Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity Evaluation
2.2. Morphological Investigations:
2.3. Cytokine Production
2.4. Are CD44 Receptors Involved in the Early Inflammatory Regulation?
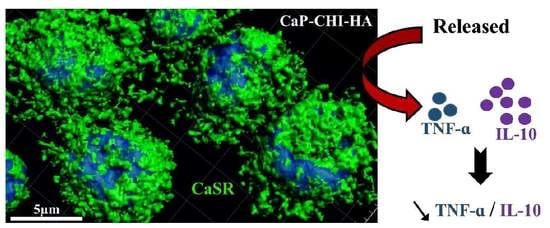
2.5. Role of CaSR Receptor in Early Inflammatory Regulation
3. Materials and Methods
3.1. Substrate Build-Up
3.2. THP-1 Culture
3.2.1. Mitochondrial Activity
3.2.2. DNA Quantification
3.2.3. Reactive Oxygen Species (ROS) Production Analysis
3.2.4. Scanning Electron Microscopy with a Field Emission Gun (FEG-SEM)
3.2.5. Cytoskeleton Visualization
3.2.6. Vinculin, CaSR and CD44 Immunolabellings
3.2.7. CD44 Quantification
3.2.8. Cytokine, Chemokine and Growth Factor Releases
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CaP | Calcium Phosphate |
| CHI | Chitosan |
| HA | Hyaluronic acid |
| CaSR | Calcium sensing receptor |
| CD | Cluster of Differentiation |
| WST-1 | Water-Soluble Tetrazolium Salt-1 |
| ROS | Reactive oxygen species |
| DNA | Deoxyribonucleic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FEG-SEM | Scanning electron microscopy with a field emission gun |
| CLSM | Confocal laser scanning microscopy |
References
- Mountziaris, P.M.; Mikos, A.G. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Anada, T.; Honda, Y.; Suzuki, O. Comparative study on in vitro biocompatibility of synthetic octacalcium phosphate and calcium phosphate ceramics used clinically. Biomed. Mater. 2012, 7, 045020. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Iviglia, G.; Cassinelli, C.; Bollati, D.; Baino, F.; Torre, E.; Morra, M.; Vitale-Brovarone, C. Engineered porous scaffolds for periprosthetic infection prevention. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Iviglia, G.; Cassinelli, C.; Torre, E.; Baino, F.; Morra, M.; Vitale-Brovarone, C. Novel bioceramic-reinforced hydrogel for alveolar bone regeneration. Acta Biomater. 2016, 44, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Boccafoschi, F.; Baino, F.; Vitale-Brovarone, C.; Vernè, E.; Barbani, N.; Ciardelli, G. Composite films of gelatin and hydroxyapatite/bioactive glass for tissue-engineering applications. J. Biomater. Sci. Polym. ED 2010, 21, 1207–1226. [Google Scholar] [CrossRef] [PubMed]
- Cado, G.; Kerdjoudj, H.; Chassepot, A.; Lefort, M.; Benmlih, K.; Hemmerlé, J.; Voegel, J.C.; Jierry, L.; Schaaf, P.; Frère, Y.; et al. Polysaccharide films built by simultaneous or alternate spray: A rapid way to engineer biomaterial surfaces. Langmuir 2012, 28, 8470–8478. [Google Scholar] [CrossRef] [PubMed]
- Rammal, H.; Dubus, M.; Aubert, L.; Reffuveille, F.; Laurent-Maquin, D.; Terryn, C.; Schaaf, P.; Alem, H.; Francius, G.; Quilès, F.; et al. Bioinspired Nanofeatured Substrates: Suitable Environment for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 12791–12801. [Google Scholar] [CrossRef] [PubMed]
- Mechiche Alami, S.; Rammal, H.; Boulagnon-Rombi, C.; Velard, F.; Lazar, F.; Drevet, R.; Laurent Maquin, D.; Gangloff, S.C.; Hemmerlé, J.; Voegel, J.C.; et al. Harnessing Wharton’s jelly stem cell differentiation into bone-like nodule on calcium phosphate substrate without osteoinductive factors. Acta Biomater. 2017, 49, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, K.S. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin. Immunol. 2008, 20, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Jiang, L.H.; Foster, R.; Yang, X.B. Immunological Responses to Total Hip Arthroplasty. J. Funct. Biomater. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Sun, X.; Qu, Y.; Man, Y. Collagen Membrane and Immune Response in Guided Bone Regeneration: Recent Progress and Perspectives. Tissue Eng. Part B Rev. 2017, 23, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Jubeli, E.; Bakar, J.; Tortolano, L.; Saunier, J.; Yagoubi, N. Biocompatibility assessment of cyclic olefin copolymers: Impact of two additives on cytotoxicity, oxidative stress, inflammatory reactions, and hemocompatibility. J. Biomed. Mater. Res. A 2017, 105, 3333–3349. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Stachelek, S.J.; Tomczyk, N.; Finley, M.J.; Composto, R.J.; Eckmann, D.M. Correlating macrophage morphology and cytokine production resulting from biomaterial contact. J. Biomed. Mater. Res. A 2013, 101, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, H.; Liu, Y.; He, Y.; Wang, W.; Du, Y.; Yang, C.; Gao, F. CD44 clustering is involved in monocyte differentiation. Acta Biochim. Biophys. Sin 2014, 46, 540–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Wei, Y.; Zhang, X.; Meng, S.; Mo, X.; Liu, X.; Deng, X.; Zhang, L.; Deng, X. Attenuating Immune Response of Macrophage by Enhancing Hydrophilicity of Ti Surface. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Andorko, J.I.; Jewell, C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng. Transl. Med. 2017, 2, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.A.; Chang, D.T.; Meyerson, H.; Colton, E.; Kwon, I.K.; Matsuda, T.; Anderson, J.M. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J. Biomed. Mater. Res. A 2007, 83, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Muswe, R.; Oktedalen, O.; Zhou, D.T.; Zinyando, E.; Shawarira-Bote, S.; Stray-Pedersen, B.; Siziba, A.; Gomo, Z.A.R. Inflammatory Markers and Plasma Lipids in HIV Patients: A Correlation Analysis Study. Open Biochem. J. 2017, 11, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.G.; Magalhães, L.M.D.; Giunchetti, R.C.; Dutra, W.O.; Gollob, K.J. Infection of Human Monocytes with Leishmania infantum Strains Induces a Down modulated Response when Compared with Infection with Leishmania braziliensis. Front. Immunol. 2017, 8, 1896. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.S.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Lesley, J.; Hyman, R.; Kincade, P.W. CD44 and its interaction with extracellular matrix. Adv. Immunol. 1993, 54, 271–335. [Google Scholar] [CrossRef] [PubMed]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, function, and association with the malignant process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar] [CrossRef] [PubMed]
- Klein, G.L.; Castro, S.M.; Garofalo, R.P. The calcium-sensing receptor as a mediator of inflammation. Cell Dev. Biol. 2016, 49, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, U.; Lawson, C.; Dabare, M.; Syndercombe-Court, D.; Newland, A.C.; Howells, G.L.; Macey, M.G. Human peripheral blood monocytes express protease receptor-2 and respond to receptor activation by production of IL-6, IL-8, and IL-1β. J. Leukoc. Biol. 2005, 78, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Steven, R.; Crilly, A.; Lockhart, J.C.; Ferrell, W.R.; Mc Innes, I.B. Proteinase-activated receptor-2 modulates human macrophage differentiation and effector function. Innate Immunity 2013, 19, 663–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rammal, H.; Bour, C.; Dubus, M.; Entz, L.; Aubert, L.; Gangloff, S.C.; Audonnet, S.; Bercu, N.B.; Boulmedais, F.; Mauprivez, C.; et al. Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response. Int. J. Mol. Sci. 2018, 19, 3458. https://doi.org/10.3390/ijms19113458
Rammal H, Bour C, Dubus M, Entz L, Aubert L, Gangloff SC, Audonnet S, Bercu NB, Boulmedais F, Mauprivez C, et al. Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response. International Journal of Molecular Sciences. 2018; 19(11):3458. https://doi.org/10.3390/ijms19113458
Chicago/Turabian StyleRammal, Hassan, Camille Bour, Marie Dubus, Laura Entz, Léa Aubert, Sophie C. Gangloff, Sandra Audonnet, Nicolae B. Bercu, Fouzia Boulmedais, Cedric Mauprivez, and et al. 2018. "Combining Calcium Phosphates with Polysaccharides: A Bone-Inspired Material Modulating Monocyte/Macrophage Early Inflammatory Response" International Journal of Molecular Sciences 19, no. 11: 3458. https://doi.org/10.3390/ijms19113458