Evaluation of Toxicity and Neural Uptake In Vitro and In Vivo of Superparamagnetic Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoparticle Size and Size Distribution
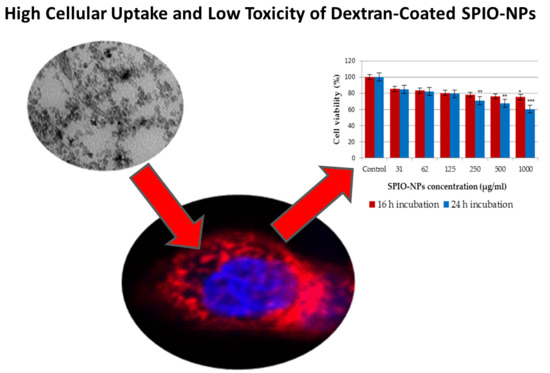
2.2. In Vitro Toxicity of Coated and Fluorescent Iron Oxide Nanoparticles by MTT Assay
2.3. In Vitro, In Vivo, and Ex Vivo Studies of Uptake of Coated and Fluorescent Iron Oxide Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Superparamagnetic Iron Oxide Nanoparticles
3.3. Determination of Particle Size, Zeta Potential, and Morphology
3.4. Cell Cultures of C6 Glioma Cells
3.5. Culturing of Neural Primary Cells
3.6. MTT Assay
3.7. Animal Model
3.7.1. In Vivo Confocal Neuroimaging (ICON)
3.7.2. Whole Mount (Ex Vivo)
3.8. Statistics
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | annular dark-field |
| BBB | blood–brain barrier |
| DI | deionize |
| DLS | dynamic light scattering |
| DMEM | dulbecco’s modified eagle’s medium |
| DMSO | dimethyl sulfoxide |
| FBS | fetal bovine serum |
| FDA | federal drug association |
| HAADF | high-angle annular dark-field |
| ICON | in vivo confocal neuroimaging |
| LCD | liquid-crystal display |
| LDH | lactate dehydrogenase |
| MNPs | magnetic nanoparticles |
| MRI | magnetic resonance imaging |
| NPs | nanoparticles |
| OD | optical density |
| PDI | polydispersity index |
| PTFE | polytetrafluoroethylene |
| ROS | reactive oxygen species |
| SPIO-NPs | superparamagnetic iron oxide nanoparticles |
| TEM | transmission electron microscopy |
| UV | ultraviolet |
References
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005, 74, 489–520. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Konwar, R.; Ahmed, A.B. Nanoparticle: An overview of preparation, characterization and application. Int. Res. J. Pharm. 2013, 4, 47–57. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chemie Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Chadha, T.S.; Haddad, K.; Biswas, P. Perspective on nanoparticle technology for biomedical use. Curr. Pharm. Des. 2016, 22, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Liu, X.; Qiu, G.; Zhang, N.; Shi, R.; Yi, R.; Tang, M.; Che, R. A simple solvothermal synthesis and characterization of round-biscuit-like Fe3O4 nanoparticles with adjustable sizes. Solid State Commun. 2007, 144, 315–318. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Q. Synthesis of magnetite nanoparticles by precipitation with forced mixing. J. Nanopart. Res. 1999, 1, 393–396. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Kotal, A.; Mandal, T.K.; Giri, S.; Nakamura, H.; Kohara, T. Size-controlled synthesis of magnetite nanoparticles in the presence of polyelectrolytes. Chem. Mater. 2004, 16, 3489–3496. [Google Scholar] [CrossRef]
- Pang, Y.L.; Lim, S.; Ong, H.C.; Chong, W.T. Research progress on iron oxide-based magnetic materials: Synthesis techniques and photocatalytic applications. Ceram. Int. 2016, 42, 9–34. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, Z.; Tang, Y.; Liang, B. Review on the synthesis and applications of Fe3O4 nanomaterials. J. Nanomater. 2013, 2013, 1–7. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Pérez, N.; Labarta, A.; Batlle, X. Controlled synthesis of iron oxide nanoparticles over a wide size range. Langmuir 2009, 26, 5843–5847. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Ulrih, N.P. Multifunctional superparamagnetic iron oxide nanoparticles: Promising tools in cancer theranostics. Cancer Lett. 2013, 336, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, J.; You, C.; Song, Z.; Yu, B.; Shen, Y. Influences of different synthesis conditions on properties of Fe3O4 nanoparticles. Mater. Chem. Phys. 2009, 113, 46–52. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Shin, D.K.; Lee, H.J.; Oh, K.S.; Lee, J.H.; Riu, D.H. Nano magnetite particles prepared under the combined addition of urea and ammonia. Key Eng. Mater. 2006, 317–318, 203–206. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Zhang, L.; Yang, M.; Bai, L.; Dai, Y.; Yu, Z.; Pan, Y. Functional magnetic hybrid nanomaterials for biomedical diagnosis and treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gyergyek, S.; Makovec, D.; Jagodič, M.; Drofenik, M.; Schenk, K.; Jordan, O.; Kovač, J.; Dražič, G.; Hofmann, H. Hydrothermal growth of iron oxide NPs with a uniform size distribution for magnetically induced hyperthermia: Structural, colloidal and magnetic properties. J. Alloys Compd. 2017, 694, 261–271. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Kovac, J.; Kubovcikova, M.; Antal, I.; Kopcansky, P.; Bednarikova, M.; Muckova, M. The cytotoxicity of iron oxide nanoparticles with different modifications evaluated in vitro. J. Magn. Magn. Mater. 2015, 380, 85–89. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, A.; Crayton, S.H.; Warden-Rothman, R.; Tsourkas, A. Quantitative comparison of tumor delivery for multiple targeted nanoparticles simultaneously by multiplex ICP-MS. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.B.; Blaufox, M.D. Blood volume in the rat. J. Nucl. Med. 1985, 26, 72–76. [Google Scholar] [PubMed]
- Balas, M.; Ciobanu, C.S.; Burtea, C.; Stan, M.S.; Bezirtzoglou, E.; Predoi, D.; Dinischiotu, A. Synthesis, characterization, and toxicity evaluation of dextran-coated iron oxide nanoparticles. Metals 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Ciobanu, C.S.; Chifiriuc, M.C.; Stoicea, M.; Predoi, D. Iron oxide magnetic nanoparticles: Characterization and toxicity evaluation by in vitro and in vivo assays. J. Nanomater. 2013, 2013, 1–10. [Google Scholar]
- Costa, C.; Brandão, F.; Bessa, M.J.; Costa, S.; Valdiglesias, V.; Kiliç, G.; Fernández-Bertólez, N.; Quaresma, P.; Pereira, E.; Pásaro, E.; et al. In vitro cytotoxicity of superparamagnetic iron oxide nanoparticles on neuronal and glial cells. Evaluation of nanoparticle interference with viability tests. J. Appl. Toxicol. 2016, 36, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Mafakheri, H.; Khoshgard, K.; Montazerabadi, A.; Mohammadbeigi, A.; Oubari, F. The cytotoxicity of Dextran-coated iron oxide nanoparticles on Hela and MCF-7 cancerous cell lines. Iran. J. Toxicol. 2017, 11, 31–36. [Google Scholar] [CrossRef]
- Kouchesfehani, H.M.; Kiani, S.; Rostami, A.A.; Fakheri, R. Cytotoxic effect of iron oxide nanoparticles on mouse embryonic stem cells by MTT assay. Iran. J. Toxicol. 2013, 7, 849–853. [Google Scholar]
- Bae, J.E.; Huh, M.I.; Ryu, B.K.; Do, J.Y.; Jin, S.U.; Moon, M.J.; Jung, J.C.; Chang, Y.; Kim, E.; Chi, S.G.; et al. The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 2011, 32, 9401–9414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Grigartzik, L.; Heiduschka, P.; van Wachem, B.; Sable, B.A.; Hintz, W.; Henrich-Noack, P. Nanoparticle delivery of Caspase-3 si-RNA to the retina inhibits pro-apoptotic gene expression after traumatic nerve injury. Manuscript in preparation.
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Lefebure, S.; Dubois, E.; Cabuil, V.; Neveu, S.; Massart, R. Monodisperse magnetic nanoparticles: Preparation and dispersion in water and oils. J. Mater. Res. 1998, 13, 2975–2981. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Soflaee, F. Synthesis and characterization of α-Fe2O3 nanoparticles by simple co-Precipitation method. Phys. Chem. Res. 2015, 3, 191–196. [Google Scholar]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Pieper, M.; Aman, S.; Hintz, W.; Tomas, J. Optimization of a continuous precipitation process to produce nanoscale BaSO4. Chem. Eng. Technol. 2011, 34, 1567–1574. [Google Scholar] [CrossRef]
- Rahmawati, R.; Permana, M.G.; Harison, B.; Yuliarto, B.; Kurniadi, D. Optimization of frequency and stirring rate for synthesis of magnetite (Fe3O4) nanoparticles by using coprecipitation-ultrasonic irradiation methods. Procedia Eng. 2017, 170, 55–59. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.K.; Asad, M.; Henrich-Noack, P.; Sokolov, M.; Hintz, W.; Grigartzik, L.; Zhang, E.; Dityatev, A.; Van Wachem, B.; Sabel, B.A. Evaluation of Toxicity and Neural Uptake In Vitro and In Vivo of Superparamagnetic Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2613. https://doi.org/10.3390/ijms19092613
Khalid MK, Asad M, Henrich-Noack P, Sokolov M, Hintz W, Grigartzik L, Zhang E, Dityatev A, Van Wachem B, Sabel BA. Evaluation of Toxicity and Neural Uptake In Vitro and In Vivo of Superparamagnetic Iron Oxide Nanoparticles. International Journal of Molecular Sciences. 2018; 19(9):2613. https://doi.org/10.3390/ijms19092613
Chicago/Turabian StyleKhalid, Muhammad Kamran, Muhammad Asad, Petra Henrich-Noack, Maxim Sokolov, Werner Hintz, Lisa Grigartzik, Enqi Zhang, Alexander Dityatev, Berend Van Wachem, and Bernhard A. Sabel. 2018. "Evaluation of Toxicity and Neural Uptake In Vitro and In Vivo of Superparamagnetic Iron Oxide Nanoparticles" International Journal of Molecular Sciences 19, no. 9: 2613. https://doi.org/10.3390/ijms19092613