The Rab Geranylgeranyl Transferase Beta Subunit Is Essential for Embryo and Seed Development in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
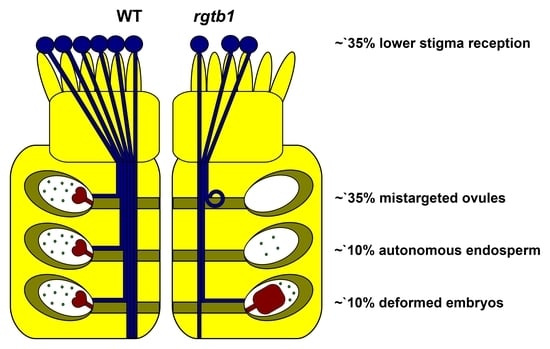
2.1. rgtb1 Mutants Form Fewer Seeds than WT Plants
2.2. Pre-Fertilization Events May Account for Failure of Seed Formation in rgtb1 Mutants
2.3. Autonomous Endosperm Formation Is More Frequent in rgtb1 Than in WT Plants
2.4. Large Malformed Embryos Form in Seeds of rgtb1 Plant
2.5. Deformations in Embryo Shape in rgtb1 × WT Crosses Correlate with Lower and Diffused Auxin Maxima at the Cotyledon Tips
2.6. PIN1 Efflux Protein Is Mislocalized in the Deformed rgtb1 × WT Embryos
2.7. Seed Coat Morphology in rgtb1 Mutants Is Defective
2.8. Expression of the RGT-Encoding Genes Change during Embryo Development
3. Discussion
3.1. Mislocalized and Weak Auxin Maxima Inhibit the Development of Multiple Reproductive Structures in rgtb1 Mutants
3.2. Increased Auxin Levels but Not Auxin-Dependent Transcription in the Central Cell Triggers Autonomous Endosperm Formation in the rgtb1 Mutant
3.3. Deficiency in Vesicular Transport in the Sporophyte Affects Embryo Size and Shape in the rgtb1 Mutant
3.4. The Imbalance of Proteins Building the RGT Complex May Influence Rab Functions in the Embryo Development
3.5. Both Pre- and Post-Fertilization Events Limit the Seed Set in rgtb1 Mutants
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Scanning Electron Microscopy
4.3. Aniline Blue Staining
4.4. Ruthenium Red Staining
4.5. Alexander Staining of Pollinated Stigma
4.6. Semi-Thin Sections Preparation
4.7. Sample Clearing
4.8. Fluorescence Analysis of Ovules
4.9. Statistical Analysis
4.10. Transcriptomic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faure, J.-E.; Rotman, N.; Fortuné, P.; Dumas, C. Fertilization in Arabidopsis thaliana wild type: Developmental stages and time course. Plant J. 2002, 30, 481–488. [Google Scholar] [CrossRef]
- Sørensen, M.B.; Mayer, U.; Lukowitz, W.; Robert, H.; Chambrier, P.; Jürgens, G.; Somerville, C.; Lepiniec, L.; Berger, F. Cellularisation in the endosperm of Arabidopsis thaliana is coupled to mitosis and shares multiple components with cytokinesis. Development 2002, 129, 5567–5576. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A. Controlling Apomixis: Shared Features and Distinct Characteristics of Gene Regulation. Genes 2020, 11, 329. [Google Scholar] [CrossRef] [Green Version]
- Hand, M.L.; Koltunow, A.M. The genetic control of apomixis: Asexual seed formation. Genetics 2014, 197, 441–450. [Google Scholar] [CrossRef] [Green Version]
- De Arias, M.M.; Gao, L.; Sherwood, D.A.; Dwivedi, K.K.; Price, B.J.; Jamison, M.; Kowallis, B.M.; Carman, J.G. Whether Gametophytes are Reduced or Unreduced in Angiosperms Might Be Determined Metabolically. Genes 2020, 11, 1449. [Google Scholar] [CrossRef]
- Hands, P.; Rabiger, D.S.; Koltunow, A. Mechanisms of endosperm initiation. Plant Reprod. 2016, 29, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Rojek, J.; Pawełko, Ł.; Kapusta, M.; Naczk, A.; Bohdanowicz, J. Exogenous steroid hormones stimulate full development of autonomous endosperm in Arabidopsis thaliana. Acta Soc. Bot. Pol. 2015, 84, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Larsson, E.; Vivian-Smith, A.; Offringa, R.; Sundberg, E. Auxin Homeostasis in Arabidopsis Ovules Is Anther-Dependent at Maturation and Changes Dynamically upon Fertilization. Front. Plant Sci. 2017, 8, 1735. [Google Scholar] [CrossRef]
- Lora, J.; Laux, T.; Hormaza, I. The role of the integuments in pollen tube guidance in flowering plants. New Phytol. 2019, 221, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Lopes, A.L.; Coimbra, S. Arabinogalactan Proteins as Interactors along the Crosstalk between the Pollen Tube and the Female Tissues. Front. Plant Sci. 2016, 7, 1895. [Google Scholar] [CrossRef] [PubMed]
- Golz, J.F.; Allen, P.J.; Li, S.F.; Parish, R.W.; Jayawardana, N.U.; Bacic, A.; Doblin, M.S. Layers of regulation—Insights into the role of transcription factors controlling mucilage production in the Arabidopsis seed coat. Plant Sci. 2018, 272, 179–192. [Google Scholar] [CrossRef]
- Ezquer, I.; Mizzotti, C.; Nguema-Ona, E.; Gotté, M.; Beauzamy, L.; Viana, V.E.; Dubrulle, N.; De Oliveira, A.C.; Caporali, E.; Koroney, A.-S.; et al. The Developmental Regulator SEEDSTICK Controls Structural and Mechanical Properties of the Arabidopsis Seed Coat. Plant Cell 2016, 28, 2478–2492. [Google Scholar] [CrossRef] [Green Version]
- Shirley, N.; Aubert, M.K.; Wilkinson, L.; Bird, D.C.; Lora, J.; Yang, X.; Tucker, M. Translating auxin responses into ovules, seeds and yield: Insight from Arabidopsis and the cereals. J. Integr. Plant Biol. 2019, 61, 310–336. [Google Scholar] [CrossRef] [Green Version]
- Larsson, E.; Roberts, C.J.; Claes, A.R.; Franks, R.G.; Sundberg, E. Polar Auxin Transport Is Essential for Medial versus Lateral Tissue Specification and Vascular-Mediated Valve Outgrowth in Arabidopsis Gynoecia. Plant Physiol. 2014, 166, 1998–2012. [Google Scholar] [CrossRef] [Green Version]
- Rojek, J.; Tucker, M.R.; Pinto, S.C.; Rychłowski, M.; Lichocka, M.; Soukupova, H.; Nowakowska, J.; Bohdanowicz, J.; Surmacz, G.; Gutkowska, M. Rab-dependent vesicular traffic affects female gametophyte development in Arabidopsis. J. Exp. Bot. 2021, 72, 320–340. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Xiao, Q.; Zhu, J.; Cheung, A.Y.; Yuan, L.; Vierling, E.; Xu, S. Auxin efflux controls orderly nucellar degeneration and expansion of the female gametophyte in Arabidopsis. New Phytol. 2021, 230, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, L.; Masiero, S.; Roy, D.S.; Bencivenga, S.; Roig-Villanova, I.; Ditengou, F.A.; Palme, K.; Simon, R.; Colombo, L. Maternal Control of PIN1 Is Required for Female Gametophyte Development in Arabidopsis. PLoS ONE 2013, 8, e66148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Tian, L.; Yang, J.; Zhao, Y.-N.; Zhu, Y.-X.; Dai, X.; Zhao, Y.; Yang, Z.-N. Auxin production in diploid microsporocytes is necessary and sufficient for early stages of pollen development. PLoS Genet. 2018, 14, e1007397. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, V.; Celebrin, D.; Napoli, N.; Ghelli, R.; Brunetti, P.; Costantino, P.; Cardarelli, M. An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol. 2017, 213, 1194–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchetti, V.; Altamura, M.M.; Falasca, G.; Costantino, P.; Cardarelli, M. Auxin Regulates Arabidopsis Anther Dehiscence, Pollen Maturation, and Filament Elongation. Plant Cell 2008, 20, 1760–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, R.A.; Figueiredo, D.D.; Santos-González, J.; Köhler, C. Auxin regulates endosperm cellularization in Arabidopsis. Genes Dev. 2019, 33, 466–476. [Google Scholar] [CrossRef] [Green Version]
- Matilla, A.J. Auxin: Hormonal Signal Required for Seed Development and Dormancy. Plants 2020, 9, 705. [Google Scholar] [CrossRef]
- Robert, H.S. Molecular Communication for Coordinated Seed and Fruit Development: What Can We Learn from Auxin and Sugars? Int. J. Mol. Sci. 2019, 20, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, D.D.; Batista, R.A.; Roszak, P.J.; Hennig, L.; Köhler, C. Auxin production in the endosperm drives seed coat development in Arabidopsis. eLife 2016, 5, e20542. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.D.; Kohler, C. Bridging the generation gap: Communication between maternal sporophyte, female gametophyte and fertilization products. Curr. Opin. Plant Biol. 2016, 29, 16–20. [Google Scholar] [CrossRef]
- Figueiredo, D.D.; Batista, R.A.; Roszak, P.; Köhler, C. Auxin production couples endosperm development to fertilization. Nat. Plants 2015, 1, 15184. [Google Scholar] [CrossRef]
- Panoli, A.; Martín, M.V.; Alandete-Saez, M.; Simon, M.; Neff, C.; Swarup, R.; Bellido, A.; Yuan, L.; Pagnussat, G.C.; Sundaresan, V. Auxin Import and Local Auxin Biosynthesis Are Required for Mitotic Divisions, Cell Expansion and Cell Specification during Female Gametophyte Development in Arabidopsis thaliana. PLoS ONE 2015, 10, e0126164. [Google Scholar] [CrossRef] [PubMed]
- Lituiev, D.; Krohn, N.G.; Müller, B.; Jackson, D.; Hellriegel, B.; Dresselhaus, T.; Grossniklaus, U. Theoretical and experimental evidence indicates that there is no detectable auxin gradient in the angiosperm female gametophyte. Development 2013, 140, 4544–4553. [Google Scholar] [CrossRef] [Green Version]
- Robert, H.; Grunewald, W.; Sauer, M.; Cannoot, B.; Soriano, M.; Swarup, R.; Weijers, D.; Bennett, M.; Boutilier, K.; Friml, J. Plant embryogenesis requires AUX/LAX-mediated auxin influx. Development 2015, 142, 702–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radoeva, T.; Lokerse, A.S.; Llavata-Peris, C.I.; Wendrich, J.R.; Xiang, D.; Liao, C.-Y.; Vlaar, L.; Boekschoten, M.; Hooiveld, G.; Datla, R.; et al. A Robust Auxin Response Network Controls Embryo and Suspensor Development through a Basic Helix Loop Helix Transcriptional Module. Plant Cell 2019, 31, 52–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, H.S.; Park, C.; Gutièrrez, C.L.; Wójcikowska, B.; Pěnčík, A.; Novák, O.; Chen, J.; Grunewald, W.; Dresselhaus, T.; Friml, J.; et al. Maternal auxin supply contributes to early embryo patterning in Arabidopsis. Nat. Plants 2018, 4, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.E.; Weijers, D. The role of auxin signaling in early embryo pattern formation. Curr. Opin. Plant Biol. 2015, 28, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.; Grones, P.; Stepanova, A.; Robles, L.M.; Lokerse, A.S.; Alonso, J.; Weijers, D.; Friml, J. Local Auxin Sources Orient the Apical-Basal Axis in Arabidopsis Embryos. Curr. Biol. 2013, 23, 2506–2512. [Google Scholar] [CrossRef] [Green Version]
- Aida, M.; Vernoux, T.; Furutani, M.; Traas, J.; Tasaka, M. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 2002, 129, 3965–3974. [Google Scholar] [CrossRef]
- Steinmann, T.; Geldner, N.; Grebe, M.; Mangold, S.; Jackson, C.L.; Paris, S.; Gälweiler, L.; Palme, K.; Jürgens, G. Coordinated Polar Localization of Auxin Efflux Carrier PIN1 by GNOM ARF GEF. Science 1999, 286, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Z.; Chua, N.H. Auxin Polar Transport Is Essential for the Establishment of Bilateral Symmetry during Early Plant Embryogenesis. Plant Cell 1993, 5, 621–630. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nat. Cell Biol. 2005, 433, 39–44. [Google Scholar] [CrossRef]
- Friml, J.; Vieten, A.; Sauer, M.; Weijers, D.; Schwarz, H.; Hamann, T.; Offringa, R.; Jürgens, G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nat. Cell Biol. 2003, 426, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Weijers, D.; Sauer, M.; Meurette, O.; Friml, J.; Ljung, K.; Sandberg, G.; Hooykaas, P.; Offringa, R. Maintenance of Embryonic Auxin Distribution for Apical-Basal Patterning by PIN-FORMED–Dependent Auxin Transport in Arabidopsis. Plant Cell 2005, 17, 2517–2526. [Google Scholar] [CrossRef] [Green Version]
- Vieten, A.; Vanneste, S.; Wiśniewska, J.; Benková, E.; Benjamins, R.; Beeckman, T.; Luschnig, C.; Friml, J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 2005, 132, 4521–4531. [Google Scholar] [CrossRef] [Green Version]
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, H.; Nodzynski, T.; Kitakura, S.; Feraru, M.I.; Sasabe, M.; Ishikawa, T.; Kleine-Vehn, J.; Kakimoto, T.; Friml, J. BEX1/ARF1A1C is Required for BFA-Sensitive Recycling of PIN Auxin Transporters and Auxin-Mediated Development in Arabidopsis. Plant Cell Physiol. 2014, 55, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Naramoto, S.; Nodzynski, T.; Dainobu, T.; Takatsuka, H.; Okada, T.; Friml, J.; Fukuda, H. VAN4 Encodes a Putative TRS120 That is Required for Normal Cell Growth and Vein Development in Arabidopsis. Plant Cell Physiol. 2014, 55, 750–763. [Google Scholar] [CrossRef] [Green Version]
- Drdova, E.J.; Synek, L.; Pecenkova, T.; Hála, M.; Kulich, I.; Fowler, J.E.; Murphy, A.S.; Žárský, V. The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J. 2013, 73, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Feraru, E.; Feraru, M.I.; Asaoka, R.; Paciorek, T.; De Rycke, R.; Tanaka, H.; Nakano, A.; Friml, J. BEX5/RabA1b Regulates trans-Golgi Network-to-Plasma Membrane Protein Trafficking in Arabidopsis. Plant Cell 2012, 24, 3074–3086. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, S.R. Rab GTPases: Master regulators that establish the secretory and endocytic pathways. Mol. Biol. Cell 2017, 28, 712–715. [Google Scholar] [CrossRef]
- Novick, P. Regulation of membrane traffic by Rab GEF and GAP cascades. Small GTPases 2016, 7, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, E. The Small GTPase Superfamily in Plants: A Conserved Regulatory Module with Novel Functions. Annu. Rev. Plant Biol. 2020, 71, 247–272. [Google Scholar] [CrossRef]
- Leung, K.F.; Baron, R.; Seabra, M. Thematic review series: Lipid Posttranslational Modifications. Geranylgeranylation of Rab GTPases. J. Lipid Res. 2006, 47, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvius, J.R.; I’Heureux, F. Fluorimetric evaluation of the affinities of isoprenylated peptides for lipid bilayers. Biochemistry 1994, 33, 3014–3022. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Ali, B.R.; Ramalho, J.; Godfrey, R.F.; Barral, D.; Hume, A.; Seabra, M.C. Membrane Targeting of Rab GTPases Is Influenced by the Prenylation Motif. Mol. Biol. Cell 2003, 14, 1882–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, J.P.; Hume, A.; Seabra, M.C. Prenylation of Rab GTPases: Molecular mechanisms and involvement in genetic disease. FEBS Lett. 2001, 498, 197–200. [Google Scholar] [CrossRef]
- Hala, M.; Soukupova, H.; Synek, L.; Zarsky, V. Arabidopsis RAB geranylgeranyl transferase beta-subunit mutant is constitutively photomorphogenic, and has shoot growth and gravitropic defects. Plant J. 2010, 62, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Gutkowska, M.; Wnuk, M.; Nowakowska, J.; Lichocka, M.; Stronkowski, M.M.; Swiezewska, E. Rab geranylgeranyl transferase beta subunit is essential for male fertility and tip growth in Arabidopsis. J. Exp. Bot. 2015, 66, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Z.; Dresselhaus, T. Friend or foe: Signaling mechanisms during double fertilization in flowering seed plants. Curr. Top. Dev. Biol. 2019, 131, 453–496. [Google Scholar]
- Heisler, M.; Ohno, C.; Das, P.; Sieber, P.; Reddy, G.V.; Long, J.A.; Meyerowitz, E.M. Patterns of Auxin Transport and Gene Expression during Primordium Development Revealed by Live Imaging of the Arabidopsis Inflorescence Meristem. Curr. Biol. 2005, 15, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Brunoud, G.; Wells, D.; Oliva, M.; Larrieu, A.; Mirabet, V.; Burrow, A.H.; Beeckman, T.; Kepinski, S.; Traas, J.; Bennett, M.; et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nat. Cell Biol. 2012, 482, 103–106. [Google Scholar] [CrossRef]
- Furutani, M.; Vernoux, T.; Traas, J.; Kato, T.; Tasaka, M.; Aida, M. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 2004, 131, 5021–5030. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Attuluri, V.P.S.; Robert, H.S. An Essential Function for Auxin in Embryo Development. Cold Spring Harb. Perspect. Biol. 2021, 13, a039966. [Google Scholar] [CrossRef]
- Govindaraju, P.; Verna, C.; Zhu, T.; Scarpella, E. Vein patterning by tissue-specific auxin transport. Development 2020, 147. [Google Scholar] [CrossRef]
- Shimada, T.; Kunieda, T.; Sumi, S.; Koumoto, Y.; Tamura, K.; Hatano, K.; Ueda, H.; Hara-Nishimura, I. The AP-1 Complex is Required for Proper Mucilage Formation in Arabidopsis Seeds. Plant Cell Physiol. 2018, 59, 2331–2338. [Google Scholar] [CrossRef]
- Gendre, D.; McFarlane, H.; Johnson, E.; Mouille, G.; Sjödin, A.; Oh, J.; Levesque-Tremblay, G.; Watanabe, Y.; Samuels, A.L.; Bhalerao, R.P. Trans-Golgi Network Localized ECHIDNA/Ypt Interacting Protein Complex Is Required for the Secretion of Cell Wall Polysaccharides in Arabidopsis. Plant Cell 2013, 25, 2633–2646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, F.; Schon, M.A.; Nodine, M.D. The embryonic transcriptome of Arabidopsis thaliana. Plant Reprod. 2019, 32, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Zeng, Q.; Kunkel, B.N.; Running, M.P. Arabidopsis Rab Geranylgeranyltransferases Demonstrate Redundancy and Broad Substrate Specificity in Vitro. J. Biol. Chem. 2016, 291, 1398–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmonte, M.F.; Kirkbride, R.; Stone, S.L.; Pelletier, J.M.; Bui, A.Q.; Yeung, E.C.; Hashimoto, M.; Fei, J.; Harada, C.M.; Munoz, M.D.; et al. Comprehensive developmental profiles of gene activity in regions and subregions of the Arabidopsis seed. Proc. Natl. Acad. Sci. USA 2013, 110, E435–E444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, J.; Liu, X.-G.; Yang, K.-Z.; Chen, X.-L.; Zou, J.-J.; Wang, H.-Z.; Wang, M.; Vanneste, S.; Morita, M.; Tasaka, M.; et al. Auxin transport and activity regulate stomatal patterning and development. Nat. Commun. 2014, 5, 3090. [Google Scholar] [CrossRef] [Green Version]
- Galvan-Ampudia, C.S.; Cerutti, G.; Legrand, J.; Brunoud, G.; Martin-Arevalillo, R.; Azais, R.; Bayle, V.; Moussu, S.; Wenzl, C.; Jaillais, Y.; et al. Temporal integration of auxin information for the regulation of patterning. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Guo, X.; Dong, J. Asymmetric cell division in plants: Mechanisms of symmetry breaking and cell fate determination. Cell. Mol. Life Sci. 2016, 73, 4213–4229. [Google Scholar] [CrossRef] [Green Version]
- Shao, W.; Dong, J. Polarity in plant asymmetric cell division: Division orientation and cell fate differentiation. Dev. Biol. 2016, 419, 121–131. [Google Scholar] [CrossRef]
- Di Mambro, R.; De Ruvo, M.; Pacifici, E.; Salvi, E.; Sozzani, R.; Benfey, P.A.; Busch, A.; Novak, O.; Ljung, K.; Di Paola, A.; et al. Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc. Natl. Acad. Sci. USA 2017, 114, E7641–E7649. [Google Scholar] [CrossRef] [Green Version]
- Sorefan, K.; Girin, T.; Liljegren, S.J.; Ljung, K.; Robles, P.; Galván-Ampudia, C.S.; Offringa, R.; Friml, J.; Yanofsky, M.F.; Østergaard, L. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nat. Cell Biol. 2009, 459, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Fendrych, M.; Akhmanova, M.; Merrin, J.; Glanc, M.; Hagihara, S.; Takahashi, K.; Uchida, N.; Torii, K.U.; Friml, J. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat. Plants 2018, 4, 453–459. [Google Scholar] [CrossRef]
- Godel-Jędrychowska, K.; Kulinska-Lukaszek, K.; Horstman, A.; Soriano, M.; Li, M.; Malota, K.; Boutilier, K.; Kurczynska, E.U. Symplasmic isolation marks cell fate changes during somatic embryogenesis. J. Exp. Bot. 2020, 71, 2612–2628. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hyun, T.K.; Zhang, M.; Kumar, R.; Koh, E.-J.; Kang, B.-H.; Lucas, W.J.; Kim, J.-Y. Auxin-Callose-Mediated Plasmodesmal Gating Is Essential for Tropic Auxin Gradient Formation and Signaling. Dev. Cell 2014, 28, 132–146. [Google Scholar] [CrossRef] [Green Version]
- Kubes, M.; Napier, R. Non-canonical auxin signalling: Fast and curious. J. Exp. Bot. 2019, 70, 2609–2614. [Google Scholar] [CrossRef]
- Ellinger, D.; Glöckner, A.; Koch, J.; Naumann, M.; Stürtz, V.; Schütt, K.; Manisseri, C.; Somerville, S.C.; Voigt, C.A. Interaction of the Arabidopsis GTPase RabA4c with Its Effector PMR4 Results in Complete Penetration Resistance to Powdery Mildew. Plant Cell 2014, 26, 3185–3200. [Google Scholar] [CrossRef] [Green Version]
- Zientara-Rytter, K.; Sirko, A. To deliver or to degrade—An interplay of the ubiquitin-proteasome system, autophagy and vesicular transport in plants. FEBS J. 2016, 283, 3534–3555. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Smet, W.; Brunoud, G.; Yoshida, S.; Vernoux, T.; Weijers, D. Reporters for sensitive and quantitative measurement of auxin response. Nat. Methods 2015, 12, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Simonini, S.; Deb, J.; Moubayidin, L.; Stephenson, P.; Valluru, M.; Freire-Rios, A.; Sorefan, K.; Weijers, D.; Friml, J.; Østergaard, L. A noncanonical auxin-sensing mechanism is required for organ morphogenesis in Arabidopsis. Genes Dev. 2016, 30, 2286–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellor, N.L.; Voß, U.; Janes, G.; Bennett, M.J.; Wells, D.M.; Band, L.R. Auxin fluxes through plasmodesmata modify root-tip auxin distribution. Development 2020, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, N.R. The Importance of Being Absent: Auxin Minima Are Required for Axillary Meristem Formation. Plant Cell 2014, 26, 1836. [Google Scholar] [CrossRef] [Green Version]
- Petrella, R.; Cucinotta, M.; Mendes, M.A.; Underwood, C.J.; Colombo, L. The emerging role of small RNAs in ovule development, a kind of magic. Plant Reprod. 2021, 1–17. [Google Scholar] [CrossRef]
- Kalantidis, K.; Schumacher, H.T.; Alexiadis, T.; Helm, J.M. RNA silencing movement in plants. Biol. Cell 2008, 100, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svoboda, P. Key Mechanistic Principles and Considerations Concerning RNA Interference. Front. Plant Sci. 2020, 11, 1237. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef]
- Yao, X.; Chen, J.; Zhou, J.; Yu, H.; Ge, C.; Zhang, M.; Gao, X.; Dai, X.; Yang, Z.-N.; Zhao, Y. An Essential Role for miRNA167 in Maternal Control of Embryonic and Seed Development. Plant Physiol. 2019, 180, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.H.; Han, S.J.; Yoon, E.K.; Lee, W.S. Evidence of an auxin signal pathway, microRNA167-ARF8-GH3, and its response to exogenous auxin in cultured rice cells. Nucleic Acids Res. 2006, 34, 1892–1899. [Google Scholar] [CrossRef]
- Wu, M.-F.; Tian, Q.; Reed, J. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 2006, 133, 4211–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böttcher, C.; Boss, P.; Davies, C. Acyl substrate preferences of an IAA-amido synthetase account for variations in grape (Vitis vinifera L.) berry ripening caused by different auxinic compounds indicating the importance of auxin conjugation in plant development. J. Exp. Bot. 2011, 62, 4267–4280. [Google Scholar] [CrossRef]
- Mallory, A.C.; Bartel, D.P.; Bartel, B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 2005, 17, 1360–1375. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Kobayashi, K.; Cho, E.; Zambryski, P.C. Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 11945–11950. [Google Scholar] [CrossRef] [Green Version]
- Stadler, R.; Lauterbach, C.; Sauer, N. Cell-to-Cell Movement of Green Fluorescent Protein Reveals Post-Phloem Transport in the Outer Integument and Identifies Symplastic Domains in Arabidopsis Seeds and Embryos. Plant Physiol. 2005, 139, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Hempel, F.D.; Sha, K.; Pfluger, J.; Zambryski, P.C. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development 2002, 129, 1261–1272. [Google Scholar] [CrossRef]
- Carluccio, A.V.; Zicca, S.; Stavolone, L. Hitching a Ride on Vesicles: Cauliflower Mosaic Virus Movement Protein Trafficking in the Endomembrane System. Plant Physiol. 2014, 164, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.; Okada, T.; Hu, Y.; Scholefield, A.; Taylor, J.M.; Koltunow, A.M.G. Somatic small RNA pathways promote the mitotic events of megagametogenesis during female reproductive development in Arabidopsis. Development 2012, 139, 1399–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pylypenko, O.; Rak, A.; Durek, T.; Kushnir, S.; Dursina, B.E.; Thomae, N.H.; Constantinescu, A.T.; Brunsveld, L.; Watzke, A.; Waldmann, H.; et al. Structure of doubly prenylated Ypt1:GDI complex and the mechanism of GDI-mediated Rab recycling. EMBO J. 2006, 25, 13–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomä, N.H.; Iakovenko, A.; Goody, R.; Alexandrov, K. Phosphoisoprenoids Modulate Association of Rab Geranylgeranyltransferase with REP-1. J. Biol. Chem. 2001, 276, 48637–48643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, Efflux-Dependent Auxin Gradients as a Common Module for Plant Organ Formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Kuroiwa, H.; Higashiyama, T.; Kuroiwa, T. Generative cell specific 1 is essential for angiosperm fertilization. Nat. Cell Biol. 2005, 8, 64–71. [Google Scholar] [CrossRef]
- Kulich, I.; Cole, R.; Drdova, E.J.; Cvrčková, F.; Soukup, A.; Fowler, J.; Žárský, V. Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytol. 2010, 188, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Lalanne, E.; Honys, D.; Johnson, A.; Borner, G.H.H.; Lilley, K.S.; Dupree, P.; Grossniklaus, U.; Twell, D. SETH1 and SETH2, Two Components of the Glycosylphosphatidylinositol Anchor Biosynthetic Pathway, Are Required for Pollen Germination and Tube Growth in Arabidopsis. Plant Cell 2004, 16, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojek, J.; Kapusta, M.; Kozieradzka-Kiszkurno, M.; Majcher, D.; Górniak, M.; Sliwinska, E.; Sharbel, T.F.; Bohdanowicz, J. Establishing the cell biology of apomictic reproduction in diploid Boechera stricta (Brassicaceae). Ann. Bot. 2018, 122, 513–539. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojek, J.; Tucker, M.R.; Rychłowski, M.; Nowakowska, J.; Gutkowska, M. The Rab Geranylgeranyl Transferase Beta Subunit Is Essential for Embryo and Seed Development in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 7907. https://doi.org/10.3390/ijms22157907
Rojek J, Tucker MR, Rychłowski M, Nowakowska J, Gutkowska M. The Rab Geranylgeranyl Transferase Beta Subunit Is Essential for Embryo and Seed Development in Arabidopsis thaliana. International Journal of Molecular Sciences. 2021; 22(15):7907. https://doi.org/10.3390/ijms22157907
Chicago/Turabian StyleRojek, Joanna, Matthew R. Tucker, Michał Rychłowski, Julita Nowakowska, and Małgorzata Gutkowska. 2021. "The Rab Geranylgeranyl Transferase Beta Subunit Is Essential for Embryo and Seed Development in Arabidopsis thaliana" International Journal of Molecular Sciences 22, no. 15: 7907. https://doi.org/10.3390/ijms22157907