Effect of Glutathione Bio-Molecule on Tooth Discoloration Associated with Silver Diammine Fluoride
Abstract
:1. Introduction
2. Results
2.1. Spectrophotometric Measurement of Color Changes
2.2. SEM Observation Results
2.3. EDS Elemental Analysis
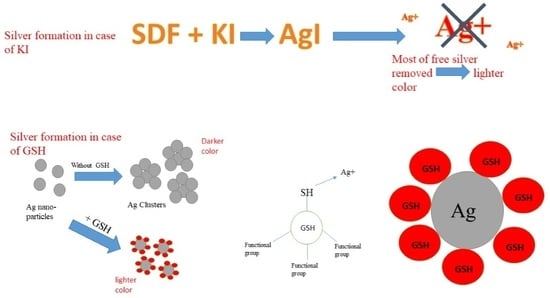
3. Discussion
4. Materials and Methods
4.1. Specimens Preparation
4.2. Material Application
- 1-
- Thirty-eight percent SDF: applied to the tooth surface and agitated using a micro-brush for 1 min, left for 2 min and rinsed with a copious amount of distilled water for 30 s;
- 2-
- SDF followed by KI: SDF was applied to the tooth surface, immediately followed by application of a saturated KI solution until creamy white precipitates turned clear, then washed with copious amounts of distilled water for 30 s; and,
- 3-
- SDF mixed with 20% GSH: under vigorous stirring, SDF was mixed with 20% GSH by weight until the solution was clear without any precipitates. Then applied in the same manner as the SDF.
4.3. Color Assessment
4.4. .Morphology Observation
4.5. Elemental Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shah, S.; Bhaskar, V.; Venkataraghavan, K.; Choudhary, P.; Ganesh, M.; Trivedi, K. Efficacy of silver diamine fluoride as an antibacterial as well as antiplaque agent compared to fluoride varnish and acidulated phosphate fluoride gel: An in vivo study. Indian J. Dent. Res. 2013, 24, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Hollands, K. Nano Silver Fluoride for preventing caries. Evid. Based Dent. 2015, 16, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Botelho, M.G.; Matinlinna, J.P. Silver compounds used in dentistry for caries management: A review. J. Dent. 2012, 40, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nishida, M.; Sobue, S.; Moriwaki, Y. Effects of diammine silver fluoride on tooth enamel. J. Osaka Univ. Dent. Sch. 1974, 14, 61–72. [Google Scholar] [PubMed]
- Savas, S.; Kucukyilmaz, E.; Celik, E.U.; Ates, M. Effects of different antibacterial agents on enamel in a biofilm caries model. J. Oral Sci. 2015, 57, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Zhang, S.; Mei, M.L.; Lo, E.C.; Chu, C.H. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment—A systematic review. BMC Oral Health. 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblatt, A.; Stamford, T.C.; Niederman, R. Silver diamine fluoride: A caries “silver-fluoride bullet”. J. Dent. Res. 2009, 88, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.L.; Ito, L.; Cao, Y.; Li, Q.L.; Lo, E.C.; Chu, C.H. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J. Dent. 2013, 41, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G.; Mulyani; Zilm, P.S.; Gully, N.J. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int. 2009, 40, 155–161. [Google Scholar] [PubMed]
- Li, R.; Lo, E.C.; Liu, B.Y.; Wong, M.C.; Chu, C.H. Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J. Dent. 2016, 51, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, I.S.; Mei, M.L.; Burrow, M.F.; Lo, E.C.; Chu, C.H. Effect of Silver Diamine Fluoride and Potassium Iodide Treatment on Secondary Caries Prevention and Tooth Discolouration in Cervical Glass Ionomer Cement Restoration. Int. J. Mol. Sci. 2017, 18, 340. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Hiraishi, N.; Shimokawa, H.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. The inhibition effect of non-protein thiols on dentinal matrix metalloproteinase activity and HEMA cytotoxicity. J. Dent. 2014, 42, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Amato, E.; Diaz-Fernandez, Y.A.; Taglietti, A.; Pallavicini, P.; Pasotti, L.; Cucca, L.; Milanese, C.; Grisoli, P.; Dacarro, C.; Fernandez-Hechavarria, J.M.; et al. Synthesis, characterization and antibacterial activity against Gram positive and Gram negative bacteria of biomimetically coated silver nanoparticles. Langmuir 2011, 27, 9165–9173. [Google Scholar] [CrossRef] [PubMed]
- Taglietti, A.; Diaz Fernandez, Y.A.; Amato, E.; Cucca, L.; Dacarro, G.; Grisoli, P.; Necchi, V.; Pallavicini, P.; Pasotti, L.; Patrini, M. Antibacterial activity of glutathione-coated silver nanoparticles against Gram positive and Gram negative bacteria. Langmuir 2012, 28, 8140–8148. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.M.; McIntyre, J.M.; Craig, G.G.; Mulyani; Zilm, P.S.; Gully, N.J. Differences between normal and demineralized dentine pretreated with silver fluoride and potassium iodide after an in vitro challenge by Streptococcus mutans. Aust. Dent. J. 2007, 52, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.; Thibodeaux, D.; Cui, X.; Martin, V.; Watson, M.; Knowlton, J. Instrumental and operational impacts on spectrophotometer color measurements. J. Cotton Sci. 2008, 12, 287–297. [Google Scholar]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.B.; Haiter-Neto, F.; Carlo, H.L.; Soares, C.J.; Sinhoreti, M.A.; Puppin-Rontani, R.M.; Correr-Sobrinho, L. Radiodensity and hardness of enamel and dentin of human and bovine teeth, varying bovine teeth age. Arch. Oral Biol. 2008, 53, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Ghisi, A.C.; Kopper, P.M.; Baldasso, F.E.; Sturmer, C.P.; Rossi-Fedele, G.; Steier, L.; de Figueiredo, J.A.; Morgental, R.D.; Vier-Pelisser, F.V. Effect of superoxidized water and sodium hypochlorite, associated or not with EDTA, on organic and inorganic components of bovine root dentin. J. Endod. 2015, 41, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.A.; Ellenikiotis, H.; Milgrom, P.L. UCSF Protocol for Caries Arrest Using Silver Diamine Fluoride: Rationale, Indications and Consent. J. Calif. Dent. Assoc. 2016, 44, 16–28. [Google Scholar] [PubMed]
- Lou, Y.L.; Botelho, M.G.; Darvell, B.W. Reaction of silver diamine [corrected] fluoride with hydroxyapatite and protein. J. Dent. 2011, 39, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Lopez, L.A.; Quock, R.L. Silver diamine fluoride, potassium iodide, and esthetic perception: An in vitro pilot study. Am. J. Dent. 2016, 29, 248–250. [Google Scholar] [PubMed]
- Zhao, I.S.; Mei, M.L.; Burrow, M.F.; Lo, E.C.; Chu, C.H. Prevention of secondary caries using silver diamine fluoride treatment and casein phosphopeptide-amorphous calcium phosphate modified glass-ionomer cement. J. Dent. 2017, 57, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; De Peralta, T.; Handy, R.D. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology 2014, 8, 745–754. [Google Scholar] [CrossRef] [PubMed]








| Enamel | Exposed to Light (Wavelength 410–460 nm) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| SDF | SDF + GSH | SDF + KI | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Immediate | 4.68 a | 1.44 | 1.20 b | 0.34 | 4.65 a | 0.84 | ≤0.001 * |
| 3 h | 14.17 a | 3.05 | 2.17 c | 0.58 | 5.28 b | 0.71 | ≤0.001 * |
| 6 h | 21.95 a | 3.53 | 3.32 b | 0.71 | 5.51 b | 0.81 | ≤0.001 * |
| 24 h | 34.52 a | 1.93 | 7.62 b | 2.11 | 6.23 b | 0.86 | ≤0.001 * |
| 48 h | 40.32 a | 2.40 | 9.76 b | 2.29 | 6.55 c | 1.06 | ≤0.001 * |
| 72 h | 42.62 a | 2.38 | 10.94 b | 2.07 | 6.90 c | 1.42 | ≤0.001 * |
| 7 days | 43.81 a | 3.27 | 13.03 b | 2.46 | 7.08 c | 1.97 | ≤0.001 * |
| 10 days | 45.68 a | 4.06 | 13.98 b | 2.54 | 6.88 c | 1.24 | ≤0.001 * |
| 14 days | 46.77 a | 3.45 | 14.76 b | 2.35 | 6.99 c | 1.39 | ≤0.001 * |
| Enamel | Dark | p-Value | |||||
|---|---|---|---|---|---|---|---|
| SDF | SDF + GSH | SDF + KI | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Immediate | 2.63 b | 0.88 | 2.01 b | 0.52 | 4.36 a | 1.03 | ≤0.001 * |
| 3 h | 2.79 b | 0.99 | 1.64 b | 0.54 | 5.29 a | 1.09 | ≤0.001 * |
| 6 h | 2.61 b | 0.83 | 2.61 b | 0.89 | 5.36 a | 1.01 | ≤0.001 * |
| 24 h | 4.66 ab | 1.66 | 3.23 b | 0.92 | 5.68 a | 1.28 | 0.009 * |
| 48 h | 5.65 ab | 1.91 | 4.13 b | 0.67 | 6.13 a | 1.23 | 0.033 * |
| 72 h | 6.95 | 2.37 | 4.95 | 1.02 | 6.46 | 1.50 | 0.117 NS |
| 7 days | 8.35 | 2.67 | 5.86 | 1.53 | 6.25 | 1.58 | 0.134 NS |
| 10 days | 11.02 a | 2.39 | 6.55 b | 1.20 | 6.74 b | 1.60 | 0.003 * |
| 14 days | 11.87 a | 2.32 | 7.45 b | 1.56 | 6.91 b | 1.73 | 0.004 * |
| Dentin | Light | p-Value | |||||
|---|---|---|---|---|---|---|---|
| SDF | SDF + GSH | SDF + KI | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Immediate | 2.47 b | 0.57 | 3.38 a | 1.18 | 4.50 a | 1.00 | 0.002 * |
| 3 h | 8.43 | 1.23 | 7.12 | 0.74 | 7.82 | 1.42 | 0.095 NS |
| 6 h | 9.65 a | 1.32 | 8.17 b | 1.09 | 8.33 b | 1.00 | 0.016 * |
| 24 h | 14.40 a | 1.67 | 11.50 b | 1.12 | 9.12 c | 0.92 | ≤0.001 * |
| 48 h | 19.16 a | 1.72 | 12.98 b | 1.10 | 10.42 c | 0.73 | ≤0.001 * |
| 72 h | 21.30 a | 1.21 | 15.36 b | 0.71 | 11.60 c | 0.99 | ≤0.001 * |
| 7 days | 23.91a | 1.94 | 18.55 b | 0.77 | 13.04 c | 0.83 | ≤0.001 * |
| 10 days | 27.94 a | 1.18 | 21.63 b | 0.58 | 12.74 c | 0.72 | ≤0.001 * |
| 14 days | 27.52 a | 1.15 | 25.67 b | 0.45 | 13.96 c | 1.12 | ≤0.001 * |
| Dentin | Dark | p-Value | |||||
|---|---|---|---|---|---|---|---|
| SDF | SDF + GSH | SDF + KI | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Immediate | 2.05 | 0.61 | 2.99 | 0.91 | 2.69 | 1.43 | 0.129 NS |
| 3 h | 6.12 | 2.18 | 5.14 | 1.15 | 6.57 | 1.64 | 0.268 NS |
| 6 h | 8.45 a | 0.84 | 5.52 b | 1.28 | 8.00 a | 1.04 | ≤0.001 * |
| 24 h | 10.30 a | 0.80 | 7.72 c | 0.73 | 8.84 b | 0.86 | ≤0.001 * |
| 48 h | 11.64 a | 1.04 | 9.87 b | 0.84 | 9.64 b | 0.55 | ≤0.001 * |
| 72 h | 13.32 a | 1.11 | 12.36 b | 0.66 | 10.69 c | 0.76 | ≤0.001 * |
| 7 days | 15.96 a | 0.57 | 15.69 a | 0.91 | 12.54 b | 0.91 | ≤0.001 * |
| 10 days | 19.32 a | 0.54 | 18.62 b | 0.37 | 12.84 c | 0.85 | ≤0.001 * |
| 14 days | 22.29 a | 00.81 | 20.90 b | 0.82 | 13.61 c | 1.02 | ≤0.001 * |
| Code | Materials | Composition | Manufacturer |
|---|---|---|---|
| SDF | Saforide | 38% Silver diamine fluoride | Bee Brand Medico Dental, Osaka, Japan |
| F = 44,880 ppm, Ag = 253,870 ppm | |||
| KI | Potassium Iodide (KI) | White, crystalline powder of KI | Wako Pure Chemical Industries, Osaka, Japan |
| GSH | Glutathione | l-Glutathione reduced | Sigma-Aldrich, St. Louis, MO, USA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, M.; Matsui, N.; Hiraishi, N.; Nikaido, T.; Burrow, M.F.; Tagami, J. Effect of Glutathione Bio-Molecule on Tooth Discoloration Associated with Silver Diammine Fluoride. Int. J. Mol. Sci. 2018, 19, 1322. https://doi.org/10.3390/ijms19051322
Sayed M, Matsui N, Hiraishi N, Nikaido T, Burrow MF, Tagami J. Effect of Glutathione Bio-Molecule on Tooth Discoloration Associated with Silver Diammine Fluoride. International Journal of Molecular Sciences. 2018; 19(5):1322. https://doi.org/10.3390/ijms19051322
Chicago/Turabian StyleSayed, Mahmoud, Naoko Matsui, Noriko Hiraishi, Toru Nikaido, Michael F. Burrow, and Junji Tagami. 2018. "Effect of Glutathione Bio-Molecule on Tooth Discoloration Associated with Silver Diammine Fluoride" International Journal of Molecular Sciences 19, no. 5: 1322. https://doi.org/10.3390/ijms19051322