delayed Bone Healing. Tissue Eng. A 2009, 15, 2947–2954. [Google Scholar] [CrossRef]
- Castano-Izquierdo, H.; Álvarez-Barreto, J.; van den Dolder, J.; Jansen, J.A.; Mikos, A.G.; Sikavitsas, V.I. Pre-Culture Period of Mesenchymal Stem Cells in Osteogenic Media Influences Their In Vivo Bone Forming Potential. J. Biomed. Mater. Res. A 2006, 79, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Yin, X.; Yang, D.; Tan, J.; Liu, G. Ectopic Bone Regeneration by Human Bone Marrow Mononucleated Cells, Undifferentiated and Osteogenically Differentiated Bone Marrow Mesenchymal Stem Cells in Beta-Tricalcium Phosphate Scaffolds. Tissue Eng. C Methods 2012, 18, 545–556. [Google Scholar] [CrossRef]
- Ghali, O.; Broux, O.; Falgayrac, G.; Haren, N.; van Leeuwen, J.P.T.M.; Penel, G.; Hardouin, P.; Chauveau, C. Dexamethasone in Osteogenic Medium Strongly Induces Adipocyte Differentiation of Mouse Bone Marrow Stromal Cells and Increases Osteoblast Differentiation. BMC Cell Biol. 2015, 16, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoch, A.I.; Mittal, V.; Mitra, D.; Vollmer, N.; Zikry, C.A.; Leach, J.K. Cell-Secreted Matrices Perpetuate the Bone-Forming Phenotype of Differentiated Mesenchymal Stem Cells. Biomaterials 2016, 74, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yan, J.; He, F.; Zhong, D.; Yang, H.; Pei, M.; Luo, Z.-P. Mechanical Stretch Induces Antioxidant Responses and Osteogenic Differentiation in Human Mesenchymal Stem Cells Through. Free Radic. Biol. Med. 2018, 126, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Byun, M.R.; Kim, A.R.; Kim, K.M.; Cho, H.J.; Lee, Y.H.; Kim, J.; Jeong, M.G.; Hwang, E.S.; Hong, J.H. Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaw, J.S.; Xue, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation of Titanium to Promote Stem Cell Orientation, Elongation and Osteogenesis. Acta Biomater. 2021, 139, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.M.; Gadegaard, N.; Burgess, K.; White, K.; Reynolds, P.; Herzyk, P.; Oreffo, R.; Dalby, M.J. Nanotopographical Effects on Mesenchymal Stem Cell Morphology and Phenotype. J. Cell. Biochem. 2014, 115, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Nikukar, H.; Reid, S.; Tsimbouri, P.M.; Riehle, M.O.; Curtis, A.S.G.; Dalby, M.J. Osteogenesis of Mesenchymal Stem Cells by Nanoscale Mechanotransduction. ACS Nano 2013, 7, 2758–2767. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, T.; Tsimbouri, P.M.; Llopis-Hernandez, V.; Campsie, P.; Scurr, D.; Childs, P.G.; Phillips, D.; Donnelly, S.; Wells, J.A.; O’Brien, F.J.; et al. The Use of Nanovibration to Discover Specific and Potent Bioactive Metabolites That Stimulate Osteogenic Differentiation in Mesenchymal Stem Cells. Sci. Adv. 2021, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Minary-Jolandan, M.; Yu, M.F. Uncovering Nanoscale Electromechanical Heterogeneity in the Subfibrillar Structure of Collagen Fibrils Responsible for the Piezoelectricity of Bone. ACS Nano 2009, 3, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric Materials for Tissue Regeneration: A Review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Marino, A.; Becker, R.O. Piezoelectric Effect and Growth Control in Bone. Nature 1970, 228, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Basset, A.C.; Pawluk, R.J. Effets of Electric Currents on Bone in Vivo. Nature 1964, 204, 652–654. [Google Scholar] [CrossRef]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive Poly (Vinylidene Fluoride)-Based Structures for Advanced Applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Pärssinen, J.; Hammarén, H.; Rahikainen, R.; Sencadas, V.; Ribeiro, C.; Vanhatupa, S.; Miettinen, S.; Lanceros-Méndez, S.; Hytönen, V.P. Enhancement of Adhesion and Promotion of Osteogenic Differentiation of Human Adipose Stem Cells by Poled Electroactive Poly (Vinylidene Fluoride). J. Biomed. Mater. Res. A 2015, 103, 919–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobreiro-Almeida, R.; Tamaño-Machiavello, M.; Carvalho, E.; Cordón, L.; Doria, S.; Senent, L.; Correia, D.; Ribeiro, C.; Lanceros-Méndez, S.; Serra, R.S.; et al. Human Mesenchymal Stem Cells Growth and Osteogenic Differentiation on Piezoelectric Poly(Vinylidene Fluoride) Microsphere Substrates. Int. J. Mol. Sci. 2017, 18, 1–17. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-Dimensional Piezoelectric Fibrous Scaffolds Selectively Promote Mesenchymal Stem Cell Differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.M.; Correia, D.M.; Ribeiro, C.; Castro, N.; Correia, V.; Lanceros-Mendez, S. Bioinspired Three-Dimensional Magnetoactive Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 45265–45275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, W.; He, T.; Qian, L.; Tan, G.; Ning, C. Polarization of an Electroactive Functional Film on Titanium for Inducing Osteogenic Differentiation. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yu, P.; Zhou, L.; Tu, L.; Fan, L.; Zhang, F.; Dai, C.; Liu, Y.; Ning, C.; Du, J.; et al. Polypyrrole Nanocones and Dynamic Piezoelectric Stimulation-Induced Stem Cell Osteogenic Differentiation. ACS Biomater. Sci. Eng. 2019, 5, 4386–4392. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Martins, P.; Correia, D.M.; Sencadas, V.; Vilas, J.L.; León, L.M.; Botelho, G.; Lanceros-Méndez, S. Development of Magnetoelectric CoFe2O4/Poly(Vinylidene Fluoride) Microspheres. RSC Adv. 2015, 5, 35852–35857. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.; Martins, P.; Moya, X.; Ghidini, M.; Sencadas, V.; Botelho, G.; Mathur, N.D.; Lanceros-Mendez, S. Magnetoelectric CoFe2O4 /Polyvinylidene Fluoride Electrospun Nanofibres. Nanoscale 2015, 7, 8058–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baji, A.; Mai, Y.W.; Yimnirun, R.; Unruan, S. Electrospun Barium Titanate/Cobalt Ferrite Composite Fibers with Improved Magnetoelectric Performance. RSC Adv. 2014, 4, 55217–55223. [Google Scholar] [CrossRef]
- Maceiras, A.; Martins, P.; Gonҫalves, R.; Botelho, G.; Ramana, E.V.; Mendiratta, S.K.; Sebastián, M.S.; Vilas, J.L.; Lanceros-Mendez, S.; León, L.M. High-Temperature Polymer Based Magnetoelectric Nanocomposites. Eur. Polym. J. 2015, 64, 224–228. [Google Scholar] [CrossRef]
- Martins, P.; Costa, C.M.; Lanceros-Mendez, S. Nucleation of Electroactive β-Phase Poly(Vinilidene Fluoride) with CoFe2O4 and NiFe2O4 Nanofillers: A New Method for the Preparation of Multiferroic Nanocomposites. Appl. Phys. A Mater. Sci. Process. 2011, 103, 233–237. [Google Scholar] [CrossRef]
- Martins, P.; Costa, C.M.; Benelmekki, M.; Botelho, G.; Lanceros-Mendez, S. On the Origin of the Electroactive Poly (Vinylidene Fluoride) β-Phase Nucleation by Ferrite Nanoparticles via Surface Electrostatic Interactions. CrystEngComm 2012, 14, 2807–2811. [Google Scholar] [CrossRef]
- Guillot-Ferriols, M.; Rodríguez-Hernández, J.C.; Correia, D.M.; Carabineiro, S.A.C.; Lanceros-Méndez, S.; Ribelles, J.L.G.; Ferrer, G.G. Poly (Vinylidene) Fluoride Membranes Coated by Heparin/Collagen Layer-by-Layer, Smart Biomimetic Approaches for Mesenchymal Stem Cell Culture. Mater. Sci. Eng. C 2020, 117, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.A.; Ródenas-Rochina, J.; Salazar, D.; Cardoso, V.F.; Ribelles, J.L.G.; Lanceros-Mendez, S. Microfluidic Processing of Piezoelectric and Magnetic Responsive Electroactive Microspheres. ACS Appl. Polym. Mater. 2022, 4, 5368–5379. [Google Scholar] [CrossRef]
- Bock, N.; Woodruff, M.A.; Hutmacher, D.W.; Dargaville, T.R. Electrospraying, a Reproducible Method for Production of Polymeric Microspheres for Biomedical Applications. Polymers 2011, 3, 131–149. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.M.; Gonçalves, R.; Ribeiro, C.; Sencadas, V.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S. Electrosprayed Poly (Vinylidene Fluoride) Microparticles for Tissue Engineering Applications. RSC Adv. 2014, 4, 33013–33021. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly (Vinylidene Fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Boccaccio, T.; Bottino, A.; Capannelli, G.; Piaggio, P. Characterization of PVDF Membranes by Vibrational Spectroscopy. J. Memb. Sci. 2002, 210, 315–329. [Google Scholar] [CrossRef]
- Imamura, R.; Silva, A.B.; Gregorio, J. Gamma to Beta Phase Transformation Induced in Poly (Vinylidene Fluoride) by Stretching. J. Appl. Polym. Sci. 2008, 110, 3242–3246. [Google Scholar] [CrossRef]
- Gregorio, R.; Cestari, M. Effect of Crystallization Temperature on the Crystalline Phase Content and Morphology of Poly (Vinylidene Fluoride). J. Polym. Sci. Part B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Sencadas, V.; Filho, R.G.; Lanceros-Mendez, S. Processing and Characterization of a Novel Nonporous Poly (Vinilidene Fluoride) Films in the β Phase. J. Non. Cryst. Solids 2006, 352, 2226–2229. [Google Scholar] [CrossRef]
- Davis, G.T.; Mckinney, J.E.; Broadhurst, M.G.; Roth, S.C. Electric-Field-Induced Phase Changes in Poly (Vinylidene Fluoride). J. Appl. Phys. 1978, 49, 4998–5002. [Google Scholar] [CrossRef]
- Moise, S.; Céspedes, E.; Soukup, D.; Byrne, J.M.; Haj, A.J.E.; Telling, N.D. The Cellular Magnetic Response and Biocompatibility of Biogenic Zinc- and Cobalt-Doped Magnetite Nanoparticles. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vaca-González, J.J.; Clara-Trujillo, S.; Guillot-Ferriols, M.; Ródenas-Rochina, J.; Sanchis, M.J.; Ribelles, J.-L.G.; Garzón-Alvarado, D.A.; Ferrer, G.G. Effect of Electrical Stimulation on Chondrogenic Differentiation of Mesenchymal Stem Cells Cultured in Hyaluronic Acid—Gelatin Injectable Hydrogels. Bioelectrochemistry 2020, 134, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hermenegildo, B.; Ribeiro, C.; Pérez-Álvarez, L.; Vilas, J.L.; Learmonth, D.A.; Sousa, R.A.; Martins, P.; Lanceros-Méndez, S. Hydrogel-Based Magnetoelectric Microenvironments for Tissue Stimulation. Coll. Surf. B Biointerfaces 2019, 181, 1041–1047. [Google Scholar] [CrossRef]
- Carvalho, E.O.; Ribeiro, C.; Correia, D.M.; Botelho, G.; Lanceros-Mendez, S. Biodegradable Hydrogels Loaded with Magnetically Responsive Microspheres as 2D and 3D Scaffolds. Nanomaterials 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Moulisová, V.; Poveda-Reyes, S.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Ferrer, G.G. Hybrid Protein-Glycosaminoglycan Hydrogels Promote Chondrogenic Stem Cell Differentiation. ACS Omega 2017, 2, 7609–7620. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP Signaling and Other Molecular Events: Regulation of Osteoblastogenesis and Bone Formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef] [Green Version]
- Guillot-Ferriols, M.; Lanceros-Méndez, S.; Ribelles, J.L.G.; Gallego-Ferrer, G. Electrical Stimulation: Effective Cue to Direct Osteogenic Differentiation of Mesenchymal Stem Cells? Biomater. Adv. 2022, 138, 1–18. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Hermann, B.P.; Bizios, R. Adult Human Mesenchymal Stem Cell Differentiation at the Cell Population and Single-Cell Levels Under Alternating Electric Current. Tissue Eng. C Methods 2016, 22, 155–164. [Google Scholar] [CrossRef]
- Zhu, S.; Jing, W.; Hu, X.; Huang, Z.; Cai, Q.; Ao, Y.; Yang, X. Time-Dependent Effect of Electrical Stimulation on Osteogenic Differentiation of Bone Mesenchymal Stromal Cells Cultured on Conductive Nanofibers. J. Biomed. Mater. Res. A 2017, 105, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Chen, T.C.; Tsao, C.W.; Cheng, Y.C. The Effects of Substrate-Mediated Electrical Stimulation on the Promotion of Osteogenic Differentiation and Its Optimization. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, C.; Lin, Y.; Hu, P.; Shen, Y.; Wang, K.; Meng, S.; Chai, Y.; Dai, X.; Liu, X.; et al. Nanocomposite Membranes Enhance Bone Regeneration Through Restoring Physiological Electric Microenvironment. ACS Nano 2016, 10, 7279–7286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, W.; Cao, C.; Zhang, F.; Tang, Q.; Ma, S.; Zhao, J.J.; Hu, L.; Shen, Y.; Chen, L. Modulating Surface Potential by Controlling the β Phase Content in Poly (Vinylidene Fluoridetrifluoroethylene) Membranes Enhances Bone Regeneration. Adv. Healthc. Mater. 2018, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ning, C.; Zhang, Y.; Tan, G.; Lin, Z.; Liu, S.; Wang, X.; Yang, H.; Li, K.; Yi, X.; et al. Bone-Inspired Spatially Specific Piezoelectricity Induces Bone Regeneration. Theranostics 2017, 7, 3387–3397. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Peng, S.; Qi, F.; Zan, J.; Liu, G.; Zhao, Z.; Shuai, C. Graphene-Assisted Barium Titanate Improves Piezoelectric Performance of Biopolymer Scaffold. Mater. Sci. Eng. C 2020, 116, 111195. [Google Scholar] [CrossRef]
- Li, Y.; Dai, X.; Bai, Y.; Liu, Y.; Wang, Y.; Liu, O.; Yan, F.; Tang, Z.; Zhang, X.; Deng, X. Electroactive BaTiO3 Nanoparticle-Functionalized Fibrous Scaffolds Enhance Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Nanomed. 2017, 12, 4007–4018. [Google Scholar] [CrossRef] [Green Version]
- McCann, J.T.; Marquez, M.; Xia, Y. Highly Porous Fibers by Electrospinning into a Cryogenic Liquid. J. Am. Chem. Soc. 2006, 128, 1436–1437. [Google Scholar] [CrossRef]
- Lovinger, A.J. Poly (Vinylidene Fluoride). In Developments in Crystalline Polymers—1; Basset, D., Ed.; Springer: Dordrecht, The netherlands, 1981; pp. 195–273. ISBN 978-94-009-7345-9. [Google Scholar]
- Poveda-Reyes, S.; Moulisova, V.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Ferrer, G.G. Gelatin—Hyaluronic Acid Hydrogels with Tuned Stiffness to Counterbalance Cellular Forces and Promote Cell Differentiation. Macromol. Biosci. 2016, 16, 1311–1324. [Google Scholar] [CrossRef]
- Castro, N.; Fernandes, M.M.; Ribeiro, C.; Correia, V.; Minguez, R.; Lanceros-Mendez, S. Magnetic Bioreactor for Magneto-, Mechano- and Electroactive Tissue Engineering Strategies. Sensors 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Ribeiro, S.; Ribeiro, C.; Carvalho, E.O.; Tubio, C.R.; Castro, N.; Pereira, N.; Correia, V.; Gomes, A.C.; Lanceros-Méndez, S. Magnetically Activated Electroactive Microenvironments for Skeletal Muscle Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 4239–4252. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. Quantification Strategies in Real-Time PCR. In A–Z of Quantitative PCR.; Bustin, S.A., Ed.; International University Line: La Jolla, CA, USA, 2004; pp. 87–112. [Google Scholar]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.H.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.B.; Moorman, A.F.M. Amplification Efficiency: Linking Baseline and Bias in the Analysis of Quantitative PCR Data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillot-Ferriols, M.; García-Briega, M.I.; Tolosa, L.; Costa, C.M.; Lanceros-Méndez, S.; Gómez Ribelles, J.L.; Gallego Ferrer, G. Magnetically Activated Piezoelectric 3D Platform Based on Poly(Vinylidene) Fluoride Microspheres for Osteogenic Differentiation of Mesenchymal Stem Cells. Gels 2022, 8, 680. https://doi.org/10.3390/gels8100680
Guillot-Ferriols M, García-Briega MI, Tolosa L, Costa CM, Lanceros-Méndez S, Gómez Ribelles JL, Gallego Ferrer G. Magnetically Activated Piezoelectric 3D Platform Based on Poly(Vinylidene) Fluoride Microspheres for Osteogenic Differentiation of Mesenchymal Stem Cells. Gels. 2022; 8(10):680. https://doi.org/10.3390/gels8100680
Chicago/Turabian StyleGuillot-Ferriols, Maria, María Inmaculada García-Briega, Laia Tolosa, Carlos M. Costa, Senentxu Lanceros-Méndez, José Luis Gómez Ribelles, and Gloria Gallego Ferrer. 2022. "Magnetically Activated Piezoelectric 3D Platform Based on Poly(Vinylidene) Fluoride Microspheres for Osteogenic Differentiation of Mesenchymal Stem Cells" Gels 8, no. 10: 680. https://doi.org/10.3390/gels8100680