Conjugates of Gold Nanoparticles and Antitumor Gold(III) Complexes as a Tool for Their AFM and SERS Detection in Biological Tissue
Abstract
:1. Introduction
2. Results
2.1. Prediction of Gold(III) Complexes Binding to Citrate-Capped AuNPs
2.2. Spectrophotometric Study of the Interaction Between Gold(III) Complexes and AuNPs
2.3. AFM Images of AuNPs /Au(III) Complexes Assembly
2.4. SERS Spectra of Au(III) Complexes
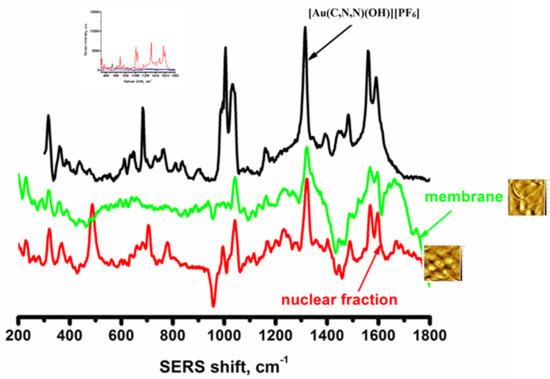
2.5. AFM and SERS Tracking of Gold(III) Complexes in the Cytosolic, Nuclear, and Plasma Membrane Fractions of Human Lymphocytes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of AuNPs
4.3. Subcellular Fractionation of Human Lymphocytes
4.4. Apparatus
4.5. DFT Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| (C:N) | deprotonated 6-(1,1-dimethylbenzyl)-pyridine |
| (C,N,N) | deprotonated 6-(1,1-dimethylbenzyl)-2,2′-bipyridine |
| (N,N) | 2,2′-bipyridine |
| PBS | Phosphate buffer solution |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| DFT | Density functional theory |
| CLS | Compact laser system |
| AFM | Atomic Force Microscopy |
| SERS | Surface-enhanced Raman spectroscopy |
| LSPR | Localized surface Plasmon resonance |
| NPs | Nanoparticles |
| AuNPs | Gold nanoparticles |
| AgNPs | Silver nanoparticles |
References
- Bertrand, B.; Williams, M.R.M.; Bochmann, M. Gold(III) Complexes for Antitumor Applications: An Overview. Chem. Eur. J. 2018, 24, 11840–11851. [Google Scholar] [CrossRef] [PubMed]
- Nardon, C.; Boscutti, G.; Fregona, D. Beyond Platinums: Gold Complexes as Anticancer Agents. Anticancer Res. 2014, 34, 487–492. [Google Scholar] [PubMed]
- Marcon, G.; Carotti, S.; Coronnello, M.; Messori, L.; Mini, E.; Orioli, P.; Mazzei, T.; Cinellu, M.A.; Minghetti, G. Gold(III) Complexes with Bipyridyl Ligands: Solution Chemistry, Cytotoxicity, and DNA Binding Properties. J. Med. Chem. 2002, 45, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Law, B.Y.K.; Qu, Y.Q.; Mok, S.W.F.; Liu, H.; Zeng, W.; Han, Y.; Gordillo-Martinez, F.; Chan, W.K.; Wong, K.M.; Wong, V.K.W. New perspectives of cobalt tris(bipyridine) system: Anti-cancer effect and its collateral sensitivity towards multidrug-resistant (MDR) cancers. Oncotarget 2017, 8, 55003–55021. [Google Scholar] [CrossRef] [PubMed]
- Amani, V.; Abedi, A.; Ghabeshi, S.; Khavasi, H.R.; Hosseini, S.M.; Safari, N. Synthesis and characterization of a series of gold(III) complexes with the 4,4′-dimethyl-2,2′-bipyridine ligand: Counterion influence on the cytotoxicity of gold(III) complexes. Polyhedron 2014, 79, 104–115. [Google Scholar] [CrossRef]
- Casini, A.; Hartinger, C.; Gabbiani, C.; Mini, E.; Dyson, P.J.; Keppler, B.K.; Messori, L. Gold(III) compounds as anticancer agents: Relevance of gold-protein interactions for their mechanism of action. J. Inorg. Biochem. 2008, 102, 564–575. [Google Scholar] [CrossRef]
- Simpson, D.H.; Scott, P. Seven-Antimicrobial Metallodrugs. In Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells, 1st ed.; Lo, K.K.-W., Ed.; Academic Press: Hong Kong, China, 2017; Volume 7, pp. 205–243. [Google Scholar]
- Renfrew, A.K. Spectroscopic Approaches to Tracking Metal-based Drugs in Cells and Tissue. Chima 2017, 71, 112–119. [Google Scholar] [CrossRef]
- Dean, K.M.; Qin, Y.; Palmer, A.E. Visualizing metal ions in cells: An overview of analytical techniques, approaches, and probes. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1406–1415. [Google Scholar] [CrossRef] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Eljack, N.D.; Ma, H.Y.; Drucker, J.; Shen, C.; Hambley, T.W.; New, E.J.; Friedrich, T.; Clarke, R.J. Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin. Metallomics 2014, 6, 2126–2133. [Google Scholar] [CrossRef] [Green Version]
- Coronnello, M.; Mini, E.; Caciagli, B.; Cinellu, M.A.; Bindoli, A.; Gabbiani, C.; Messori, L. Mechanisms of Cytotoxicity of Selected Organogold(III) Compounds. J. Med. Chem. 2005, 48, 6761–6765. [Google Scholar] [CrossRef] [PubMed]
- Gabbiani, C.; Mastrobuoni, G.; Sorrentino, F.; Dani, B.; Rigobello, M.P.; Bindoli, A.; Cinellu, M.A.; Pieraccini, G.; Messori, L.; Casini, A. Thioredoxin reductase, an emerging target for anticancer metallodrugs. Enzyme inhibition by cytotoxic gold(III) compounds studied with combined mass spectrometry and biochemical assays. Med. Chem. Comm. 2011, 2, 50–54. [Google Scholar] [CrossRef]
- Sreedhar, A.; Zhao, Y. Dysregulated metabolic enzymes and metabolic reprogramming in cancer cells. Biomed. Rep. 2018, 8, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Alevizopoulos, K.; Calogeropoulou, T.; Lang, F.; Stournaras, C. Na+/K+-ATPase inhibitors in cancer. Curr. Drug Targets 2014, 15, 988–1000. [Google Scholar] [CrossRef]
- Lefranc, F.; Mijatovic, T.; Kondo, Y.; Sauvage, S.; Roland, I.; Debeir, O.; Krstic, D.; Vasic, V.; Gailly, P.; Kondo, S.; et al. Targeting the alpha 1 subunit of the sodium pump to combat glioblastoma cells. Neurosurgery 2008, 62, 211–221. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Čolović, M.B.; Janjić, G.V.; Zarić, B.; Petrović, S.; Krstić, D.Z.; Marzo, T.; Messori, L.; Vasić, V.M. The influence of oxo-bridged binuclear gold(III) complexes on Na/K-ATPase activity: A joint experimental and theoretical approach. J. Biol. Inorg. Chem. 2017, 22, 819–832. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Janjić, G.V.; Dramićanin, M.D.; Messori, L.; Massai, L.; Parac Vogt, T.N.; Vasić, V.M. Na/K-ATPase as a target for anticancer metal based drugs: Insights into molecular interactions with selected gold(iii) complexes. Metallomics 2017, 9, 292–300. [Google Scholar]
- Vujačić Nikezić, A.V.; Janjić, G.V.; Bondžić, A.M.; Zarić, B.L.; Vasić-Anićijević, D.D.; Momić, T.G.; Vasić, V.M. Interaction of Au(III) and Pt(II) complexes with Na/K-ATPase: Experimental and theoretical study of reaction stoichiometry and binding sites. Metallomics 2018, 10, 1003–1015. [Google Scholar] [CrossRef]
- Felippe Gonçalves-de-Albuquerque, C.; Ribeiro Silva, A.; Ignácio da Silva, C.; Caire Castro-Faria-Neto, H.; Burth, P. Na/K Pump and Beyond: Na/K-ATPase as a Modulator of Apoptosis and Autophagy. Molecules 2017, 22, 578. [Google Scholar] [CrossRef] [Green Version]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Delivery 2012, 3, 457–478. [Google Scholar] [CrossRef] [Green Version]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of Surface Enhanced Raman Spectroscopy (SERS) in Therapeutic Drug Monitoring (TDM). A Critical Review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- D’ Acunto, M.; Dinarelli, S.; Cricenti, A.; Luce, M. Optical Detection of core-gold nanoshells inside biosystems. Nanospectroscopy 2016, 1, 97–105. [Google Scholar]
- Liu, L.; Tang, Y.; Dai, S.; Kleitz, F.; Qiao, S.Z. Smart surface-enhanced Raman scattering traceable drug delivery systems. Nanoscale 2016, 8, 12803–12811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’ Acunto, M.; Cricenti, A.; Danti, S.; Dinarelli, S.; Luce, M.; Moroni, D.; Salvetti, O. Detection and localization of gold nanoshells inside cells: Near-field approximation. Appl. Opt. 2016, 55, D11–D16. [Google Scholar] [CrossRef]
- Huang, X.; He, J.; Liu, M.; Zhou, C. The influence of aminophylline on the nanostructure and nanomechanics of T lymphocytes: An AFM study. Nanoscale Res. Lett. 2014, 9, 518. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Wang, H. The structure and function of cell membranes examined by atomic force microscopy and single-molecule force spectroscopy. Chem. Soc. Rev. 2015, 44, 3617–3638. [Google Scholar] [CrossRef]
- Craig, G.E.; Brown, S.D.; Lamprou, D.A.; Graham, D.; Wheate, N.J. Cisplatin-tethered gold nanoparticles that exhibit enhanced reproducibility, drug loading, and stability: A step closer to pharmaceutical approval? Inorg. Chem. 2012, 51, 3490–3497. [Google Scholar] [CrossRef] [Green Version]
- Muller, D.J. AFM: A nanotool in membrane biology. Biochemistry 2008, 47, 7986–7998. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Vujačić, A.; Vasić, V.; Dramićanin, M.; Sovilj, S.P.; Bibić, N.; Milonjić, S.; Vodnik, V. Fluorescence quenching of 5,5′-disulfopropyl-3,3′- dichlorothiacyanine dye adsorbed on gold nanoparticles. J. Phys. Chem. C 2013, 117, 6567–6577. [Google Scholar] [CrossRef]
- Cricenti, A.; Generosi, R. Air operating atomic force-scanning tunneling microscope suitable to study semiconductors, metals, and biological samples. Rev. Sci. Instrum. 1995, 66, 2843–2847. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Kokalj, A. XCrySDen—a new program for displaying crystalline structures and electron densities. J. Mol. Graphics Modell. 1999, 17, 176–179. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Casini, A.; Diawara, M.C.; Scopelliti, R.; Zakeeruddin, S.M.; Grätzel, M.; Dyson, P.J. Synthesis, characterisation and biological properties of gold(III) compounds with modified bipyridine and bipyridylamine ligands. Dalton Trans. 2010, 39, 2239–2245. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1994; pp. 1–264. [Google Scholar]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Chegel, V.; Rachkov, O.; Lopatynskyi, A.; Ishihara, S.; Yanchuk, I.; Nemoto, Y.; Hill, J.P.; Ariga, K. Gold Nanoparticles Aggregation: Drastic Effect of Cooperative Functionalities in a Single Molecular Conjugate. J. Phys. Chem. C 2012, 116, 2683–2690. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Vujačić Nikezić, A.V.; Klekotka, U.; Marković, M.M.; Vodnik, V.V.; Kalska, B.; Vasić, V.M. Insight into the Interaction between Selected Antitumor Gold(III) Complexes and Citrate Stabilized Gold Nanoparticles. Russ. J. Phys. Chem. A 2019, 93, 189–194. [Google Scholar]
- Zakaria, N.S.; Siti, R.M.; Aziz, A.A.; Razak, K.A. Imaging of Colloidal Gold Nanoparticle Using Atomic Force Microscope. Nano Hybrids 2013, 4, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Darwich, S.; Mougin, K.; Rao, A.; Gnecco, E.; Jayaraman, S.; Haidara, H. Manipulation of gold colloidal nanoparticles with atomic force microscopy in dynamic mode: Influence of particle-substrate chemistry and morphology, and of operating conditions. Beilstein, J. Nanotech. 2011, 2, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, S.; El-Sayed, M.A. Optical Properties and Ultrafast Dynamics of Metallic Nanocrystals. Annu. Rev. Phys. Chem. 2003, 54, 331–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Brolossy, T.; Abdallah, T.; Mohamed, M.; Abdallah, S.; Easawi, K.; Negm, S.; Talaat, M.H. Shape and size dependence of the surface plasmon resonance of gold nanoparticles studied by Photoacoustic technique. Eur. Phys. J. Spec. Top. 2008, 153, 361–364. [Google Scholar] [CrossRef]
- Zuo, C.; Jagodzinski, P.W. Surface-Enhanced Raman Scattering of Pyridine Using Different Metals: Differences and Explanation Based on the Selective Formation of α-Pyridyl on Metal Surfaces. J. Phys. Chem. B 2005, 109, 1788–1793. [Google Scholar] [CrossRef]
- Strekas, T.C.; Diamandopoulos, P.S. Surface-enhanced raman spectroscopy of bipyridines and phenylpyridines. J. Phys. Chem. 1990, 94, 1986–1991. [Google Scholar] [CrossRef]
- Brolo, A.; Jiang, Z.; Irish, D.E. The Orientation of 2,2′-Bipyridine Adsorbed at a SERS-active Au(Ш) Electrode Surface. J. Electroanal. Chem. 2003, 547, 163–172. [Google Scholar] [CrossRef]
- Yan, X.; Li, P.; Yang, L.; Liu, J. Time-dependent SERS spectra monitoring the dynamic adsorption behavior of bipyridine isomerides combined with bianalyte method. Analyst 2016, 141, 5189–5194. [Google Scholar] [CrossRef]
- McConnell, A.A.; Brown, D.H.; Smith, W.E. Resonance enhancement of the Raman spectrum of bipyridyl gold(III) chloro complexes. Spectrochim. Acta A 1982, 38, 737–741. [Google Scholar] [CrossRef]
- Bilić, A.; Reimers, J.R.; Hush, N.S. Adsorption of Pyridine on the Gold(111) Surface: Implications for “Alligator Clips” for Molecular Wires. J. Phys. Chem. B 2002, 106, 6740–6747. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Cardini, G.; Schettino, V. Surface-enhanced Raman spectra of pyridine and pyrazolide on silver colloids: Chemical and electromagnetic effects. Theor. Chem. Acc. 2004, 111, 264–269. [Google Scholar] [CrossRef]
- Joo, S.W. Adsorption of Bipyridine Compounds on Gold Nanoparticle Surfaces Investigated by UV-Vis Absorbance Spectroscopy and Surface Enhanced Raman Scattering. Spectrosc. Lett. 2006, 39, 85–96. [Google Scholar] [CrossRef]
- Gong, X.; Taszarek, M.; Schefzig, L.; Reissig, H.-U.; Thierbach, S.; Wassermann, B.; Maria Graf, C.; Mollenhauer, D.; Ruehl, E. Adsorption of Mono- and Divalent 4-(Dimethylamino)pyridines on Gold Surfaces: Studies by Surface-Enhanced Raman Scattering and Density Functional Theory. Langmuir 2019, 35, 8517–8874. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Amatori, S.; Porchia, M.; Tisato, F.; Bondino, F.; Magnano, E.; Pellei, M.; et al. Highly Hydrophilic Gold Nanoparticles as Carrier for Anticancer Copper(I) Complexes: Loading and Release Studies for Biomedical Applications. Nanomaterials 2019, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Thambiraj, S.; Hema, S.; Ravi Shankaran, D. Functionalized gold nanoparticles for drug delivery applications. In Materials Today, Proceedings of the International Conference on Advanced Materials, Amrita University, Coimbatore, India, 19–21 December 2016; Ramachandran, T., Ed.; Elsevier: Amsterdam, the Netherland, 2018; Materials today: Proceedings 5; pp. 16059–16868. [Google Scholar]
- Brolo, A.G.; Irish, D.E.; Lipkowski, J. Surface-Enhanced Raman Spectra of Pyridine and Pyrazine Adsorbed on a Au(210) Single-Crystal Electrode. J. Phys. Chem. B 1997, 101, 3906–3909. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Bizzotto, D.; Lipkowski, J.; Pettinger, B.; Mirwald, S. Electrochemical and Second Harmonic Generation Studies of 2,2′-Bipyridine Adsorption at the Au(Ш) Electrode Surface. J. Phys. Chem. 1994, 98, 7083–7089. [Google Scholar] [CrossRef]
- Mollenhauer, D.; Gaston, N.; Voloshina, E.; Paulus, B. Interaction of Pyridine Derivatives with a Gold (111) Surface as a Model for Adsorption to Large Nanoparticles. J. Phys. Chem. C 2013, 117, 4470–4479. [Google Scholar] [CrossRef]
- Mezni, A.; Dammak, T.; Fkiri, A.; Mlayah, A.; Abid, Y.; Smiri, L.S. Photochemistry at the Surface of Gold Nanoprisms from Surface-Enhanced Raman Scattering Blinking. J. Phys. Chem. C 2014, 118, 17956–17967. [Google Scholar] [CrossRef]
- Yang, R.; Xi, N.; Fung, C.K.M.; Seiffert-Sinha, K.; Lai, K.W.C.; Sinha, A.A. The Emergence of AFM Applications to Cell Biology: How new technologies are facilitating investigation of human cells in health and disease at the nanoscale. J. Nanosci. Lett. 2011, 1, 87–101. [Google Scholar] [PubMed]
- Dorney, K.M.; Sizemore, I.E.; Alqahtani, T.; Adragna, N.C.; Lauf, P.K. Surface-enhanced Raman spectroscopy (SERS) tracking of chelerythrine, a Na(+)/K(+) pump inhibitor, into cytosol and plasma membrane fractions of human lens epithelial cell cultures. Cell. Physiol. Biochem. 2013, 32, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Xu, X.; Ji, L.; Ma, R.; Dang, Z.; Yang, H. Surface-Enhanced Raman Spectroscopy to study the biological activity of anticancer agent. Cancer Transl. Med. 2019, 5, 37–41. [Google Scholar]








| Complex + Surface | Closest Complex-Surface Distance (Å) | Eads (eV) | Charge Received by Complex Molecule (e) | Optimized Binding Geometry |
|---|---|---|---|---|
| [Au(N,N)(OH)2]+ + Au111 (parallel) | 3.27 | −6.09 | −0.118 |  |
| [Au(N,N)(OH)2]+ + Au111 (vertical) | 2.55 | −4.07 | −0.097 |  |
| [Au(N,N)(OH)2]+Cl + Au111 | 3.88 | −0.43 | −0.231 |  |
| [Au(C,N)(AcO)2] + Au111 | 2.83 | −0.36 | −0.012 |  |
| [Au(C,N,N)(OH)]+ + Au111 | 2.13 | −4.48 | −0.110 |  |
| Complex | Assignment a | Raman Vibrational Mode a (cm−1) | SERS Vibrational Mode (cm−1) | Membrane Fraction (cm−1) | Nuclear Fraction (cm−1) |
|---|---|---|---|---|---|
| [Au(C,N,N)(OH)] [PF6] | Au–N stretching | 324 | 316 | 321 | 321 |
| 602 | 602 | 610 | − | ||
| In-plane ring deformation | 741 | 756 | − | 778 | |
| C–H ring breathing | 1049 | 1031 | 1040 | 1043 | |
| Aromatic C–C stretching | 1327 | 1315 | 1319 | 1319 | |
| 1496 | 1484 | 1486 | 1487 | ||
| 1575 | 1559 | 1568 | 1568 | ||
| 1603 | 1592 | 1591 | 1601 | ||
| [Au(C,N)(AcO)2] | Au–N stretching | 317 | 319 | ||
| − | 361 | ||||
| − | 460 | ||||
| 523 | 549 | ||||
| 660 | 652 | ||||
| In-plane ring deformation | − | 758 | |||
| C–H ring breathing | 1046 | 1037 | |||
| 1272 | 1270 | ||||
| Aromatic C–C stretching | − | 1592 | |||
| 1616 | |||||
| [Au(N,N)(OH)2] [PF6] | Au–N stretching | 342 | 357 | 342 | |
| 475 | 421 | 482 | |||
| 725 | − | − | |||
| Ring deformation in the plane | 757 | 757 | 707 | ||
| 882 | 1006 | 996 | |||
| − | 1021 | 1026 | |||
| C–H ring breathing | 1051 | 1055 | 1168 | ||
| 1326 | 1308 | 1315 | |||
| 1504 | 1484 | 1443 | |||
| Aromatic C–C stretching | 1572 | 1559 | 1547 | ||
| 1609 | 1592 | 1608 | |||
| 2,2′-bipyridine b | Out-of-plane ring deformation | 328 | 354 | ||
| 548 | − | ||||
| 611 | 646 | ||||
| In-plane ring deformation | 752 | 761 | |||
| 992 | 1010 | ||||
| 1043 | 1057 | ||||
| C–H ring breathing | 1298 | 1301 | |||
| 1480 | 1479 | ||||
| 1570 | 1562 | ||||
| Aromatic C–C stretching | 1588 | 1586 | |||
| Pyridine b | Out-of-plane ring deformation | 389 | |||
| 385 | |||||
| In-plane ring deformation | 618 | 636 | |||
| C–H ring breathing | 1002 | 1009 | |||
| 1036 | 1035 | ||||
| 1220 | 1213 | ||||
| Aromatic C–C stretching | 1583 | 1597 | |||
| - | 1639 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondžić, A.M.; Leskovac, A.R.; Petrović, S.Ž.; Vasić Anićijević, D.D.; Luce, M.; Massai, L.; Generosi, A.; Paci, B.; Cricenti, A.; Messori, L.; et al. Conjugates of Gold Nanoparticles and Antitumor Gold(III) Complexes as a Tool for Their AFM and SERS Detection in Biological Tissue. Int. J. Mol. Sci. 2019, 20, 6306. https://doi.org/10.3390/ijms20246306
Bondžić AM, Leskovac AR, Petrović SŽ, Vasić Anićijević DD, Luce M, Massai L, Generosi A, Paci B, Cricenti A, Messori L, et al. Conjugates of Gold Nanoparticles and Antitumor Gold(III) Complexes as a Tool for Their AFM and SERS Detection in Biological Tissue. International Journal of Molecular Sciences. 2019; 20(24):6306. https://doi.org/10.3390/ijms20246306
Chicago/Turabian StyleBondžić, Aleksandra M., Andreja R. Leskovac, Sandra Ž. Petrović, Dragana D. Vasić Anićijević, Marco Luce, Lara Massai, Amanda Generosi, Barbara Paci, Antonio Cricenti, Luigi Messori, and et al. 2019. "Conjugates of Gold Nanoparticles and Antitumor Gold(III) Complexes as a Tool for Their AFM and SERS Detection in Biological Tissue" International Journal of Molecular Sciences 20, no. 24: 6306. https://doi.org/10.3390/ijms20246306