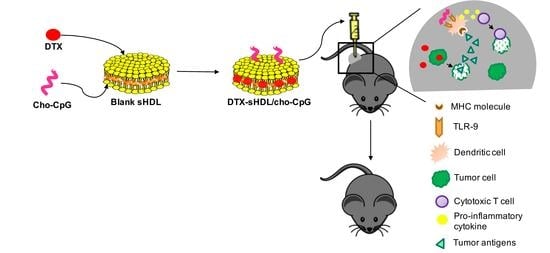
Synthetic HDL Nanoparticles Delivering Docetaxel and CpG for Chemoimmunotherapy of Colon Adenocarcinoma
Abstract
:1. Introduction
2. Results
2.1. Drug and Adjuvant Loading into sHDL do not Affect Size or Shape of sHDL
2.2. SR-B1 Is Highly Expressed by MC38 Cells and Plays a Role in Cellular Uptake of sHDL
2.3. Delivery of DTX by sHDL Maintains the Cytotoxic Effect of DTX on MC38 Cells
2.4. Combination of Immunostimulatory Agent with DTX Increases Antitumor Effects and Prolongs Survival
3. Discussion
4. Materials & Methods
4.1. Materials
4.2. Formulation and Characterization of DTX-sHDL
4.3. In Vitro Uptake Assays
4.4. In Vitro Cytotoxicity Assay
4.5. Western Blot of SR-B1 Expression on Murine Cancer Cells
4.6. In Vivo Treatment Using Combination Chemotherapy and Immunotherapy
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gong, M.; Zhang, Q.; Zhao, Q.; Zheng, J.; Li, Y.; Wang, S.; Yuan, Y. Development of synthetic high-density lipoprotein-based apoa-i mimetic peptide-loaded docetaxel as a drug delivery nanocarrier for breast cancer chemotherapy. Drug Deliv. 2019, 26, 708–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConathy, W.J.; Nair, M.P.; Paranjape, S.; Mooberry, L.; Lacko, A.G. Evaluation of synthetic/reconstituted high-density lipoproteins as delivery vehicles for paclitaxel. Anti-Cancer Drugs 2008, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lacko, A.G.; Nair, M.; Paranjape, S.; Johnson, S.; McConathy, W.J. High density lipoprotein complexes as delivery vehicles for anticancer drugs. Anticancer Res. 2002, 22, 2045–2050. [Google Scholar] [PubMed]
- Buhtoiarov, I.N.; Sondel, P.M.; Wigginton, J.M.; Buhtoiarova, T.N.; Yanke, E.M.; Mahvi, D.A.; Rakhmilevich, A.L. Anti-tumour synergy of cytotoxic chemotherapy and anti-cd40 plus cpg-odn immunotherapy through repolarization of tumour-associated macrophages. Immunology 2011, 132, 226–239. [Google Scholar] [CrossRef]
- Lollo, G.; Vincent, M.; Ullio-Gamboa, G.; Lemaire, L.; Franconi, F.; Couez, D.; Benoit, J.-P. Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and cpg-odn in the treatment of glioblastoma. Int. J. Pharm. 2015, 495, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Weigel, B.J.; Rodeberg, D.A.; Krieg, A.M.; Blazar, B.R. Cpg oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin. Cancer Res. 2003, 9, 3105–3114. [Google Scholar]
- Zheng, Y.; Liu, Y.; Jin, H.; Pan, S.; Qian, Y.; Huang, C.; Zeng, Y.; Luo, Q.; Zeng, M.; Zhang, Z. Scavenger receptor b1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by hdl-mimetic nanoparticles. Theranostics 2013, 3, 477–486. [Google Scholar] [CrossRef]
- Shahzad, M.M.; Mangala, L.S.; Han, H.D.; Lu, C.; Bottsford-Miller, J.; Nishimura, M.; Mora, E.M.; Lee, J.-W.; Stone, R.L.; Pecot, C.V. Targeted delivery of small interfering rna using reconstituted high-density lipoprotein nanoparticles. Neoplasia 2011, 13, IN303–IN319. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, J.; Ding, L.; Jin, H.; Lovell, J.F.; Corbin, I.R.; Cao, W.; Lo, P.C.; Yang, M.; Tsao, M.S. Hdl-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small 2010, 6, 430–437. [Google Scholar] [CrossRef]
- Kuai, R.; Sun, X.; Yuan, W.; Ochyl, L.J.; Xu, Y.; Hassani Najafabadi, A.; Scheetz, L.; Yu, M.Z.; Balwani, I.; Schwendeman, A.; et al. Dual tlr agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J. Control. Release 2018, 282, 131–139. [Google Scholar] [CrossRef]
- Scheetz, L.; Park, K.S.; Li, Q.; Lowenstein, P.R.; Castro, M.G.; Schwendeman, A.; Moon, J.J. Engineering patient-specific cancer immunotherapies. Nat. Biomed. Eng. 2019, 3, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Sun, X.; Yuan, W.; Xu, Y.; Schwendeman, A.; Moon, J.J. Subcutaneous nanodisc vaccination with neoantigens for combination cancer immunotherapy. Bioconjug. Chem. 2018, 29, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Ochyl, L.J.; Bahjat, K.S.; Schwendeman, A.; Moon, J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017, 16, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Liao, X.-L.; Wu, M.-P.; Cheng, P.-F.; Yin, C.-Y.; Fei, Z. High-density lipoprotein as a potential carrier for delivery of a lipophilic antitumoral drug into hepatoma cells. World J. Gastroenterol. 2005, 11, 954–959. [Google Scholar] [CrossRef]
- Rink, J.S.; Plebanek, M.P.; Tripathy, S.; Thaxton, C.S. Update on current and potential nanoparticle cancer therapies. Curr. Opin. Oncol. 2013, 25, 646–651. [Google Scholar] [CrossRef] [Green Version]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-density lipoproteins: Nature’s multifunctional nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.K. Targeting endogenous apo A-I—A new approach for raising hdl. Nat. Rev. Cardiol. 2011, 8, 187. [Google Scholar] [CrossRef]
- Tardif, J.-C.; Grégoire, J.; L’Allier, P.L.; Ibrahim, R.; Lespérance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.-A.; et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosisa randomized controlled trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Cerenis Therapeutics, S. Cer-001 Therapy as a Novel Approach to Treat Genetic Orphan Diseases (TANGO). Available online: ClinicalTrials.gov/ct2/show/NCT02697136 (accessed on 11 February 2019).
- Li, D.; Gordon, S.; Schwendeman, A.; Remaley, A.T. Apolipoprotein mimetic peptides for stimulating cholesterol efflux. In Apolipoprotein Mimetics in the Management of Human Disease; Springer: Berlin/Heidelberg, Germany, 2015; pp. 29–42. [Google Scholar]
- Yang, S.; Damiano, M.G.; Zhang, H.; Tripathy, S.; Luthi, A.J.; Rink, J.S.; Ugolkov, A.V.; Singh, A.T.K.; Dave, S.S.; Gordon, L.I.; et al. Biomimetic, synthetic hdl nanostructures for lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 2511–2516. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Wen, J.; Tang, J.; Kan, Q.; Ackermann, R.; Olsen, K.; Schwendeman, A. Synthetic high-density lipoproteins for delivery of 10-hydroxycamptothecin. Int. J. Nanomed. 2016, 11, 6229. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, C.; Kuai, R.; Zhu, Q.; White, P.; Moon, J.J.; Schwendeman, A.; Cohen, M.S. Synthetic high-density lipoprotein nanoparticles: A novel therapeutic strategy for adrenocortical carcinomas. Surgery 2016, 159, 284–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuai, R.; Subramanian, C.; White, P.T.; Timmermann, B.N.; Moon, J.J.; Cohen, M.S.; Schwendeman, A. Synthetic high-density lipoprotein nanodisks for targeted withalongolide delivery to adrenocortical carcinoma. Int. J. Nanomed. 2017, 12, 6581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, E.E.; Li, X.-A.; Schwendeman, A. Hdl in endocrine carcinomas: Biomarker, drug carrier, and potential therapeutic. Front. Endocrinol. 2018, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, J.; Liu, J.; He, H.; Zhang, W.; Li, Z. Tumor targeting effects of a novel modified paclitaxel-loaded discoidal mimic high density lipoproteins. Drug Deliv. 2013, 20, 356–363. [Google Scholar] [CrossRef] [Green Version]
- Medina-Echeverz, J.; Vasquez, M.; Gomar, C.; Ardaiz, N.; Berraondo, P. Overexpression of apolipoprotein a-i fused to an anti-transforming growth factor beta peptide modulates the tumorigenicity and immunogenicity of mouse colon cancer cells. Cancer Immunol. Immunother. 2015, 64, 717–725. [Google Scholar] [CrossRef]
- Mooberry, L.K.; Nair, M.; Paranjape, S.; McConathy, W.J.; Lacko, A.G. Receptor mediated uptake of paclitaxel from a synthetic high density lipoprotein nanocarrier. J. Drug Target. 2010, 18, 53–58. [Google Scholar] [CrossRef]
- Leon, C.G.; Locke, J.A.; Adomat, H.H.; Etinger, S.L.; Twiddy, A.L.; Neumann, R.D.; Nelson, C.C.; Guns, E.S.; Wasan, K.M. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate 2010, 70, 390–400. [Google Scholar] [CrossRef]
- Tang, J.; Kuai, R.; Yuan, W.; Drake, L.; Moon, J.J.; Schwendeman, A. Effect of size and pegylation of liposomes and peptide-based synthetic lipoproteins on tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1869–1878. [Google Scholar] [CrossRef] [Green Version]
- Cancer, F.C. Facts and Stats about Colorectal Cancer. Available online: https://fightcolorectalcancer.org/colorectal-cancer/facts-stats/ (accessed on 12 November 2019).
- Duineveld, L.A.M.; van Asselt, K.M.; Bemelman, W.A.; Smits, A.B.; Tanis, P.J.; van Weert, H.C.P.M.; Wind, J. Symptomatic and asymptomatic colon cancer recurrence: A multicenter cohort study. Ann. Fam. Med. 2016, 14, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Krieg, A.M. Toll-like receptor 9 (tlr9) agonists in the treatment of cancer. Oncogene 2008, 27, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Kadiyala, P.; Li, D.; Nuñez, F.M.; Altshuler, D.; Doherty, R.; Kuai, R.; Yu, M.; Kamran, N.; Edwards, M.; Moon, J.J. High-density lipoprotein-mimicking nanodiscs for chemo-immunotherapy against glioblastoma multiforme. ACS Nano 2019, 13, 1365–1384. [Google Scholar] [CrossRef] [PubMed]
- Zamanian-Daryoush, M.; Lindner, D.J.; DiDonato, J.A.; Wagner, M.; Buffa, J.; Rayman, P.; Parks, J.S.; Westerterp, M.; Tall, A.R.; Hazen, S.L. Myeloid-specific genetic ablation of atp-binding cassette transporter abca1 is protective against cancer. Oncotarget 2017, 8, 71965–71980. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Jin, H.; Qiao, S.; Dai, Y.; Huang, C.; Lu, L.; Luo, Q.; Zhang, Z. Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials 2016, 98, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Kuai, R.; Yuan, W.; Son, S.; Nam, J.; Xu, Y.; Fan, Y.; Schwendeman, A.; Moon, J.J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018, 4, eaao1736. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Sun, R.; Wang, H.-X.; Shen, S.; Liu, Y.; Du, X.-J.; Zhu, Y.-H.; Jun, W. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J. Control. Release 2015, 205, 7–14. [Google Scholar] [CrossRef]
- Jin, H.; Pi, J.; Yang, F.; Wu, C.; Cheng, X.; Bai, H.; Huang, D.; Jiang, J.; Cai, J.; Chen, Z.W. Ursolic acid-loaded chitosan nanoparticles induce potent anti-angiogenesis in tumor. Appl. Microbiol. Biotechnol. 2016, 100, 6643–6652. [Google Scholar] [CrossRef]
- Camacho, K.M.; Menegatti, S.; Vogus, D.R.; Pusuluri, A.; Fuchs, Z.; Jarvis, M.; Zakrewsky, M.; Evans, M.A.; Chen, R.; Mitragotri, S. Dafodil: A novel liposome-encapsulated synergistic combination of doxorubicin and 5fu for low dose chemotherapy. J. Control. Release 2016, 229, 154–162. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheetz, L.M.; Yu, M.; Li, D.; Castro, M.G.; Moon, J.J.; Schwendeman, A. Synthetic HDL Nanoparticles Delivering Docetaxel and CpG for Chemoimmunotherapy of Colon Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1777. https://doi.org/10.3390/ijms21051777
Scheetz LM, Yu M, Li D, Castro MG, Moon JJ, Schwendeman A. Synthetic HDL Nanoparticles Delivering Docetaxel and CpG for Chemoimmunotherapy of Colon Adenocarcinoma. International Journal of Molecular Sciences. 2020; 21(5):1777. https://doi.org/10.3390/ijms21051777
Chicago/Turabian StyleScheetz, Lindsay M., Minzhi Yu, Dan Li, María G. Castro, James J. Moon, and Anna Schwendeman. 2020. "Synthetic HDL Nanoparticles Delivering Docetaxel and CpG for Chemoimmunotherapy of Colon Adenocarcinoma" International Journal of Molecular Sciences 21, no. 5: 1777. https://doi.org/10.3390/ijms21051777