Comparative Study of the Steroidogenic Effects of Human Chorionic Gonadotropin and Thieno[2,3-D]pyrimidine-Based Allosteric Agonist of Luteinizing Hormone Receptor in Young Adult, Aging and Diabetic Male Rats
Abstract
:1. Introduction
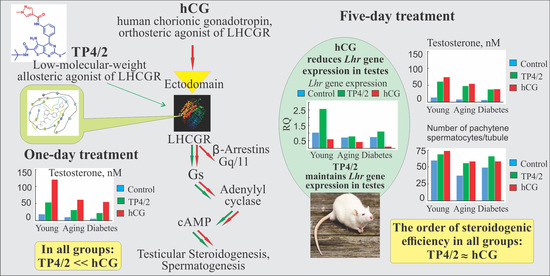
2. Results
2.1. The Metabolic and Hormonal Parameters in Diabetic and Aging Rats
2.2. The Effects of TP4/2 and hCG on AC Activity in Testicular Membranes of the Young Adult, Aging and Diabetic Rats
2.3. The Effect of a Single Administration of TP4/2 and hCG on the Blood Levels of Testosterone in the Young Adult, Aging and Diabetic Rats
2.4. The Effect of TP4/2 on the AC Activity in Thyroidal Membranes and the Thyroid Hormones Levels
2.5. The Effect of Five-Day Treatment of the Young Adult, Aging and Diabetic Rats with TP4/2 and hCG on the Blood Testosterone Level and the Intratesticular Content of Testosterone and Its Precursors
2.6. The Effect of Five-Day Treatment of Male Rats with TP4/2 and hCG on Intratesticular Expression of Genes Encoding LHCGR, StAR and Steroidogenic Enzymes
2.7. The Effect of TP4/2 and hCG Treatment on the Thickness of the Seminiferous Epithelium and the Number of Germ Cells within the Seminiferous Tubules
2.8. Rat LHCGR and TSHR Transmembrane Allosteric Site Modelling and Molecular Docking of Thienopyrimidine Derivatives
3. Discussion
4. Materials and Methods
4.1. Synthesis of 5-amino-N-tert-butyl-4-(3-(1-methylpyrazole-4-carboxamido)phenyl)-2-(methylthio)thieno[2,3-d]pyrimidine-6-carboxylic Acid Amide (TP4/2)
4.2. Animals
4.3. The Selection of the Doses and Delivery Routes for TP4/2 and hCG and the Estimation of Thyroid Hormone Levels in TP4/2-Treated Rats
4.4. The Estimation of Thyroid Hormone Levels in TP4/2-Treated Rats
4.5. Young Adult Rats and Their Treatment
4.6. Aging Rats
4.7. Diabetic Rats and Their Treatment
4.8. Chemicals and Radiochemicals
4.9. The Determination of the Blood Levels of Testosterone, Luteinizing Hormone, Insulin, Leptin, Glycated Hemoglobin (HbA1c) and Thyroid Hormones
4.10. The Determination of the Intratesticular Levels of Testosterone and Its Precursors
4.11. The Isolation of Testicular and Thyroidal Membranes
4.12. Adenylyl Cyclase Assay
4.13. The RNA Extraction, Reverse Transcription and Real-Time Quantitative PCR Analysis
4.14. Preparation of the Testis Sections
4.15. Histological Analysis of the Seminiferous Tubules
4.16. Modelling of the Rat LHCGR Structure and Thyrotropin-Stimulating Hormone (TSH) Receptor (TSHR)
4.17. Docking of Thienopyrimidine Derivatives and Parameters Selected for Analysis
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | adenylyl cyclase |
| hCG | human chorionic gonadotropin |
| LH | luteinizing hormone |
| LHCGR | LH/hCG receptor |
| LMW | low molecular weight |
| StAR | steroidogenic acute regulatory protein |
| T | testosterone |
| T1DM | type 1 diabetes mellitus |
| TP | thieno[2,3-d]pyrimidine |
| TRH | thyrotropin-releasing hormone |
| TSH | thyroid stimulating hormone |
| TSHR | thyroid stimulating hormone receptor |
References
- De Leo, V.; Musacchio, M.C.; Di Sabatino, A.; Tosti, C.; Morgante, G.; Petraglia, F. Present and future of recombinant gonadotropins in reproductive medicine. Curr. Pharm. Biotechnol. 2012, 13, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Prior, M.; Stewart, J.; McEleny, K.; Dwyer, A.A.; Quinton, R. Fertility induction in hypogonadotropic hypogonadal men. Clin. Endocrinol. 2018, 89, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, K.; Kałafut, J.; Rivero-Müller, A. The gonadotropin system, lessons from animal models and clinical cases. Minerva Ginecol. 2018, 70, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Krzastek, S.C.; Smith, R.P. Non-testosterone management of male hypogonadism: An examination of the existing literature. Transl. Androl. Urol. 2020, 9, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signaling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccetti, L.; de Pascali, F.; Gilioli, L.; Potì, F.; Giva, L.B.; Marino, M.; Tagliavini, S.; Trenti, T.; Fanelli, F.; Mezzullo, M.; et al. Human LH and hCG stimulate differently the early signalling pathways but result in equal testosterone synthesis in mouse Leydig cells in vitro. Reprod. Biol. Endocrinol. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccetti, L.; Yvinec, R.; Klett, D.; Gallay, N.; Combarnous, Y.; Reiter, E.; Simoni, M.; Casarini, L.; Ayoub, M.A. Human luteinizing hormone and chorionic gonadotropin display biased agonism at the LH and LH/CG receptors. Sci. Rep. 2017, 7, 940. [Google Scholar] [CrossRef]
- De Pascali, F.; Reiter, E. β-arrestins and biased signaling in gonadotropin receptors. Minerva Ginecol. 2018, 70, 525–538. [Google Scholar] [CrossRef]
- Shimizu-Albergine, M.; Tsai, L.C.; Patrucco, E.; Beavo, J.A. cAMP-specific phosphodiesterases 8A and 8B, essential regulators of Leydig cell steroidogenesis. Mol. Pharmacol. 2012, 81, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Shimizu-Albergine, M.; van Yserloo, B.; Golkowski, M.G.; Ong, S.E.; Beavo, J.A.; Bornfeldt, K.E. SCAP/SREBP pathway is required for the full steroidogenic response to cyclic AMP. Proc. Natl. Acad. Sci. USA 2016, 113, 5685–5693. [Google Scholar] [CrossRef] [Green Version]
- Eacker, S.M.; Agrawal, N.; Qian, K.; Dichek, H.L.; Gong, E.Y.; Lee, K.; Braun, R.E. Hormonal regulation of testicular steroid and cholesterol homeostasis. Mol. Endocrinol. 2008, 22, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyson, M.T.; Kowalewski, M.P.; Manna, P.R.; Stocco, D.M. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol. Cell Endocrinol. 2009, 300, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cailleux-Bounacer, A.; Reznik, Y.; Cauliez, B.; Menard, J.F.; Duparc, C.; Kuhn, J.M. Evaluation of endocrine testing of Leydig cell function using extractive and recombinant human chorionic gonadotropin and different doses of recombinant human LH in normal men. Eur. J. Endocrinol. 2008, 159, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhuis, J.D.; Liu, P.Y.; Takahashi, P.Y.; Keenan, D.M. Dynamic testosterone responses to near-physiological LH pulses are determined by the time pattern of prior intravenous LH infusion. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Latronico, A.C.; Arnhold, I.J.P. Gonadotropin resistance. Endocr. Dev. 2013, 24, 25–32. [Google Scholar] [CrossRef]
- Van Koppen, C.J.; Zaman, G.J.; Timmers, C.M.; Kelder, J.; Mosselman, S.; van de Lagemaat, R.; Smit, M.J.; Hanssen, R.G. A signaling-selective, nanomolar potent allosteric low molecular weight agonist for the human luteinizing hormone receptor. Naunyn Schmiedebergs Arch. Pharmacol. 2008, 378, 503–514. [Google Scholar] [CrossRef]
- Nataraja, S.G.; Yu, H.N.; Palmer, S.S. Discovery and development of small molecule allosteric modulators of glycoprotein hormone receptors. Front. Endocrinol. 2015, 6, 142. [Google Scholar] [CrossRef] [Green Version]
- Van Straten, N.C.; Schoonus-Gerritsma, G.G.; van Someren, R.G.; Draaijer, J.; Adang, A.E.; Timmers, C.M.; Hanssen, R.G.; van Boeckel, C.A. The first orally active low molecular weight agonists for the LH receptor: Thienopyr(im)idines with therapeutic potential for ovulation induction. ChemBioChem 2002, 3, 1023–1026. [Google Scholar] [CrossRef]
- Van de Lagemaat, R.; Raafs, B.C.; van Koppen, C.; Timmers, C.M.; Mulders, S.M.; Hanssen, R.G. Prevention of the onset of ovarian hyperstimulation syndrome (OHSS) in the rat after ovulation induction with a low molecular weight agonist of the LH receptor compared with hCG and rec-LH. Endocrinology 2011, 152, 4350–4357. [Google Scholar] [CrossRef]
- Gerrits, M.; Mannaerts, B.; Kramer, H.; Addo, S.; Hanssen, R. First evidence of ovulation induced by oral LH agonists in healthy female volunteers of reproductive age. J. Clin. Endocrinol. Metab. 2013, 98, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Shpakov, A.O.; Dar’in, D.V.; Derkach, K.V.; Lobanov, P.S. The stimulating influence of thienopyrimidine compounds on the adenylyl cyclase systems in the rat testes. Dokl. Biochem. Biophys. 2014, 456, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Derkach, K.V.; Bakhtyukov, A.A.; Shpakov, A.A.; Dar’in, D.V.; Shpakov, A.O. Specificity of heterotrimeric G protein regulation by human chorionic gonadotropin and low-molecular agonist of luteinizing hormone receptor. Cell Tissue Biol. 2017, 11, 475–482. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Dar’in, D.V.; Shpakov, A.O. Conservation of steroidogenic effect of the low-molecular-weight agonist of luteinizing hormone receptor in the course of its long-term administration to male rats. Dokl. Biochem. Biophys. 2019, 484, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Derkach, K.V.; Dar’in, D.V.; Bakhtyukov, A.A.; Lobanov, P.S.; Shpakov, A.O. In vitro and in vivo studies of functional activity of new low molecular weight agonists of the luteinizing hormone receptor. Biochem. Moscow Suppl. Ser. B 2016, 10, 294–300. [Google Scholar] [CrossRef]
- Van Dam, E.W.; Dekker, J.M.; Lentjes, E.G.; Romijn, F.P.; Smulders, Y.M.; Post, W.J.; Romijn, J.A.; Krans, H.M.J. Steroids in adult men with type 1 diabetes: A tendency to hypogonadism. Diabetes Care 2003, 26, 1812–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossmann, M.; Thomas, M.C.; Panagiotopoulos, S.; Sharpe, K.; MacIsaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1834–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, M.; Hampton, D.; Newcombe, R.G.; Rees, D.A. Total and free testosterone concentrations are strongly influenced by age and central obesity in men with type 1 and type 2 diabetes but correlate weakly with symptoms of androgen deficiency and diabetes-related quality of life. Clin. Endocrinol. 2012, 76, 665–673. [Google Scholar] [CrossRef]
- Schoeller, E.L.; Schon, S.; Moley, K.H. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res. 2012, 349, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Jangir, R.N.; Jain, G.C. Diabetes mellitus induced impairment of male reproductive functions: A review. Curr. Diabetes Rev. 2014, 10, 147–157. [Google Scholar] [CrossRef]
- Carruthers, M. Testosterone deficiency syndrome: Cellular and molecular mechanism of action. Curr. Aging Sci. 2013, 6, 115–124. [Google Scholar] [CrossRef]
- Hylmarova, S.; Stechova, K.; Pavlinkova, G.; Peknicova, J.; Macek, M.; Kvapil, M. The impact of type 1 diabetes mellitus on male sexual functions and sex hormone levels. Endocr. J. 2020, 67, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, J.M.; Lapauw, B.; Mahmoud, A.; T’Sjoen, G.; Huhtaniemi, I.T. Aging and the male reproductive system. Endocr. Rev. 2019, 40, 906–972. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.J.; Zhao, M.J.; Yang, Y.H.; Guan, D.; Li, Z.G.; Ji, Y.D.; Zhang, B.L.; Shang, X.J.; Xiong, C.L.; Gu, Y.Q. Age-related changes in serum reproductive hormone levels and prevalence of androgen deficiency in Chinese community-dwelling middle-aged and aging men: Two cross-sectional studies in the same population. Medicine 2020, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lin, S.; Lv, Q.; Yang, Q.; Wu, G.; Hu, J.; Yang, J. Taurine recovers testicular steroidogenesis and spermatogenesis in streptozotocin-induced diabetic rats. Adv. Exp. Med. Biol. 2017, 975, 801–811. [Google Scholar] [CrossRef]
- Bakhtyukov, A.A.; Derkach, K.V.; Dar’in, D.V.; Shpakov, A.O. Thienopyrimidine derivatives specifically activate testicular steroidogenesis but do not affect thyroid functions. J. Evol. Biochem. Physiol. 2019, 55, 30–39. [Google Scholar] [CrossRef]
- Newton, C.L.; Whay, A.M.; McArdle, C.A.; Zhang, M.; van Koppen, C.J.; van de Lagemaat, R.; Segaloff, D.L.; Millar, R.P. Rescue of expression and signaling of human luteinizing hormone G protein-coupled receptor mutants with an allosterically binding small-molecule agonist. Proc. Natl. Acad. Sci. USA 2011, 108, 7172–7176. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Wei, W.; Xie, F.; Zhu, X.; Zheng, L.; Lv, Z. Steroidogenesis decline accompanied with reduced antioxidation and endoplasmic reticulum stress in mice testes during ageing. Andrologia 2018, 50. [Google Scholar] [CrossRef]
- Ghosh, S.; Chowdhury, S.; Das, A.K.; Sil, P.C. Taurine ameliorates oxidative stress induced inflammation and ER stress mediated testicular damage in STZ-induced diabetic Wistar rats. Food Chem. Toxicol. 2019, 124, 64–80. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, N.; Chen, Q.; You, X.; Liu, C.; Wang, T.; Yuan, D.; Zhang, C. Decline in testicular function in ageing rats: Changes in the unfolded protein response and mitochondrial apoptotic pathway. Exp. Gerontol. 2019, 127. [Google Scholar] [CrossRef]
- Shi, W.; Guo, Z.; Ji, Y.; Feng, J. The protective effect of recombinant globular adiponectin on testis by modulating autophagy, endoplasmic reticulum stress and oxidative stress in streptozotocin-induced diabetic mice. Eur. J. Pharmacol. 2020, 879. [Google Scholar] [CrossRef] [PubMed]
- Derkach, K.V.; Bogush, I.V.; Berstein, L.M.; Shpakov, A.O. The influence of intranasal insulin on hypothalamic-pituitary-thyroid axis in normal and diabetic rats. Horm. Metab. Res. 2015, 47, 916–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Jernigan, R.L.; Covell, D.G. A role for surface hydrophobicity in protein-protein recognition. Protein Sci. 1994, 3, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Shpakova, E.A.; Derkach, K.V.; Shpakov, A.O. Biological activity of lipophilic derivatives of peptide 562–572 of rat luteinizing hormone receptor. Dokl. Biochem. Biophys. 2013, 452, 248–250. [Google Scholar] [CrossRef]
- Troppmann, B.; Kleinau, G.; Krause, G.; Gromoll, J. Structural and functional plasticity of the luteinizing hormone/choriogonadotrophin receptor. Hum. Reprod. Update 2013, 19, 583–602. [Google Scholar] [CrossRef] [Green Version]
- Chantreau, V.; Taddese, B.; Munier, M.; Gourdin, L.; Henrion, D.; Rodien, P.; Chabbert, M. Molecular insights into the transmembrane domain of the thyrotropin receptor. PLoS ONE 2015, 10, e0142250. [Google Scholar] [CrossRef] [Green Version]
- Kleinau, G.; Worth, C.L.; Kreuchwig, A.; Biebermann, H.; Marcinkowski, P.; Scheerer, P.; Krause, G. Structural-Functional features of the thyrotropin receptor: A class a g-protein-coupled receptor at work. Front. Endocrinol. 2017, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Jorand-Lebrun, C.; Brondyk, B.; Lin, J.; Magar, S.; Murray, R.; Reddy, A.; Schroff, H.; Wands, G.; Weiser, W.; Xu, Q.; et al. Identification, synthesis, and biological evaluation of novel pyrazoles as low molecular weight luteinizing hormone receptor agonists. Bioorg. Med. Chem. Lett. 2007, 17, 2080–2085. [Google Scholar] [CrossRef]
- Heitman, L.H.; Ijzerman, A.P. G protein-coupled receptors of the hypothalamic-pituitary-gonadal axis: A case for Gnrh, LH, FSH, and GPR54 receptor ligands. Med. Res. Rev. 2008, 28, 975–1011. [Google Scholar] [CrossRef]
- Heitman, L.H.; Narlawar, R.; de Vries, H.; Willemsen, M.N.; Wolfram, D.; Brussee, J.; Ijzerman, A.P. Substituted terphenyl compounds as the first class of low molecular weight allosteric inhibitors of the luteinizing hormone receptor. J. Med. Chem. 2009, 52, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Jaeschke, H.; Kleinau, G.; Neumann, S.; Costanzi, S.; Jiang, J.K.; Childress, J.; Raaka, B.M.; Colson, A.; Paschke, R.; et al. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: Structure-activity relationships and selective binding patterns. J. Med. Chem. 2006, 49, 3888–3896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, S.; Gershengorn, M.C. Small molecule TSHR agonists and antagonists. Ann. Endocrinol. 2011, 72, 74–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, S.; Kleinau, G.; Costanzi, S.; Moore, S.; Jiang, J.K.; Raaka, B.M.; Thomas, C.J.; Krause, G.; Gershengorn, M.C. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology 2008, 149, 5945–5950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyer, I.; Haas, A.K.; Kreuchwig, A.; Schülein, R.; Krause, G. Molecular sampling of the allosteric binding pocket of the TSH receptor provides discriminative pharmacophores for antagonist and agonists. Biochem. Soc. Trans. 2013, 41, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latif, R.; Realubit, R.B.; Karan, C.; Mezei, M.; Davies, T.F. TSH receptor signaling abrogation by a novel small molecule. Front. Endocrinol. 2016, 7, 130. [Google Scholar] [CrossRef]
- Conn, P.J.; Christopoulos, A.; Lindsley, C.W. Allosteric Modulators of GPCRs as a Novel Approach to Treatment of CNS Disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Lindsley, C.W.; Emmitte, K.A.; Hopkins, C.R.; Bridges, T.M.; Gregory, K.J.; Niswender, C.M.; Conn, P.J. Practical strategies and concepts in GPCR allosteric modulator discovery: Recent advances with metabotropic glutamate receptors. Chem. Rev. 2016, 116, 6707–6741. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.J.; Conn, P.J. Allosteric modulation of GPCRs: New insights and potential utility for treatment of schizophrenia and other CNS disorders. Neuron 2017, 94, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.R.; Dyson, M.T.; Stocco, D.M. Regulation of the steroidogenic acute regulatory protein gene expression: Present and future perspectives. Mol. Hum. Reprod. 2009, 15, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, J.; Higashi, Y.; LaNasa, J.A.; Yoshida, K.; Winters, S.J.; Oshima, H.; Troen, P. Studies of the human testis. XVIII. Simultaneous measurement of nine intratesticular steroids: Evidence for reduced mitochondrial function in testis of elderly men. J. Clin. Endocrinol. Metab. 1983, 56, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Meadowcraft, L.M.; Williamson, B. Prevalence, pathophysiology, and management of androgen deficiency in men with metabolic syndrome, type 2 diabetes mellitus, or both. Pharmacotherapy 2015, 35, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chen, H.; Zirkin, B.R. Temporal relationship among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzymes (P450scc) during Leydig cells aging. J. Androl. 2005, 26, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sokanovic, S.J.; Janjic, M.M.; Stojkov, N.J.; Baburski, A.Z.; Bjelic, M.M.; Andric, S.A.; Kostic, T.S. Age related changes of cAMP and MAPK signaling in Leydig cells of Wistar rats. Exp. Gerontol. 2014, 58, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Qi, Y.W.; Hu, C.Y.; Chen, S.H.; Liu, Y. Advanced glycation end products inhibit testosterone secretion by rat Leydig cells by inducing oxidative stress and endoplasmic reticulum stress. Int. J. Mol. Med. 2016, 38, 659–665. [Google Scholar] [CrossRef]
- Dufau, M.L.; Catt, K.J. Gonadotropin receptors and regulation of steroidogenesis in the testis and ovary. Vitam. Horm. 1978, 36, 461–592. [Google Scholar] [CrossRef]
- Dufau, M.L.; Winters, C.A.; Hattori, M.; Aquilano, D.; Barañao, J.L.; Nozu, K.; Baukal, A.; Catt, K.J. Hormonal regulation of androgen production by the Leydig cell. J. Steroid Biochem. 1984, 20, 161–173. [Google Scholar] [CrossRef]
- Pakarinen, P.; Vihko, K.K.; Voutilainen, R.; Huhtaniemi, I. Differential response of luteinizing hormone receptor and steroidogenic enzyme gene expression to human chorionic gonadotropin stimulation in the neonatal and adult rat testis. Endocrinology 1990, 127, 2469–2474. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakaya, S.; Kumai, T.; Watanabe, M.; Matsumoto, N.; Kobayashi, S. Impaired testicular function in rats with diet-induced hypercholesterolemia and/or streptozotocin-induced diabetes mellitus. Endocr. Res. 2001, 27, 109–117. [Google Scholar] [CrossRef]
- Geisthövel, F.; Brabant, G.; Wickings, E.J.; Nieschlag, E. Changes in testicular hCG binding and Leydig cell function in rats throughout life. Horm. Res. 1981, 14, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.W.; Fiore, C.; Zirkin, B.R. The effect of aging on the seminiferous epithelium of the brown Norway rat. J. Androl. 1993, 14, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Serre, V.; Hermo, L.; Robaire, B. The effects of aging on the seminiferous epithelium and the blood-testis barrier of the Brown Norway rat. J. Androl. 1999, 20, 356–365. [Google Scholar] [CrossRef]
- Glenn, D.R.; McClure, N.; Lewis, S.E. The hidden impact of diabetes on male sexual dysfunction and fertility. Hum Fertil 2003, 6, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Hekim, G.N.; Arslan, M.A.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, G.J.; Li, Z.M.; Zheng, J.; Chen, J.; Han, X.X.; Wu, J.; Li, G.Y.; Chang, Q.; Li, Y.X.; Yu, J.Q. Diabetes associated with male reproductive system damages: Onset of presentation, pathophysiological mechanisms and drug intervention. Biomed. Pharmacother. 2017, 90, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Simas, J.N.; Mendes, T.B.; Paccola, C.C.; Vendramini, V.; Miraglia, S.M. Resveratrol attenuates reproductive alterations in type 1 diabetes-induced rats. Int. J. Exp. Pathol. 2017, 98, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Miranda, E.P.; Lorenzini, F.; Neves, B.; Melchioretto, E.F.; Hota, T.; Fraga, R. Stereological and morphological analysis of the effects of aging on spermatogenesis in rat testis. Acta Cir. Bras. 2018, 33, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, A.; Nakamura, M.; Namiki, M.; Aono, T.; Matsumoto, K.; Utsunomiya, M.; Yoshioka, T.; Itoh, H.; Itatani, H.; Mizutani, S. Testicular responsiveness to long-term administration of hCG and hMG in patients with hypogonadotrophic hypogonadism. Horm. Res. 1986, 23, 21–30. [Google Scholar] [CrossRef]
- Kim, E.D.; Crosnoe, L.; Bar-Chama, N.; Khera, M.; Lipshultz, L.I. The treatment of hypogonadism in men of reproductive age. Fertil. Steril. 2013, 99, 718–724. [Google Scholar] [CrossRef]
- Moss, J.L.; Crosnoe, L.E.; Kim, E.D. Effect of rejuvenation hormones on spermatogenesis. Fertil. Steril. 2013, 99, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Pitteloud, N.; Dwyer, A. Hormonal control of spermatogenesis in men: Therapeutic aspects in hypogonadotropic hypogonadism. Ann. Endocrinol. 2014, 75, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Rohayem, J.; Sinthofen, N.; Nieschlag, E.; Kliesch, S.; Zitzmann, M. Causes of hypogonadotropic hypogonadism predict response to gonadotropin substitution in adults. Andrology 2016, 4, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.F.; Liu, Z.X.; Nie, M.; Wang, X.; Xu, H.L.; Huang, B.K.; Zheng, J.J.; Min, L.; Kaiser, U.B.; Wu, X.Y. Pulsatile gonadotropin-releasing hormone therapy is associated with earlier spermatogenesis compared to combined gonadotropin therapy in patients with congenital hypogonadotropic hypogonadism. Asian J. Androl. 2017, 19, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, R.G.J.M.; Timmers, C.M. Thieno[2,3-d]pyrimidines with Combined LH and FSH Agonistic Activity. Patent WO2003020726, 13 March 2003. [Google Scholar]
- Holzer, W.; Seiringer, G. N-1 Substituted ethyl 4-pyrazolecarboxylates: Synthesis and spectroscopic investigations. J. Heterocycl. Chem. 1993, 30, 865–872. [Google Scholar] [CrossRef]
- Derkach, K.V.; Bakhtyukov, A.A.; Romanova, I.V.; Zorina, I.I.; Bayunova, L.V.; Bondareva, V.M.; Morina, I.Y.; Roy, V.K.; Shpakov, A.O. The effect of metformin treatment on the basal and gonadotropin-stimulated steroidogenesis in male rats with type 2 diabetes mellitus. Andrologia 2020, e13816. [Google Scholar] [CrossRef]
- Heyma, P.; Harrison, L.C. Precipitation of the thyrotropin receptor and identification of thyroid autoantigens using Graves’ disease immunoglobulins. J. Clin. Investig. 1984, 74, 1090–1097. [Google Scholar] [CrossRef] [Green Version]
- Shpakov, A.O.; Derkach, K.V.; Bondareva, V.M. A decrease in the sensitivity of adenylyl cyclase and heterotrimeric G-proteins to chorionic gonadotrophin and peptide hormones action in the tissues of reproductive system of rats with experimental type 2 diabetes. Biochem. Moscow Suppl. Ser. B 2010, 4, 258–263. [Google Scholar] [CrossRef]
- Salomon, Y.; Londos, C.; Rodbell, M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974, 58, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Derkach, K.V.; Bondareva, V.M.; Chistyakova, O.V.; Berstein, L.M.; Shpakov, A.O. The effect of long-term intranasal serotonin treatment on metabolic parameters and hormonal signaling in rats with high-fat diet/low-dose streptozotocin-induced type 2 diabetes. Int. J. Endocrinol. 2015, 2015, 245459. [Google Scholar] [CrossRef] [Green Version]
- Derkach, K.; Zakharova, I.; Zorina, I.; Bakhtyukov, A.; Romanova, I.; Bayunova, L.; Shpakov, A. The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLoS ONE 2019, 14, e0213779. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, M.; Shoorei, H.; Soltani, M.; Abtahi-Eivari, S.H.; Salimnejad, R.; Moghimian, M. Protective effects of the hydroalcoholic extract of Fumaria parviflora on testicular injury induced by torsion/detorsion in adult rats. Andrologia 2018, 50, e13047. [Google Scholar] [CrossRef] [PubMed]
- Shoorei, H.; Khaki, A.; Khaki, A.A.; Hemmati, A.A.; Moghimian, M.; Shokoohi, M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed. Pharmacother. 2019, 111, 568–578. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Pandy-Szekeres, G.; Munk, C.; Tsonkov, T.M.; Mordalski, S.; Harpsoe, K.; Hauser, A.S.; Bojarski, A.J.; Gloriam, D.E. GPCRdb in 2018: Adding GPCR structure models and ligands. Nucleic Acids Res. 2018, 46, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins 2004, 55, 351–367. [Google Scholar] [CrossRef] [Green Version]
- Bowers, K.; Chow, E.; Xu, H.; Dror, R.; Eastwood, M.; Gregersen, B.; Klepeis, J.; Kolossváry, I.; Moraes, M.; Sacerdoti, F.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Heitman, L.H.; Kleinau, G.; Brussee, J.; Krause, G.; Ijzerman, A.P. Determination of different putative allosteric binding pockets at the lutropin receptor by using diverse drug-like low molecular weight ligands. Mol. Cell. Endocrinol. 2012, 351, 326–336. [Google Scholar] [CrossRef]
- Marcinkowski, P.; Hoyer, I.; Specker, E.; Furkert, J.; Rutz, C.; Neuenschwander, M.; Sobottka, S.; Sun, H.; Nazare, M.; Berchner-Pfannschmidt, U.; et al. A new highly thyrotropin receptor-selective small-molecule antagonist with potential for the treatment of Graves’ orbitopathy. Thyroid 2019, 29, 111–123. [Google Scholar] [CrossRef]
- Fournier, T. Human chorionic gonadotropin: Different glycoforms and biological activity depending on its source of production. Ann. Endocrinol. 2016, 77, 75–81. [Google Scholar] [CrossRef] [PubMed]










| Groups | Body Weight, g | Testes Weight, g | GSI, arb. Units | Glucose, mmol/L @ | HbA1c, % | Insulin, ng/mL # | Leptin, ng/mL # | LH, pg/mL # |
|---|---|---|---|---|---|---|---|---|
| Young+dmso | 315 ± 8 | 3.84 ± 0.15 | 1.22 ± 0.03 | 5.2 ± 0.2 | 4.10 ± 0.11 | 0.70 ± 0.16 | 2.45 ± 0.35 | 1.97 ± 0.27 |
| Young+TP | 320 ± 5 | 4.02 ± 0.11 | 1.26 ± 0.02 | 5.3 ± 0.2 | 4.20 ± 0.13 | 0.76 ± 0.14 | 2.34 ± 0.40 | 1.30 ± 0.17 |
| Young+saline | 329 ± 7 | 3.90 ± 0.05 | 1.19 ± 0.02 | 5.4 ± 0.1 | 4.24 ± 0.12 | 0.80 ± 0.14 | 2.60 ± 0.32 | 1.94 ± 0.24 |
| Young+hCG | 322 ± 8 | 4.34 ± 0.14 a | 1.35 ± 0.03 a | 5.5 ± 0.2 | 4.12 ± 0.09 | 0.98 ± 0.15 | 2.64 ± 0.25 | 0.77 ± 0.11 a |
| Aging+dmso | 451 ± 14 | 4.14 ± 0.19 | 0.92 ± 0.03 | 6.4 ± 0.4 | 4.78 ± 0.29 | 1.07 ± 0.15 | 3.23 ± 0.33 | 2.11 ± 0.27 |
| Aging+TP | 454 ± 9 a | 4.36 ± 0.13 | 0.96 ± 0.02 a | 6.1 ± 0.3 | 4.48 ± 0.25 | 0.99 ± 0.16 | 3.13 ± 0.42 | 1.76 ± 0.23 |
| Aging+saline | 458 ± 13 a | 4.16 ± 0.10 | 0.91 ± 0.05 a | 6.2 ± 0.3 | 4.68 ± 0.22 | 1.14 ± 0.20 | 3.38 ± 0.41 | 2.37 ± 0.32 |
| Aging+hGC | 450 ± 6 a | 4.80 ± 0.18 a,b | 1.07 ± 0.04 a | 6.4 ± 0.3 | 4.46 ± 0.29 | 1.08 ± 0.17 | 2.92 ± 0.26 | 0.83 ± 0.09 a,b |
| Diab+dmso | 284 ± 6 | 3.26 ± 0.07 | 1.15 ± 0.01 | 22.3 ± 2.1 | 7.36 ± 0.59 | 0.14 ± 0.04 | 1.39 ± 0.22 | 1.16 ± 0.19 |
| Diab+TP | 283 ± 7 a | 3.44 ± 0.07 a | 1.22 ± 0.02 | 20.4 ± 1.8 a | 6.66 ± 0.20 a | 0.20 ± 0.05 a | 1.55 ± 0.21 a | 1.01 ± 0.20 a |
| Diab+saline | 292 ± 8 | 3.30 ± 0.10 a | 1.13 ± 0.04 | 21.8 ± 1.6 a | 7.10 ± 0.42 a | 0.17 ± 0.06 a | 1.49 ± 0.22 a | 1.22 ± 0.22 |
| Diab+hCG | 290 ± 7 a | 3.66 ± 0.11 | 1.26 ± 0.01 b | 19.1 ± 1.9 a | 6.44 ± 0.39 a | 0.16 ± 0.04 a | 1.32 ± 0.28 a | 0.72 ± 0.14 a |
| Group | Progesterone, nmol/g of Tissue | 17-OH-Progesterone, nmol/g of Tissue | Androstenedione, pmol/g of Tissue | Testosterone, nmol/g of Tissue |
|---|---|---|---|---|
| Young+dmso | 0.536 ± 0.036 | 0.063 ± 0.004 | 53.8 ± 4.2 | 0.995 ± 0.105 |
| Young+TP | 0.534 ± 0.088 | 0.173 ± 0.012 | 148.6 ± 10.1 a | 2.174 ± 0.244 a |
| Young+saline | 0.569 ± 0.041 | 0.066 ± 0.005 | 55.6 ± 6.7 | 0.910 ± 0.086 |
| Young+hCG | 0.545 ± 0.066 | 0.395 ± 0.055 a,c | 214.3 ± 11.0 a,c | 2.810 ± 0.132 a |
| Aging+dmso | 0.728 ± 0.064 a | 0.025 ± 0.005 a | 32.1 ± 5.6 | 0.255 ± 0.044 a |
| Aging+TP | 0.856 ± 0.043 a | 0.075 ± 0.005 | 129.1 ± 6.7 a,b | 1.441 ± 0.078 a,b |
| Aging+saline | 0.699 ± 0.034 | 0.019 ± 0.003 a | 30.4 ± 3.0 | 0.237 ± 0.043 a |
| Aging+hGC | 1.558 ± 0.164 a,b,c | 0.271 ± 0.022 a,b,c | 233.9 ± 16.5 a,b,c | 1.869 ± 0.123 a,b,c |
| Diab+dmso | 0.377 ± 0.030 | 0.049 ± 0.006 | 32.7± 5.1 | 0.287 ± 0.032 a |
| Diab+TP | 0.521 ± 0.048 | 0.083 ± 0.006a | 100.3 ± 16.4 a,b | 1.308 ± 0.097 a,b |
| Diab+saline | 0.387 ± 0.026 | 0.057 ± 0.008 | 35.1± 5.6 | 0.293 ± 0.044 a |
| Diab+hGC | 0.480 ± 0.087 | 0.142 ± 0.011 a,b,c | 141.5 ± 10.0 a,b | 1.356 ± 0.117 a,b |
| Group | Thickness of the Seminiferous Epithelium, µm | Number of Spermatogonia/Seminiferous Tubule, Units | Number of Pachytenic Spermatocytes/Seminiferous Tubule, Units |
|---|---|---|---|
| Young+dmso | 82.64 ± 0.94 | 59.18 ± 0.77 | 59.4 ± 1.48 |
| Young+TP | 81.91 ± 0.68 | 60.60 ± 0.96 | 68.54 ± 1.20 a |
| Young+saline | 83.21 ± 0.63 | 58.78 ± 0.85 | 60.32 ± 1.33 |
| Young+hCG | 81.15 ± 0.87 | 64.00 ± 1.02 a | 73.60 ± 1.34 a |
| Aging+dmso | 66.95 ± 0.88 a | 38.40 ± 1.43 a | 37.22 ± 0.86 a |
| Aging+TP | 76.12 ± 0.88 a,b | 60.80 ± 1.60 b | 55.68 ± 1.60 b |
| Aging+saline | 67.60 ± 1.07 a | 39.28 ± 1.20 a | 38.44 ± 1.06 a |
| Aging+hGC | 72.08 ± 0.93 a,b,c | 61.10 ± 1.09 b | 57.90 ± 1.27 b |
| Diab+dmso | 68.52 ± 0.71 a | 49.28 ± 1.24 a | 49.06 ± 0.80 a |
| Diab+TP | 75.39 ± 0.95 a,b | 64.36 ± 0.72 a,b | 65.84 ± 0.76 a,b |
| Diab+saline | 68.30 ± 0.84 a | 48.80 ± 0.86 a | 48.46 ± 0.98 a |
| Diab+hGC | 73.45 ± 0.95 a,b | 59.98 ± 0.59 b,c | 57.92 ± 1.04 b,c |
| Compound | GlideScore | Emodel | Lipo | Coulomb | H-Bond |
|---|---|---|---|---|---|
| Rat LHCGR | |||||
| Org 43553 | −5.53 | −51.36 | −3.23 | −4.87 | −0.28 |
| TP4/2 | −5.07 | −52.84 | −2.47 | −1.12 | 0.00 |
| Rat TSHR | |||||
| Org 43553 | −4.06 | −57.83 | −1.33 | −6.44 | 0.00 |
| TP4/2 | −3.82 | −38.71 | −0.84 | −6.66 | −0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhtyukov, A.A.; Derkach, K.V.; Gureev, M.A.; Dar’in, D.V.; Sorokoumov, V.N.; Romanova, I.V.; Morina, I.Y.; Stepochkina, A.M.; Shpakov, A.O. Comparative Study of the Steroidogenic Effects of Human Chorionic Gonadotropin and Thieno[2,3-D]pyrimidine-Based Allosteric Agonist of Luteinizing Hormone Receptor in Young Adult, Aging and Diabetic Male Rats. Int. J. Mol. Sci. 2020, 21, 7493. https://doi.org/10.3390/ijms21207493
Bakhtyukov AA, Derkach KV, Gureev MA, Dar’in DV, Sorokoumov VN, Romanova IV, Morina IY, Stepochkina AM, Shpakov AO. Comparative Study of the Steroidogenic Effects of Human Chorionic Gonadotropin and Thieno[2,3-D]pyrimidine-Based Allosteric Agonist of Luteinizing Hormone Receptor in Young Adult, Aging and Diabetic Male Rats. International Journal of Molecular Sciences. 2020; 21(20):7493. https://doi.org/10.3390/ijms21207493
Chicago/Turabian StyleBakhtyukov, Andrey A., Kira V. Derkach, Maxim A. Gureev, Dmitry V. Dar’in, Viktor N. Sorokoumov, Irina V. Romanova, Irina Yu. Morina, Anna M. Stepochkina, and Alexander O. Shpakov. 2020. "Comparative Study of the Steroidogenic Effects of Human Chorionic Gonadotropin and Thieno[2,3-D]pyrimidine-Based Allosteric Agonist of Luteinizing Hormone Receptor in Young Adult, Aging and Diabetic Male Rats" International Journal of Molecular Sciences 21, no. 20: 7493. https://doi.org/10.3390/ijms21207493