Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Salivary MicroRNA (miRNA) in OSCC Diagnosis and Prognosis
2.1. Exosomal miRNAs with Diagnostic Capacity
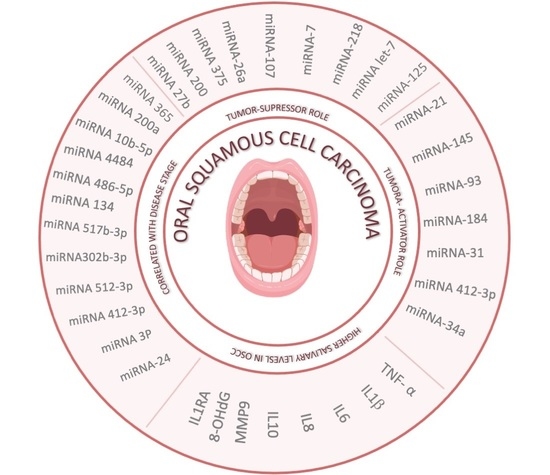
2.2. MiRNAs with Tumor-Suppressor Role
2.3. MiRNAs with Tumor-Activator Role
2.4. Circular RNA in OSCC Diagnosis
3. Salivary Cytokines in OSCC Diagnosis and Prognosis
3.1. IL-6
3.2. IL-8
3.3. TNF-α
3.4. MMP-9
3.5. IL-1-β
3.6. IL-1-Ra
3.7. IL-10
3.8. 8-OHdG (8-Oxo-dG)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSCC | Oral squamous cell carcinoma |
| EVs | Extracellular vesicles |
| EZH2 | Enhancer of zeste homolog 2 |
| EGFR | Epidermal growth factor receptor |
| PKCε | Protein kinase Cε |
| TGF | Transforming growth factor |
| circRNAs | Circular RNAs |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor alpha |
| IL-8 | Interleukin-8 |
| MMP-9 | Matrix metallopeptidase 9 |
| IL-1-β | Interleukin-1-β |
| IL-1-Ra | IL-1 receptor antagonist |
| IL-10 | Interleukin-10 |
| 8-OHdG (8-oxo-dG) | 8-Oxo-2′-deoxyguanosine |
References
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Guha, N.; Warnakulasuriya, S.; Vlaanderen, J.; Straif, K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int. J. Cancer 2014, 135, 1433–1443. [Google Scholar] [CrossRef]
- Jeng, J.H.; Chang, M.C.; Hahn, L.J. Role of areca nut in betel quid-associated chemical carcinogenesis: Current awareness and future perspectives. Oral Oncol. 2001, 37, 477–492. [Google Scholar] [CrossRef]
- Bellairs, J.A.; Hasina, R.; Agrawal, N. Tumor DNA: An emerging biomarker in head and neck cancer. Cancer Metastasis Rev. 2017, 36, 515–523. [Google Scholar] [CrossRef]
- Woolgar, J.A. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006, 42, 229–239. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Perron, M.P.; Provost, P. Protein interactions and complexes in human microRNA biogenesis and function. Front. Biosci. 2008, 13, 2537–2547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faur, C.I.; Rotaru, H.; Osan, C.; Jurj, A.; Roman, R.C.; Moldovan, M.; Chirila, M.; Hedesiu, M. Salivary exosomal microRNAs as biomarkers for head and neck cancer detection-a literature review. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 19. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ping, F.; Fan, Z.; Zhang, C.; Deng, M.; Cheng, B.; Xia, J. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharmacother. 2020, 121, 109553. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef] [Green Version]
- Byun, J.-S.; Hong, S.-H.; Choi, J.-K.; Jung, J.-K.; Lee, H.-J. Diagnostic profiling of salivary exosomal microRNAs in oral lichen planus patients. Oral Dis. 2015, 21, 987–993. [Google Scholar] [CrossRef]
- Coon, J.; Kingsley, K.; Howard, K.M. miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 5317. [Google Scholar] [CrossRef] [PubMed]
- Al Rawi, N.; Elmabrouk, N.; Abu Kou, R.; Mkadmi, S.; Rizvi, Z.; Hamdoon, Z. The role of differentially expressed salivary microRNA in oral squamous cell carcinoma. A systematic review. Arch. Oral Biol. 2021, 125, 105108. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Bala, S. Emerging role of non-coding RNA in oral cancer. Cell. Signal. 2018, 42, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Jimenez, L.; Kawachi, N.; Fan, J.-B.; Chen, J.; Belbin, T.; Ramnauth, A.; Loudig, O.; Keller, C.E.; Smith, R.; et al. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am. J. Pathol. 2012, 180, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinowski, F.C.; Giles, K.M.; Candy, P.A.; Ali, A.; Ganda, C.; Epis, M.R.; Webster, R.J.; Leedman, P.J. Regulation of epidermal growth factor receptor signaling and erlotinib sensitivity in head and neck cancer cells by miR-7. PLoS ONE 2012, 7, e47067. [Google Scholar] [CrossRef]
- Lu, Z.-M.; Lin, Y.-F.; Jiang, L.; Chen, L.-S.; Luo, X.-N.; Song, X.-H.; Chen, S.-H.; Zhang, S.-Y. Micro-ribonucleic acid expression profiling and bioinformatic target gene analyses in laryngeal carcinoma. OncoTargets Ther. 2014, 7, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Smith, A.; Lang, J.C.; Islam, M.; Dutt, D.; Teknos, T.N.; Pan, Q. microRNA-107 functions as a candidate tumor-suppressor gene in head and neck squamous cell carcinoma by downregulation of protein kinase Cɛ. Oncogene 2012, 31, 4045–4053. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Nohata, N.; Hanazawa, T.; Kikkawa, N.; Yamamoto, N.; Yoshino, H.; Itesako, T.; Enokida, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. Br. J. Cancer 2013, 109, 2636–2645. [Google Scholar] [CrossRef] [Green Version]
- Alajez, N.M.; Shi, W.; Wong, D.; Lenarduzzi, M.; Waldron, J.; Weinreb, I.; Liu, F.-F. Lin28b Promotes Head and Neck Cancer Progression via Modulation of the Insulin-Like Growth Factor Survival Pathway. Oncotarget 2012, 3, 1641–1652. [Google Scholar] [CrossRef] [Green Version]
- Wiklund, E.D.; Gao, S.; Hulf, T.; Sibbritt, T.; Nair, S.; Costea, D.E.; Villadsen, S.B.; Bakholdt, V.; Bramsen, J.B.; Sørensen, J.A.; et al. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS ONE 2011, 6, e27840. [Google Scholar] [CrossRef]
- Zahran, F.; Ghalwash, D.; Shaker, O.; Al-Johani, K.; Scully, C. Salivary microRNAs in oral cancer. Oral Dis. 2015, 21, 739–747. [Google Scholar] [CrossRef]
- Greither, T.; Vorwerk, F.; Kappler, M.; Bache, M.; Taubert, H.; Kuhnt, T.; Hey, J.; Eckert, A.W. Salivary miR-93 and miR-200a as post-radiotherapy biomarkers in head and neck squamous cell carcinoma. Oncol. Rep. 2017, 38, 1268–1275. [Google Scholar] [CrossRef] [Green Version]
- Maheswari, T.N.U.; Venugopal, A.; Sureshbabu, N.M.; Ramani, P. Salivary micro RNA as a potential biomarker in oral potentially malignant disorders: A systematic review. Tzu-Chi Med. J. 2018, 30, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xin, Z.; Guo, S.; Li, S.; Cheng, J.; Jiang, H. Blood and Salivary MicroRNAs for Diagnosis of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2021, 79, 1082.e1–1082.e13. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-S.; Liu, C.-J.; Chou, C.-S.; Kao, S.-Y.; Yang, C.-C.; Chang, K.-W.; Chiu, T.-H.; Lin, S.-C. miR-146a Enhances the Oncogenicity of Oral Carcinoma by Concomitant Targeting of the IRAK1, TRAF6 and NUMB Genes. PLoS ONE 2013, 8, e79926. [Google Scholar] [CrossRef]
- Larrea, E.; Sole, C.; Manterola, L.; Goicoechea, I.; Armesto, M.; Arestin, M.; Caffarel, M.M.; Araujo, A.M.; Araiz, M.; Fernandez-Mercado, M.; et al. New Concepts in Cancer Biomarkers: Circulating miRNAs in Liquid Biopsies. Int. J. Mol. Sci. 2016, 17, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giner, M.; Montoya, M.J.; Vázquez, M.A.; Miranda, C.; Miranda, M.J.; Pérez-Cano, R. ¿Qué son los microARNs?: Posibles biomarcadores y dianas terapéuticas en la enfermedad osteoporótica. Rev. Osteoporos. Metab. Min. 2016, 8, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Xiong, X.; Jia, J.; Zhang, W. MicroRNAs: New actors in the oral cancer scene. Oral Oncol. 2011, 47, 314–319. [Google Scholar] [CrossRef]
- Sannigrahi, M.K.; Sharma, R.; Singh, V.; Panda, N.K.; Rattan, V.; Khullar, M. Role of Host miRNA Hsa-miR-139-3p in HPV-16-Induced Carcinomas. Clin. Cancer Res. 2017, 23, 3884–3895. [Google Scholar] [CrossRef] [Green Version]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, A.F.; Sabry, D.; Hassabou, N.F.; Alaa EL-Din, Y. MicroRNA-134/MicroRNA-200a Derived Salivary Exosomes are Novel Diagnostic Biomarkers of Oral Squamous Cell Carcinoma. Egypt. Dent. J. 2021, 67, 367–377. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.; Alaouna, M.; Mbatha, S.; Bhayat, A.; Mabongo, M.; Chatziioannou, A.; Hull, R. Genetic Drivers of Head and Neck Squamous Cell Carcinoma: Aberrant Splicing Events, Mutational Burden, HPV Infection and Future Targets. Genes 2021, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-Y.; Wang, J.; Ouyang, S.-B.; Huang, Z.-K.; Liao, L. Salivary Circular RNAs Hsa_Circ_0001874 and Hsa_Circ_0001971 as Novel Biomarkers for the Diagnosis of Oral Squamous Cell Carcinoma. Cell. Physiol. Biochem. 2018, 47, 2511–2521. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [Green Version]
- Loo, S.W.; Pui, T.-S. Cytokine and Cancer Biomarkers Detection: The Dawn of Electrochemical Paper-Based Biosensor. Sensors 2020, 20, 1854. [Google Scholar] [CrossRef] [Green Version]
- Dikova, V.; Jantus-Lewintre, E.; Bagan, J. Potential Non-Invasive Biomarkers for Early Diagnosis of Oral Squamous Cell Carcinoma. J. Clin. Med. 2021, 10, 1658. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Vu, H.; Lee, J.-H.; Kim, H.-D. Diagnostic and prognostic ability of salivary MMP-9 for oral squamous cell carcinoma: A pre-/post-surgery case and matched control study. PLoS ONE 2021, 16, e0248167. [Google Scholar] [CrossRef]
- Lee, L.T.; Wong, Y.K.; Hsiao, H.Y.; Wang, Y.W.; Chan, M.Y.; Chang, K.W. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef]
- Vesty, A.; Gear, K.; Biswas, K.; Radcliff, F.J.; Taylor, M.W.; Douglas, R.G. Microbial and inflammatory-based salivary biomarkers of head and neck squamous cell carcinoma. Clin. Exp. Dent. Res. 2018, 4, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Khyani, I.A.M.; Qureshi, M.A.; Mirza, T.; Farooq, M.U. Detection of interleukins-6 and 8 in saliva as potential biomarkers of oral pre-malignant lesion and oral carcinoma: A breakthrough in salivary diagnostics in Pakistan. Pak. J. Pharm. Sci. 2017, 30, 817–823. [Google Scholar]
- Csősz, É.; Lábiscsák, P.; Kalló, G.; Márkus, B.; Emri, M.; Szabó, A.; Tar, I.; Tőzsér, J.; Kiss, C.; Márton, I. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS ONE 2017, 12, e0177282. [Google Scholar] [CrossRef] [Green Version]
- Ghallab, N.A.; Shaker, O.G. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin. Oral Investig. 2017, 21, 937–947. [Google Scholar] [CrossRef]
- Peisker, A.; Raschke, G.-F.; Fahmy, M.-D.; Guentsch, A.; Roshanghias, K.; Hennings, J.; Schultze-Mosgau, S. Salivary MMP-9 in the detection of oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e270–e275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleber-Netto, F.O.; Yakob, M.; Li, F.; Feng, Z.; Dai, J.; Kao, H.-K.; Chang, Y.-L.; Chang, K.-P.; Wong, D.T.W. Salivary Biomarkers for Detection of Oral Squamous Cell Carcinoma in a Taiwanese Population. Clin. Cancer Res. 2016, 22, 3340–3347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Jacobs, R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Arduino, P.G.; Menegatti, E.; Cappello, N.; Martina, E.; Gardino, N.; Tanteri, C.; Cavallo, F.; Scully, C.; Broccoletti, R. Possible role for interleukins as biomarkers for mortality and recurrence in oral cancer. Int. J. Biol. Markers 2015, 30, e262–e266. [Google Scholar] [CrossRef] [PubMed]
- Lisa Cheng, Y.-S.; Jordan, L.; Gorugantula, L.M.; Schneiderman, E.; Chen, H.-S.; Rees, T. Salivary interleukin-6 and -8 in patients with oral cancer and patients with chronic oral inflammatory diseases. J. Periodontol. 2014, 85, 956–965. [Google Scholar] [CrossRef]
- Krishnan, R.; Thayalan, D.K.; Padmanaban, R.; Ramadas, R.; Annasamy, R.K.; Anandan, N. Association of serum and salivary tumor necrosis factor-α with histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac. J. Cancer Prev. 2014, 15, 7141–7148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Zilic-Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e10–e15. [Google Scholar] [CrossRef]
- Korostoff, A.; Reder, L.; Masood, R.; Sinha, U.K. The role of salivary cytokine biomarkers in tongue cancer invasion and mortality. Oral Oncol. 2011, 47, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Katakura, A.; Kamiyama, I.; Takano, N.; Shibahara, T.; Muramatsu, T.; Ishihara, K.; Takagi, R.; Shouno, T. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull. Tokyo Dent. Coll. 2007, 48, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodus, N.L.; Ho, V.; Miller, C.S.; Myers, S.; Ondrey, F. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 2005, 29, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; John, M.A.R.S.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.K.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.-H.; et al. Salivary Transcriptome Diagnostics for Oral Cancer Detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, O.; Kastratovic, D.A.; Dimitrijevic, M.V.; Konstantinovic, V.S.; Jelovac, D.B.; Antic, J.; Nesic, V.S.; Markovic, S.Z.; Martinovic, Z.R.; Akin, D.; et al. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncol. 2011, 47, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Kamatani, T.; Shiogama, S.; Yoshihama, Y.; Kondo, S.; Shirota, T.; Shintani, S. Interleukin-1 beta in unstimulated whole saliva is a potential biomarker for oral squamous cell carcinoma. Cytokine 2013, 64, 497–502. [Google Scholar] [CrossRef]
- Niklander, S.E. Inflammatory Mediators in Oral Cancer: Pathogenic Mechanisms and Diagnostic Potential. Front. Oral Health 2021, 2, 2. [Google Scholar] [CrossRef]
- Shiiba, M.; Saito, K.; Yamagami, H.; Nakashima, D.; Higo, M.; Kasamatsu, A.; Sakamoto, Y.; Ogawara, K.; Uzawa, K.; Takiguchi, Y.; et al. Interleukin-1 receptor antagonist (IL1RN) is associated with suppression of early carcinogenic events in human oral malignancies. Int. J. Oncol. 2015, 46, 1978–1984. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.; Ahmed, S.S.; Ali, A.; Khan, F.A.; Zulfiqar, G.; Iqbal, J.; Khan, A.A.; Shoaib, M. Salivary Immunosuppressive Cytokines IL-10 and IL-13 Are Significantly Elevated in Oral Squamous Cell Carcinoma Patients. Cancer Investig. 2015, 33, 318–328. [Google Scholar] [CrossRef]
- Arantes, L.M.R.B.; De Carvalho, A.C.; Melendez, M.E.; Lopes Carvalho, A. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Sung, W.-W.; Su, T.-C.; Chen, M.-K.; Wu, P.-R.; Yeh, K.-T.; Ko, J.-L.; Lee, H. High expression of interleukin 10 might predict poor prognosis in early stage oral squamous cell carcinoma patients. Clin. Chim. Acta 2013, 415, 25–30. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, N.; Guan, X.; Wu, H.; Sun, Z.; Zeng, H. Immunosuppression Induced by Chronic Inflammation and the Progression to Oral Squamous Cell Carcinoma. Mediat. Inflamm. 2016, 2016, 5715719. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pant, M.C.; Singh, H.S.; Khandelwal, S. Determinants of oxidative stress and DNA damage (8-OhdG) in squamous cell carcinoma of head and neck. Indian J. Cancer 2012, 49, 309. [Google Scholar] [CrossRef]
- Nandakumar, A.; Nataraj, P.; James, A.; Krishnan, R.; K M, M. Estimation of Salivary 8-Hydroxydeoxyguanosine (8-OHdG) as a Potential Biomarker in Assessing Progression towards Malignancy: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2325–2329. [Google Scholar] [CrossRef]
- Anderson, P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hop, H.T.; Huy, T.X.N.; Reyes, A.W.B.; Arayan, L.T.; Vu, S.H.; Min, W.; Lee, H.J.; Kang, C.K.; Kim, D.H.; Tark, D.S.; et al. Interleukin 6 Promotes Brucella abortus Clearance by Controlling Bactericidal Activity of Macrophages and CD8+ T Cell Differentiation. Infect. Immun. 2019, 87, e00431-19. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Mauer, J.; Denson, J.L.; Brüning, J.C. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015, 36, 92–101. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Sasaki, M.; Kodama, Y.; Shimoyama, Y.; Ishikawa, T.; Kimura, S. Aciduricity and acid tolerance mechanisms of Streptococcus anginosus. J. Gen. Appl. Microbiol. 2018, 64, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roi, A.; Roi, C.I.; Negruțiu, M.L.; Riviș, M.; Sinescu, C.; Rusu, L.-C. The Challenges of OSCC Diagnosis: Salivary Cytokines as Potential Biomarkers. J. Clin. Med. 2020, 9, 2866. [Google Scholar] [CrossRef]
- Duffy, S.A.; Taylor, J.M.G.; Terrell, J.E.; Islam, M.; Li, Y.; Fowler, K.E.; Wolf, G.T.; Teknos, T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008, 113, 750–757. [Google Scholar] [CrossRef]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H. Interleukin-8 in the Tumor Immune Niche: Lessons from Comparative Oncology. In Tumor Microenvironment: The Role of Interleukins—Part A; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 25–33. ISBN 978-3-030-38315-2. [Google Scholar]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045–1049. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [Green Version]
- Ben-Baruch, A. Inflammation-associated immune suppression in cancer: The roles played by cytokines, chemokines and additional mediators. Semin. Cancer Biol. 2006, 16, 38–52. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Pro- versus anti-inflammatory cytokines: Myth or reality. Cell. Mol. Biol. 2001, 47, 695–702. [Google Scholar]
- Sahibzada, H.A.; Khurshid, Z.; Sannam Khan, R.; Naseem, M.; Mahmood Siddique, K.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as Potential Diagnostic Biomarkers for Oral Cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Aderka, D.; Englemann, H.; Hornik, V.; Skornick, Y.; Levo, Y.; Wallach, D.; Kushtai, G. Increased serum levels of soluble receptors for tumor necrosis factor in cancer patients. Cancer Res. 1991, 51, 5602–5607. [Google Scholar]
- Selinsky, C.L.; Boroughs, K.L.; Halsey, W.A.; Howell, M.D. Multifaceted inhibition of anti-tumour immune mechanisms by soluble tumour necrosis factor receptor type I. Immunology 1998, 94, 88–93. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef] [Green Version]
- Kanneganti, T.-D. Intracellular innate immune receptors: Life inside the cell. Immunol. Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef]
- Radhika, T.; Jeddy, N.; Nithya, S.; Muthumeenakshi, R.M. Salivary biomarkers in oral squamous cell carcinoma—An insight. J. Oral Biol. Craniofac. Res. 2016, 6, S51–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acharya, S.; Tayaar, A.S. Analysis of clinical and histopathological profiles of oral squamous cell carcinoma in young Indian adults: A retrospective study. J. Dent. Sci. 2012, 7, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.Y.P.; Lu, X.J.D.; Zhu, Y.S.; Le, N.; Kim, H.; Poh, C.F. Plasma-Derived Inflammatory Proteins Predict Oral Squamous Cell Carcinoma. Front. Oncol. 2018, 8, 585. [Google Scholar] [CrossRef]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhamarneh, O.; Agada, F.; Madden, L.; Stafford, N.; Greenman, J. Serum IL10 and circulating CD4(+) CD25(high) regulatory T cell numbers as predictors of clinical outcome and survival in patients with head and neck squamous cell carcinoma. Head Neck 2011, 33, 415–423. [Google Scholar] [CrossRef]
- Arantes, D.A.C.; Costa, N.L.; Mendonça, E.F.; Silva, T.A.; Batista, A.C. Overexpression of immunosuppressive cytokines is associated with poorer clinical stage of oral squamous cell carcinoma. Arch. Oral Biol. 2016, 61, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Roszkowski, K.; Jozwicki, W.; Blaszczyk, P.; Mucha-Malecka, A.; Siomek, A. Oxidative damage DNA: 8-oxoGua and 8-oxodG as molecular markers of cancer. Med. Sci. Monit. 2011, 17, CR329–CR333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Farmanbar, N.; Abdollahi, M. Oxidative stress status and DNA damage in saliva of human subjects with oral lichen planus and oral squamous cell carcinoma. J. Oral Pathol. Med. 2012, 41, 736–740. [Google Scholar] [CrossRef]
- Korkmaz, K.S.; Butuner, B.D.; Roggenbuck, D. Detection of 8-OHdG as a diagnostic biomarker. J. Lab. Precis. Med. 2018, 3, 95. [Google Scholar] [CrossRef]
- Paredes-Sánchez, E.; Montiel-Company, J.M.; Iranzo-Cortés, J.E.; Almerich-Torres, T.; Bellot-Arcís, C.; Almerich-Silla, J.M. Meta-Analysis of the Use of 8-OHdG in Saliva as a Marker of Periodontal Disease. Dis. Markers 2018, 2018, 7916578. [Google Scholar] [CrossRef] [Green Version]
- Mascitti, M.; Togni, L.; Rubini, C.; Troiano, G.; Lo Muzio, L.; Santarelli, A. Tumour-associated tissue eosinophilia (TATE) in oral squamous cell carcinoma: A comprehensive review. Histol. Histopathol. 2021, 36, 113–122. [Google Scholar] [CrossRef]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef] [PubMed]
| Reference | Biomarker | Findings | Clinical Relevance |
|---|---|---|---|
| [8,9,10,11,12] | miRNA-24- miRNA 3P miRNA412-3p miRNA 512-3p miRNA302b-3p miRNA 517b-3p miRNA 134 miRNA 486-5p miRNA 4484 miRNA 10b-5p miRNA 200a miRNA 365 | Correlated with disease stage, histopathological type, and/or grade of OSCC | Possible tool for the early diagnosis of OSCC; in general, the expression of these miRNAs indicates poor prognosis and higher risk of malignant transformation and oral cancer |
| [13,14,15,16,17,18,19,20] | miRNA-27b miRNA-200 miRNA-375 miRNA-26a miRNA-7 miRNA-107 miRNA-218 miRNA let-7 miRNA-125 | Tumor-suppressor role | Increased levels of these biomarkers can be useful for the diagnosis, staging, and prognosis of OSCC; the expression of these miRNAs reduce the progession of OSCC |
| [1,21,22,23,24,25,26] | miRNA-21 miRNA-145 miRNA-93 miRNA-184 miRNA-31 miRNA-412-3p miRNA-34a | Tumor-activator role; these miRNAs acts as an oncogenes, promoting OSCC development and progression | Elevated concentrations in the saliva offer a reliable method to detect OSCC and potentially malignant oral lesions |
| Reference | Biomarker | Findings | Clinical Relevance |
|---|---|---|---|
| [38] | IL-6, IL-8 & TNF-α | Notably higher levels of these cytokines in advanced stages of OSCC compared to early stages of the disease; presence of neck metastases associated with increased levels of these molecules. | Possible tool to indicate OSCC progression |
| [39] | MMP-9 | Elevated salivary levels of MMP-9 were associated with OSCC Levels of the biomarker decreased dramatically after tumor surgery | MMP-9 as a critical diagnostic and prognostic biomarker for OSCC |
| [40] | IL-6, IL-8, IL-1β & TNF-α | Significant differences in levels of IL-6, IL-8, IL-1β, and TNF-α between OSCC patients and to controls | Useful complementary tool for the early detection of OSCC |
| [41] | IL-8 | Significantly increased levels of the cytokine in patients with head and neck squamous cell carcinoma; IL-8 levels were positively correlated with the abundance of C. albicans | A salivary microbial and inflammatory biomarker of head and neck squamous cell carcinoma that is influenced by oral health |
| [42] | IL-6 & IL-8 | Correlation of qualitative salivary detection of IL-6 and IL-8 between control and disease groups | Probable biomarker for detection of premalignant lesions and OSCC |
| [43] | IL-6 & TNF-α | Elevated levels of those cytokines compared to age-matched controls | IL-6 and TNF-α are potential biomarkers for the monitorization of OSCC |
| [44] | MMP-9 | MMP-9 levels significantly higher in OSCC patients than in controls or patients with premalignant lesions | Salivary diagnostic biomarker for the detection of premalignant oral lesions and early stages of OSCC |
| [45] | MMP-9 | Higher levels of MMP-9 in OSCC patients than in controls | MMP-9 is a good tool for the detection of OSCC |
| [46] | IL-8 | Protein concentration of IL-8 was significantly elevated in patients with OSCC than in those with dysplasia and controls | Important marker to discriminate between OSCC and control patients; IL-8 combined with H3F3A mRNA provides good discrimination between OSCC and potentially malignant oral disorders |
| [47] | IL-6, IL-8 & TNF-α | Increased levels of these cytokines in patients with oral leukoplakia, submucous fibrosis, and lichen planus than in healthy controls | Diagnostic tool for the detection of premalignant lesions |
| [48] | IL-6 | Higher pretreatment levels of IL-6 in patients with oral cancer, associated with better survival | Possible prognosis biomarker |
| [49] | IL-6 | Higher salivary levels of IL-6 in OSCC patients when compared with patients with chronic periodontitis, active oral lichen planus, inactive oral lichen planus, or healthy controls | Useful biomarker for the detection of OSCC |
| [50] | TNF-α | Increased serum and saliva TNF-α levels in OSCC patients compared with controls and those with premalignant disease | TNF-α as a useful biomarker for OSCC detection; increased levels are associated with histological grade and clinical stage, suggesting a role in the prognosis of OSCC |
| [51] | IL-6 | Increased levels | Monitoring of OSCC |
| [48] | TNF-α | No differences between control and OSCC group | - |
| [52] | IL-6, IL-8 & TNF-α | Higher levels in endophytic squamous cell carcinoma of the tongue than in exophytic squamous cell carcinoma of the tongue, correlated with decreased survival in the endophytic versus exophytic group; IL-6, IL-8, and TNF-α also higher in the exophytic group than in smoking and drinking controls | These biomarkers can identify the progression of squamous cell carcinoma of the tongue from high risk to neoplasm; important biomarker for cancer screening and early detection; correlation between these proteins and survival implies a prognostic benefit potentially useful for management decisions and future target treatments |
| [53] | IL-6 & IL-8 | Higher expression in patients with OSCC | Potential tool for OSCC diagnosis |
| [54] | IL-6, IL-8 & TNF-α | Increased levels in patients with OSCC and premalignant oral lesions | Proangiogenic and proinflammatory cytokines are elevated in patients with these lesions; diagnosis and prognosis significance of these markers |
| [55] | IL-8 & IL-1β | Increased levels in patients with OSCC | Potential use as a diagnostic tool for OSCC |
| [56] | IL-8 & IL-1β | Higher levels in OSCC patients, depending on the tumor stage | Increased levels of these biomarkers can be useful for OSCC diagnosis, staging, and prognosis |
| [57] | IL-1β | Levels significantly differ between before and after surgery | IL-1β levels may be useful for the detection of early stage OSCC |
| [58] | IL-1-Ra | Expression of IL-1-Ra is lower in OSCC and oral dysplasia cells than in normal cells | Possible use as a biomarker for prediction of malignant transformation |
| [59] | IL-1-Ra | Expression of IL-1-Ra decreases gradually with the progression of oral dysplasia | IL-1-Ra could be a reliable biomarker for the early diagnosis and follow-up of OSCC; it could be useful to discriminate between premalignant oral lesions and OSCC |
| [60] | IL-1-Ra & IL-10 | Salivary IL-10 levels are higher in OSCC patients; IL-1-Ra levels are lower in well-defined tumors than in immature tumors | IL-10 is an interesting tool for diagnosing OSCC, and IL-1-Ra can be helpful for cancer staging |
| [61] | IL-10 | High levels of IL-10 expression are found in OSCC, especially in advanced stage tumors and metastatic cells | Salivary IL-10 levels could be used as a biomarker for OSCC diagnosis; a high concentration appears to favor tumor proliferation and dissemination |
| [62] | IL-10 | High levels of IL-10 expression correlate with shorter survival, worse prognosis, and increased risk of death | Overexpression of IL-10 is associated with aggressive forms of OSCC, and its level can be used as a survival predictor |
| [63] | IL-10 | IL-10 levels increase with tumor progression | Useful as staging biomarker |
| [64] | 8-OHdG | 8-OHdG levels are approximately two-fold higher in patients with squamous head and neck cancer than in healthy controls | Quantification of 8-OHdG levels could be used as a diagnostic tool for OSCC |
| [65] | 8-OHdG | 8-OHdG levels are more than two-fold higher in in OSCC patients than in controls | 8-OHdG can be used as DNA damage biomarker to assess disease progression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano-Moreno, F.J.; Costela-Ruiz, V.J.; García-Recio, E.; Olmedo-Gaya, M.V.; Ruiz, C.; Reyes-Botella, C. Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 22, 12215. https://doi.org/10.3390/ijms222212215
Manzano-Moreno FJ, Costela-Ruiz VJ, García-Recio E, Olmedo-Gaya MV, Ruiz C, Reyes-Botella C. Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2021; 22(22):12215. https://doi.org/10.3390/ijms222212215
Chicago/Turabian StyleManzano-Moreno, Francisco Javier, Victor J. Costela-Ruiz, Enrique García-Recio, Maria Victoria Olmedo-Gaya, Concepción Ruiz, and Candelaria Reyes-Botella. 2021. "Role of Salivary MicroRNA and Cytokines in the Diagnosis and Prognosis of Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 22, no. 22: 12215. https://doi.org/10.3390/ijms222212215