Paving the Way to the Fuel of the Future—Nanostructured Complex Hydrides
Abstract
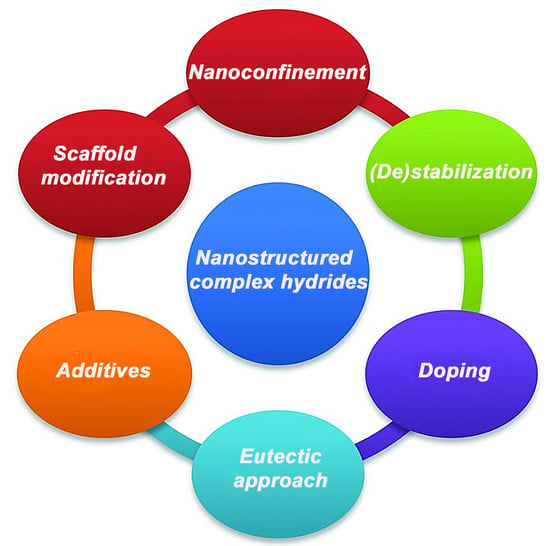
:1. Introduction
2. Classes of Complex Hydride Materials
2.1. Li-Based Systems
2.1.1. LiBH4
2.1.2. LiAlH4
2.1.3. Li3AlH6
2.1.4. LiNH2
2.1.5. Li-RHC (Reactive Hydride Composite)
2.1.6. LiBH4-Adducts—Ammoniates
2.1.7. LiNH2BH3 (Lithium Amidoborane)
2.1.8. Li-N-H System; Li3BN3H10
2.2. Na-Based Complex Hydrides
2.2.1. NaBH4
2.2.2. NaAlH4
2.3. Mg-Based Complex Hydrides
2.3.1. Mg(BH4)2
2.3.2. Mg(B3H8)2
2.3.3. Mg(BH4)2-Adducts/Ammoniates: Case of Mg(BH4)2.6NH3
2.3.4. Mg(NH2)2
2.3.5. Mg(AlH4)2
2.4. Other First Group-Derived Borohydrides
2.5. Ca-Based Complex Hydrides; Ca(BH4)2
2.6. Al-Based Complex Hydrides
2.7. TM- and RE-Based Complex Hydrides
2.7.1. Adducts/Ammoniates: Zr(BH4)4.8NH3
2.7.2. NaMgH3 and NaZn(BH4)3
2.7.3. XTiH3 (CaTiH3, MgTiH3)
2.7.4. Mg2NiH4
2.7.5. Mg2FeH6
2.7.6. K2Mn(NH2)4
2.7.7. Ti(BH4)4
2.7.8. (RE)(BH4)x
2.8. Ammonia Borane (AB) and Related Compounds
2.8.1. NH3BH3 (AB)
2.8.2. Tetraalkyl Ammonium Borohydrides [NR4][BH4]
3. Improvement Strategies and Most Encouraging Results—An Overview
3.1. Nanoconfinement
3.2. Destabilization
3.3. Cation/Anion Substitution
3.4. Eutectic Formation Approach
3.5. Doping Strategy
3.6. Electrolyte-Assisted Dehydrogenation
3.7. Additives
3.8. Electron-Tuning of the Scaffold
3.9. Host Modification
4. Outlook and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, T.; Cao, H.; Chen, P. Complex Hydrides for Energy Storage, Conversion, and Utilization. Adv. Mater. 2019, 31, 1902757. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, J.-H.; Kim, Y.-H.; Kim, J.-W.; Lee, K.-J.; Park, S.-J. Recent Progress Using Solid-State Materials for Hydrogen Storage: A Short Review. Processes 2022, 10, 304. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef] [Green Version]
- Comanescu, C. Complex Metal Borohydrides: From Laboratory Oddities to Prime Candidates in Energy Storage Applications. Materials 2022, 15, 2286. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 7111. [Google Scholar] [CrossRef]
- Hagemann, H. Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications. Molecules 2021, 26, 7425. [Google Scholar] [CrossRef]
- Hadjixenophontos, E.; Dematteis, E.M.; Berti, N.; Wołczyk, A.R.; Huen, P.; Brighi, M.; Le, T.T.; Santoru, A.; Payandeh, S.; Peru, F.; et al. A Review of the MSCA ITN ECOSTORE—Novel Complex Metal Hydrides for Efficient and Compact Storage of Renewable Energy as Hydrogen and Electricity. Inorganics 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Mohtadi, R.; Orimo, S.I. The Renaissance of Hydrides as Energy Materials. Nat. Rev. Mater. 2017, 2, 16091. [Google Scholar] [CrossRef] [Green Version]
- Comanescu, C. Recent Development in Nanoconfined Hydrides for Energy Storage. Int. J. Mol. Sci. 2022, 23, 7111. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Y.; Yang, J.; Sun, L.; Guo, D.; Xiao, P. Recent Advance of Metal Borohydrides for Hydrogen Storage. Front. Chem. 2022, 10, 945208. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Černý, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacher, F.; Jensen, T.R. Metal Borohydrides and Derivatives-Synthesis, Structure and Properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Amdisen, M.B.; Skov, L.N.; Møller, K.T.; Kristensen, L.G.; Polanski, M.; Heere, M.; Jensen, T.R. New Perspectives of Functional Metal Borohydrides. J. Alloys Compd. 2022, 896, 163014. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sandhya, K.S.; Fagg, D.P. Nanostructured Advanced Materials for Hydrogen Storage. In Nanomaterials for Sustainable Energy and Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2020; pp. 97–163. ISBN 9780128193556. [Google Scholar]
- Jepsen, L.H.; Paskevicius, M.; Jensen, T.R. 8 Nanostructured and Complex Hydrides for Hydrogen Storage. In Nanotechnology for Energy Sustainability, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 415–431, ISBN-13: 978-3527340149. [Google Scholar]
- Cuevas, F.; Amdisen, M.B.; Baricco, M.; Buckley, C.E.; Cho, Y.W.; de Jongh, P.; de Kort, L.M.; Grinderslev, J.B.; Gulino, V.; Hauback, B.C.; et al. Metallic and Complex Hydride-Based Electrochemical Storage of Energy. Prog. Energy 2022, 4, 032001. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for Hydrogen-Based Energy Storage—Past, Recent Progress and Future Outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- el Kharbachi, A.; Dematteis, E.M.; Shinzato, K.; Stevenson, S.C.; Bannenberg, L.J.; Heere, M.; Zlotea, C.; Szilágyi, P.; Bonnet, J.P.; Grochala, W.; et al. Metal Hydrides and Related Materials. Energy Carriers for Novel Hydrogen and Electrochemical Storage. J. Phys. Chem. C 2020, 124, 7599–7607. [Google Scholar] [CrossRef] [Green Version]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; An, C.; Wang, Y. Lightweight Hydrides Nanocomposites for Hydrogen Storage: Challenges, Progress and Prospects. Sci. China Mater. 2019, 62, 1597–1625. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Chen, K.; Jiang, J.; Yang, X.S.; Zhu, M. Hydrogen Storage in Light-Metal Based Systems: A Review. J. Alloys Compd. 2020, 829, 154597. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications:Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [Green Version]
- Zhao-Karger, Z.; Witter, R.; Bardají, E.G.; Wang, D.; Cossement, D.; Fichtner, M. Influence of Nanoconfinement on Reaction Pathways of Complex Metal Hydrides. Energy Procedia 2012, 29, 731–737. [Google Scholar] [CrossRef] [Green Version]
- de Kort, L.M.; Gulino, V.; de Jongh, P.E.; Ngene, P. Ionic Conductivity in Complex Metal Hydride-Based Nanocomposite Materials: The Impact of Nanostructuring and Nanocomposite Formation. J. Alloys Compd. 2022, 901, 163474. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. wen Hydrogen—A Sustainable Energy Carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Manickam, K.; Mistry, P.; Walker, G.; Grant, D.; Buckley, C.E.; Humphries, T.D.; Paskevicius, M.; Jensen, T.; Albert, R.; Peinecke, K.; et al. Future Perspectives of Thermal Energy Storage with Metal Hydrides. Int. J. Hydrogen Energy 2019, 44, 7738–7745. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q.; Li, J.; Xu, F.; Sun, L.; Zou, Y.; Chu, H.; Li, B.; Zhang, K. Enhanced Hydrogen Storage/Sensing of Metal Hydrides by Nanomodification. Mater. Today Nano 2020, 9, 100071. [Google Scholar] [CrossRef]
- Møller, K.T.; Sheppard, D.; Ravnsbæk, D.B.; Buckley, C.E.; Akiba, E.; Li, H.W.; Jensen, T.R. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef] [Green Version]
- Bannenberg, L.J.; Heere, M.; Benzidi, H.; Montero, J.; Dematteis, E.M.; Suwarno, S.; Jaron, T.; Winny, M.; Orłowski, P.A.; Wegner, W.; et al. Metal (boro-) hydrides for high energy density storage and relevant emerging technologies. Int. J. Hydrogen Energy 2020, 45, 33687–33730. [Google Scholar] [CrossRef]
- Callini, E.; Atakli, Z.Ö.K.; Hauback, B.C.; Orimo, S.I.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjörvarsson, B.; et al. Complex and Liquid Hydrides for Energy Storage. Appl. Phys. A Mater. Sci. Process 2016, 122, 353. [Google Scholar] [CrossRef]
- Salman, M.S.; Pratthana, C.; Lai, Q.; Wang, T.; Rambhujun, N.; Srivastava, K.; Aguey-Zinsou, K.-F. Catalysis in Solid Hydrogen Storage: Recent Advances, Challenges, and Perspectives. Energy Technol. 2022, 10, 2200433. [Google Scholar] [CrossRef]
- Gupta, A.; Baron, G.V.; Perreault, P.; Lenaerts, S.; Ciocarlan, R.G.; Cool, P.; Mileo, P.G.M.; Rogge, S.; van Speybroeck, V.; Watson, G.; et al. Hydrogen Clathrates: Next Generation Hydrogen Storage Materials. Energy Storage Mater. 2021, 41, 69–107. [Google Scholar] [CrossRef]
- DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 30 August 2022).
- Andersson, J.; Grönkvist, S. Large-Scale Storage of Hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, C.-G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X.J. Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage. Research 2021, 2021, 3750689. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous materials for hydrogen storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Liu, W.; Stoddart, J.F. Emergent behavior in nanoconfined molecular containers. Chem 2021, 7, 919–947. [Google Scholar] [CrossRef]
- Sreedhara, I.; Kamani, K.M.; Kamani, B.M.; Reddy, B.M.; Venugopal, A. A Bird’s Eye view on process and engineering aspects of hydrogen storage. Renew. Sust. Energy Rev. 2018, 91, 838–860. [Google Scholar] [CrossRef]
- Mohtadi, R. Beyond Typical Electrolytes for Energy Dense Batteries. Molecules 2020, 25, 1791. [Google Scholar] [CrossRef] [Green Version]
- Skripov, A.V.; Soloninin, A.V.; Babanova, O.A.; Skoryunov, R.V. Anion and Cation Dynamics in Polyhydroborate Salts: NMR Studies. Molecules 2020, 25, 2940. [Google Scholar] [CrossRef]
- Hwang, S.J.; Lee, H.S.; To, M.; Lee, Y.S.; Cho, Y.W.; Choi, H.; Kim, C. Probing Molecular Dynamics of Metal Borohydrides on the Surface of Mesoporous Scaffolds by Multinuclear High Resolution Solid State NMR. J. Alloys Compd. 2015, 645, S316–S319. [Google Scholar] [CrossRef]
- Lohstroh, W.; Heere, M. Structure and Dynamics of Borohydrides Studied by Neutron Scattering Techniques: A Review. J. Phys. Soc. Jpn. 2020, 89, 051011. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, T.; He, W.; Luo, Q.; Li, Z.; Zhang, W.; He, J.; Li, Q. Electron Microscope Investigation on Hydrogen Storage Materials: A Review. Int. J. Hydrogen Energy 2020, 45, 12048–12070. [Google Scholar] [CrossRef]
- Liu, Y.S.; Jeong, S.; White, J.L.; Feng, X.; Seon Cho, E.; Stavila, V.; Allendorf, M.D.; Urban, J.J.; Guo, J. In-Situ/Operando X-ray Characterization of Metal Hydrides. ChemPhysChem 2019, 20, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Miedema, P.S.; Ngene, P.; van der Eerden, A.M.J.; Sokaras, D.; Weng, T.C.; Nordlund, D.; Au, Y.S.; de Groot, F.M.F. In Situ X-Ray Raman Spectroscopy Study of the Hydrogen Sorption Properties of Lithium Borohydride Nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 22651–22658. [Google Scholar] [CrossRef] [Green Version]
- Sethio, D.; Daku, L.M.L.; Hagemann, H.; Kraka, E. Quantitative Assessment of B−B−B, B−Hb−B, and B−Ht Bonds: From BH3 to B12H122−. ChemPhysChem 2019, 20, 1967–1977. [Google Scholar] [CrossRef]
- Khalfaoui, M.; Knani, S.; Hachicha, M.A.; Lamine, A.B. New Theoretical Expressions for the Five Adsorption Type Isotherms Classified by BET Based on Statistical Physics Treatment. J. Colloid Interface Sci. 2003, 263, 350–356. [Google Scholar] [CrossRef]
- Broom, D.P.; Webb, C.J. Pitfalls in the characterisation of the hydrogen sorption properties of materials. Int. J. Hydrogen Energy 2017, 42, 29320–29343. [Google Scholar] [CrossRef]
- Broom, D.P.; Hirscher, M. Irreproducibility in Hydrogen Storage Material Research. Energy Env. Sci. 2016, 9, 3368–3380. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Cheng, Y.; Zhang, J. A Review of High Density Solid Hydrogen Storage Materials by Pyrolysis for Promising Mobile Applications. Ind. Eng. Chem. Res. 2021, 60, 2737–2771. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Huang, Z.; Li, H.-W.; Gao, M.; Pan, H.; Liu, Y. Recent Development of Lithium Borohydride-Based Materials for Hydrogen Storage. Adv. Energy Sustain. Res. 2021, 2, 2100073. [Google Scholar] [CrossRef]
- Puszkiel, J.; Gasnier, A.; Amica, G.; Gennari, F. Tuning LiBH4 for Hydrogen Storage: Destabilization, Additive, and Nanoconfinement Approaches. Molecules 2020, 25, 163. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Li, S.; Zhou, Y.; Chen, Z.; Yang, W.; Ma, W.; Shaw, L. LiBH4 for Hydrogen Storage—New Perspectives. Nano Mater. Sci. 2020, 2, 109–119. [Google Scholar] [CrossRef]
- Lai, Q.; Wang, T.; Sun, Y.; Aguey-Zinsou, K.F. Rational Design of Nanosized Light Elements for Hydrogen Storage: Classes, Synthesis, Characterization, and Properties. Adv. Mater Technol 2018, 3, 1700298. [Google Scholar] [CrossRef]
- Le, T.T.; Pistidda, C.; Nguyen, V.H.; Singh, P.; Raizada, P.; Klassen, T.; Dornheim, M. Nanoconfinement effects on hydrogen storage properties of MgH2 and LiBH4. Int. J. Hydrogen Energy 2021, 46, 23723–23736. [Google Scholar] [CrossRef]
- Łodziana, Z.; Błoński, P. Structure of Nanoconfined LiBH4 from First Principles 11B NMR Chemical Shifts Calculations. Int. J. Hydrogen Energy 2014, 39, 9842–9847. [Google Scholar] [CrossRef]
- Pratthana, C.; Aguey-Zinsou, K.-F. Surfactant Induced Synthesis of LiAlH4 and NaAlH4 Nanoparticles for Hydrogen Storage. Appl. Sci. 2022, 12, 4742. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.F. Synthesis of Borohydride Nanoparticles at Room Temperature by Precipitation. Int. J. Hydrogen Energy 2021, 46, 24286–24292. [Google Scholar] [CrossRef]
- Li, Y.; Chung, J.S.; Kang, S.G. First-Principles Rational Design of M-Doped LiBH4(010) Surface for Hydrogen Release: Role of Strain and Dopants (M = Na, K, Al, F, or Cl). Int. J. Hydrogen Energy 2019, 44, 6065–6073. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Z.; Ma, T.; Lu, C.T.; Ma, W.; Shaw, L. Predicting the Hydrogen Release Ability of LiBH4-Based Mixtures by Ensemble Machine Learning. Energy Storage Mater 2020, 27, 466–477. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; de Jongh, P.E.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4 Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef]
- Lambregts, S.F.H.; van Eck, E.R.H.; Suwarno; Ngene, P.; de Jongh, P.E.; Kentgens, A.P.M. Phase Behavior and Ion Dynamics of Nanoconfined LiBH4 in Silica. J. Phys. Chem. C 2019, 123, 25559–25569. [Google Scholar] [CrossRef] [Green Version]
- Zettl, R.; Gombotz, M.; Clarkson, D.; Greenbaum, S.G.; Ngene, P.; de Jongh, P.E.; Wilkening, H.M.R. Li-Ion Diffusion in Nanoconfined LiBH4-LiI/Al2O3: From 2D Bulk Transport to 3D Long-Range Interfacial Dynamics. ACS Appl. Mater. Interfaces 2020, 12, 38570–38583. [Google Scholar] [CrossRef]
- Cuan, J.; Zhou, Y.; Zhou, T.; Ling, S.; Rui, K.; Guo, Z.; Liu, H.; Yu, X. Borohydride-Scaffolded Li/Na/Mg Fast Ionic Conductors for Promising Solid-State Electrolytes. Adv. Mater. 2019, 31, 1803533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suwarno, S.; Nale, A.; Suwarta, P.; Wijayanti, I.D.; Ismail, M. Designing Nanoconfined LiBH4 for Solid-State Electrolytes. Front. Chem. 2022, 10, 866959. [Google Scholar] [CrossRef] [PubMed]
- de Kort, L.M.; Gulino, V.; Blanchard, D.; Ngene, P. Effects of LiBF4 Addition on the Lithium-Ion Conductivity of LiBH4. Molecules 2022, 27, 2187. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lee, Y.S.; Oh, K.H.; Cho, Y.W. Interface-Enhanced Li Ion Conduction in a LiBH4-SiO2 Solid Electrolyte. Phys. Chem. Chem. Phys. 2016, 18, 22540–22547. [Google Scholar] [CrossRef]
- Nguyen, J.; Fleutot, B.; Janot, R. Investigation of the Stability of Metal Borohydrides-Based Compounds LiM(BH4)3Cl (M = La, Ce, Gd) as Solid Electrolytes for Li-S Batteries. Solid State Ionics 2018, 315, 26–32. [Google Scholar] [CrossRef]
- Udovic, T.J.; Verdal, N.; Rush, J.J.; de Vries, D.J.; Hartman, M.R.; Vajo, J.J.; Gross, A.F.; Skripov, A.V. Mapping Trends in the Reorientational Mobilities of Tetrahydroborate Anions via Neutron-Scattering Fixed-Window Scans. J. Alloys Compd. 2013, 580, S47–S50. [Google Scholar] [CrossRef]
- Verkuijlen, M.H.W.; Ngene, P.; de Kort, D.W.; Barré, C.; Nale, A.; van Eck, E.R.H.; van Bentum, P.J.M.; de Jongh, P.E.; Kentgens, A.P.M. Nanoconfined LiBH4 and Enhanced Mobility of Li+ and BH4− Studied by Solid-State NMR. J. Phys. Chem. C 2012, 116, 22169–22178. [Google Scholar] [CrossRef] [Green Version]
- Breuer, S.; Uitz, M.; Wilkening, H.M.R. Rapid Li Ion Dynamics in the Interfacial Regions of Nanocrystalline Solids. J. Phys. Chem. Lett. 2018, 9, 2093–2097. [Google Scholar] [CrossRef]
- Ngene, P.; Lambregts, S.F.H.; Blanchard, D.; Vegge, T.; Sharma, M.; Hagemann, H.; de Jongh, P.E. The Influence of Silica Surface Groups on the Li-Ion Conductivity of LiBH4/SiO2 Nanocomposites. Phys. Chem. Chem. Phys. 2019, 21, 22456–22466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambregts, S.F.H.; van Eck, E.R.H.; Ngene, P.; Kentgens, A.P.M. The Nature of Interface Interactions Leading to High Ionic Conductivity in LiBH4/SiO2 Nanocomposites. ACS Appl. Energy Mater. 2022, 5, 8057–8066. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.; Nale, A.; Sveinbjörnsson, D.; Eggenhuisen, T.M.; Verkuijlen, M.H.W.; Suwarno; Vegge, T.; Kentgens, A.P.M.; de Jongh, P.E. Nanoconfined LiBH4 as a Fast Lithium Ion Conductor. Adv. Funct. Mater. 2015, 25, 184–192. [Google Scholar] [CrossRef]
- Ngene, P.; Verkuijlen, M.H.W.; Barre, C.; Kentgens, A.P.M.; de Jongh, P.E. Reversible Li-insertion in nanoscaffolds: A promising strategy to alter the hydrogen sorption properties of Li-based complex hydrides. Nano Energy 2016, 22, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Lai, Q.; Pratthana, C.; Yang, Y.; Rawal, A.; Aguey-Zinsou, K.F. Nanoconfinement of Complex Borohydrides for Hydrogen Storage. ACS Appl. Nano Mater. 2021, 4, 973–978. [Google Scholar] [CrossRef]
- Gasnier, A.; Amica, G.; Juan, J.; Troiani, H.; Gennari, F.C. N-Doped Graphene-Rich Aerogels Decorated with Nickel and Cobalt Nanoparticles: Effect on Hydrogen Storage Properties of Nanoconfined LiBH4. J. Phys. Chem. C 2020, 124, 115–125. [Google Scholar] [CrossRef]
- Lai, Q.; Yang, Y.; Aguey-Zinsou, K.-F. Nanoconfinement of borohydrides in hollow carbon spheres: Melt infiltration versus solvent impregnation for enhanced hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 23225–23238. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, X.; Liu, Y.; Zhang, L.; Hu, J.; Gao, M.; Pan, H. A Unique Double-Layered Carbon Nanobowl-Confined Lithium Borohydride for Highly Reversible Hydrogen Storage. Small 2020, 16, 2001963. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, H.-Z.; Gao, S.-C.; Wang, X.-H. Enhanced dehydrogenation kinetic properties and hydrogen storage reversibility of LiBH4 confined in activated charcoal. Trans. Nonferrous Met. Soc. China 2018, 28, 1618–1625. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Xian, K.; Li, Z.; Shen, Y.; Yao, Z.; Liu, Y.; Pan, H. LiBH4 Nanoconfined in Porous Hollow Carbon Nanospheres with High Loading, Low Dehydrogenation Temperature, Superior Kinetics, and Favorable Reversibility. ACS Appl. Energy Mater. 2020, 3, 3928–3938. [Google Scholar] [CrossRef]
- Wang, S.; Gao, M.; Yao, Z.; Liu, Y.; Wu, M.; Li, Z.; Liu, Y.; Sun, W.; Pan, H. A nanoconfined-LiBH4 system using a unique multifunctional porous scaffold of carbon wrapped ultrafine Fe3O4 skeleton for reversible hydrogen storage with high capacity. Chem. Eng. J. 2022, 428, 131056. [Google Scholar] [CrossRef]
- Sitthiwet, C.; Thiangviriya, S.; Thaweelap, N.; Meethom, S.; Kaewsuwan, D.; Chanlek, N.; Utke, R. Hydrogen sorption and permeability of compacted LiBH4 nanoconfined into activated carbon nanofibers impregnated with TiO2. J. Phys. Chem. Solids 2017, 110, 344–353. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, W.; Ren, Z.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Nano-synergy enables highly reversible storage of 9.2 wt% hydrogen at mild conditions with lithium borohydride. Nano Energy 2021, 83, 105839. [Google Scholar] [CrossRef]
- Martínez, A.A.; Gasnier, A.; Gennari, F.C. Pore Filling of a Carbon Matrix by Melt-Impregnated LiBH4. J. Phys. Chem. C 2022, 126, 66–78. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Zhang, L.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Single-pot solvothermal strategy toward support-free nanostructured LiBH4 featuring 12 wt% reversible hydrogen storage at 400 °C. Chem. Eng. J. 2022, 428, 132566. [Google Scholar] [CrossRef]
- Xian, K.; Nie, B.; Li, Z.; Gao, M.; Li, Z.; Shang, C.; Liu, Y.; Guo, Z.; Pan, H. TiO2 decorated porous carbonaceous network structures offer confinement, catalysis and thermal conductivity for effective hydrogen storage of LiBH4. Chem. Eng. J. 2021, 407, 127156. [Google Scholar] [CrossRef]
- Wu, R.; Ren, Z.; Zhang, X.; Lu, Y.; Li, H.; Gao, M.; Pan, H.; Liu, Y. Nanosheet-like Lithium Borohydride Hydrate with 10 wt% Hydrogen Release at 70 °C as a Chemical Hydrogen Storage Candidate. J. Phys. Chem. Lett. 2019, 10, 1872–1877. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Wang, S.; Zhang, X.; Gao, P.Y.; Yang, Y.X.; Sun, W.; Liu, Y.; Pan, H. In-situ introduction of highly active TiO for enhancing hydrogen storage performance of LiBH4. Chem. Eng. J. 2022, 433, 134485. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiao, L.; Liu, Y.; Guo, L.; Li, L.; Liu, H.; Wang, Y.; Yuan, H. A synergistic effect between nanoconfinement of carbon aerogels and catalysis of CoNiB nanoparticles on dehydrogenation of LiBH4. Int. J. Hydrogen Energy 2014, 39, 917–926. [Google Scholar] [CrossRef]
- Suwarno; Ngene, P.; Nale, A.; Eggenhuisen, T.M.; Oschatz, M.; Embs, J.P.; Remhof, A.; de Jongh, P.E. Confinement Effects for Lithium Borohydride: Comparing Silica and Carbon Scaffolds. J. Phys. Chem. C 2017, 121, 4197–4205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Li, Y.; Ke, D.; Lin, X.; Han, S.; Ma, M. Effect of Nano-Sized Ce2S3 on Reversible Hydrogen Storage Properties of LiBH4. Int. J. Hydrogen Energy 2016, 41, 13156–13162. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Kaewsuwan, D.; Chanlek, N.; Utke, R. Effects of Specific Surface Area and Pore Volume of Activated Carbon Nanofibers on Nanoconfinement and Dehydrogenation of LiBH4. Int. J. Hydrogen Energy 2017, 42, 6189–6201. [Google Scholar] [CrossRef]
- Zang, L.; Sun, W.; Liu, S.; Huang, Y.; Yuan, H.; Tao, Z.; Wang, Y. Enhanced Hydrogen Storage Properties and Reversibility of LiBH4 Confined in Two-Dimensional Ti3C2. ACS Appl. Mater. Interfaces 2018, 10, 19598–19604. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Zhang, Y.; Liu, D.; Wang, C.; Li, Y.; Si, T.; Zhang, Q. Enhanced Low-Temperature Hydrogen Storage in Nanoporous Ni-Based Alloy Supported LiBH4. Front. Chem. 2020, 8, 283. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, W.; Zhang, Y.; Zhao, X.; Wen, P.; Ma, D. Fe3O4 Nanoclusters Highly Dispersed on a Porous Graphene Support as an Additive for Improving the Hydrogen Storage Properties of LiBH4. RSC Adv. 2018, 8, 19353–19361. [Google Scholar] [CrossRef] [Green Version]
- Gasnier, A.; Gennari, F.C. Graphene Entanglement in a Mesoporous Resorcinol-Formaldehyde Matrix Applied to the Nanoconfinement of LiBH4 for Hydrogen Storage. RSC Adv. 2017, 7, 27905–27912. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Zhang, L.; Gao, S.; Liu, H.; Xu, L.; Wang, X.; Yan, M. Hydrogen Storage Properties of Activated Carbon Confined LiBH4 Doped with CeF3 as Catalyst. Int. J. Hydrogen Energy 2017, 42, 23010–23017. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wan, C.B.; Meng, X.H.; Ju, X. Improvement of the LiBH4 Hydrogen Desorption by Confinement in Modified Carbon Nanotubes. J. Alloys Compd. 2015, 645, S112–S116. [Google Scholar] [CrossRef]
- Shao, J.; Xiao, X.; Fan, X.; Zhang, L.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Low-Temperature Reversible Hydrogen Storage Properties of LiBH4: A Synergetic Effect of Nanoconfinement and Nanocatalysis. J. Phys. Chem. C 2014, 118, 11252–11260. [Google Scholar] [CrossRef]
- Dolotko, O.; Gupta, S.; Kobayashi, T.; McDonald, E.; Hlova, I.; Majzoub, E.; Balema, V.P.; Pruski, M.; Pecharsky, V.K. Mechanochemical Reactions and Hydrogen Storage Capacities in MBH4–SiS2 Systems (M = Li or Na). Int. J. Hydrogen Energy 2019, 44, 7381–7391. [Google Scholar] [CrossRef]
- Sofianos, M.V.; Sheppard, D.A.; Rowles, M.R.; Humphries, T.D.; Liu, S.; Buckley, C.E. Novel Synthesis of Porous Mg Scaffold as a Reactive Containment Vessel for LiBH4. RSC Adv. 2017, 7, 36340–36350. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Mao, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. Reaction Route Optimized LiBH4 for High Reversible Capacity Hydrogen Storage by Tunable Surface-Modified AlN. ACS Appl. Energy Mater. 2020, 3, 11964–11973. [Google Scholar] [CrossRef]
- Palade, P.; Comanescu, C.; Mercioniu, I. Improvements of hydrogen desorption of lithium borohydride by impregnation onto MSH-H carbon replica. J. Ovonic Res. 2012, 8, 155–160. [Google Scholar]
- Comanescu, C.; Guran, C.; Palade, P. Improvements of kinetic properties of LiBH4 by supporting on MSU-H type mesoporous silica. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 705–708. [Google Scholar]
- Vellingiri, L.; Annamalai, K.; Kandasamy, R.; Kombiah, I. Single-Walled Carbon Nanotubes/Lithium Borohydride Composites for Hydrogen Storage: Role of: In Situ Formed LiB(OH)4, Li2CO3 and LiBO2 by Oxidation and Nitrogen Annealing. RSC Adv. 2019, 9, 31483–31496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Zhu, D.; Wu, X.; Dong, D.; Jiang, X.; Xu, M. The Dehydrogenation Mechanism and Reversibility of LiBH4 Doped by Active Al Derived from AlH3. Metals 2019, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Alcántara, K.; Tena-Garcia, J.R.; Guerrero-Ortiz, R. Alanates, a Comprehensive Review. Materials 2019, 12, 2724. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Xu, F.; Zhang, C.; Wang, Z.; Ju, H.; Gao, X.; Zhang, X.; Sun, L.; Liu, Z. Enhanced Hydrogen Storage of Alanates: Recent Progress and Future Perspectives. Prog. Nat. Sci. Mater. Int. 2021, 31, 165–179. [Google Scholar] [CrossRef]
- Somo, T.R.; Mabokela, T.E.; Teffu, D.M.; Sekgobela, T.K.; Hato, M.J.; Modibane, K.D. Review on the Effect of Metal Oxides as Surface Coatings on Hydrogen Storage Properties of Porous and Non-Porous Materials. Chem. Pap. 2021, 75, 2237–2251. [Google Scholar] [CrossRef]
- Milanese, C.; Garroni, S.; Gennari, F.; Marini, A.; Klassen, T.; Dornheim, M.; Pistidda, C. Solid State Hydrogen Storage in Alanates and Alanate-Based Compounds: A Review. Metals 2018, 8, 567. [Google Scholar] [CrossRef] [Green Version]
- Weidenthaler, C. Crystal Structure Evolution of Complex Metal Aluminum Hydrides upon Hydrogen Release. J. Energy Chem. 2020, 42, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Sazelee, N.A.; Ismail, M. Recent Advances in Catalyst-Enhanced LiAlH4 for Solid-State Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2021, 46, 9123–9141. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J. Hydrogen Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, S.; Huang, Q.; Li, J.; Cen, X.; Zhang, H.; Chu, H.; Sun, L.; Xu, F.; Huang, P. Facile synthesis of NiCo2O4-anchored reduced graphene oxide nanocomposites as efficient additives for improving the dehydrogenation behavior of lithium alanate. Inorg. Chem. Front. 2020, 7, 1257–1272. [Google Scholar] [CrossRef]
- Cho, Y.J.; Li, S.; Snider, J.L.; Marple, M.A.T.; Strange, N.A.; Sugar, J.D.; Gabaly, F.E.; Schneemann, A.; Kang, S.; Kang, M.-h.; et al. Reversing the Irreversible: Thermodynamic Stabilization of LiAlH4 Nanoconfined Within a Nitrogen-Doped Carbon Host. ACS Nano 2021, 15, 10163–10174. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Sun, Y.; Sun, L.; Xu, F.; Sun, S.; Zhang, G.; Huang, P.; Du, Y.; Wang, J.; et al. Dehybridization effect in improved dehydrogenation of LiAlH4 by doping with two-dimensional Ti3C2. Mater. Today Nano 2019, 8, 100054. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Ismail, M. Catalytic effect of SrFe12O19 on the hydrogen storage properties of LiAlH4. Int. J. Hydrogen Energy 2017, 42, 19126–19134. [Google Scholar] [CrossRef]
- Li, Z.L.; Zhai, F.Q.; Qiu, H.C.; Wan, Q.; Li, P.; Qu, X.H. Dehydrogenation Characteristics of ZrC-Doped LiAlH4 with Different Mixing Conditions. Rare Met. 2020, 39, 383–391. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Zhu, D.; He, J.; Xiao, X.; Chen, L. Dehydrogenation Performances of Different Al Source Composite Systems of 2LiBH4 + M (M = Al, LiAlH4, Li3AlH6). Front. Chem. 2020, 8, 227. [Google Scholar] [CrossRef] [Green Version]
- Sazelee, N.A.; Yahya, M.S.; Ali, N.A.; Idris, N.H.; Ismail, M. Enhancement of Dehydrogenation Properties in LiAlH4 Catalysed by BaFe12O19. J. Alloys Compd. 2020, 835, 155183. [Google Scholar] [CrossRef]
- Ali, N.A.; Sazelee, N.; Yahya, M.S.; Ismail, M. Influence of K2NbF7 Catalyst on the Desorption Behavior of LiAlH4. Front. Chem. 2020, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ko, Y.; Lombardo, L.; Li, M.; Oveisí, E.; Züttel, A. Interfacial Effect between Aluminum-Based Complex Hydrides and Nickel-Containing Porous Carbon Sheets. ACS Appl. Energy Mater. 2020, 3, 9685–9695. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, M.; Wang, H.; Chen, C.; Chen, J. LiAlH4 Supported on TiO2/Hierarchically Porous Carbon Nanocomposites with Enhanced Hydrogen Storage Properties. Inorg. Chem. Front. 2016, 3, 1536–1542. [Google Scholar] [CrossRef]
- Pratthana, C.; Yang, Y.; Rawal, A.; Aguey-Zinsou, K.F. Nanoconfinement of Lithium Alanate for Hydrogen Storage. J. Alloys Compd. 2022, 926, 166834. [Google Scholar] [CrossRef]
- Humphries, T.D.; Birkmire, D.; McGrady, G.S.; Hauback, B.C.; Jensen, C.M. Regeneration of LiAlH4 at Sub-Ambient Temperatures Studied by Multinuclear NMR Spectroscopy. J. Alloys Compd. 2017, 723, 1150–1154. [Google Scholar] [CrossRef] [Green Version]
- Graetz, J.; Wegrzyn, J.; Reilly, J.J. Regeneration of Lithium Aluminum Hydride. J. Am. Chem. Soc. 2008, 130, 17790–17794. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, J.; Xiao, X.; Liu, Z.; Ren, X.; Wang, X.; Li, S.; Chen, L. Ultra-Fast Dehydrogenation Behavior at Low Temperature of LiAlH4 Modified by Fluorographite. Int. J. Hydrogen Energy 2020, 45, 28123–28133. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Isobe, S.; Ohki, T.; Hashimoto, N. Unique Hydrogen Desorption Properties of LiAlH4/h-BN Composites. Inorganics 2017, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Yahya, M.S.; Ali, N.A.; Sazelee, N.A.; Mustafa, N.S.; Halim Yap, F.A.; Ismail, M. Intensive Investigation on Hydrogen Storage Properties and Reaction Mechanism of the NaBH4-Li3AlH6 Destabilized System. Int. J. Hydrogen Energy 2019, 44, 21965–21978. [Google Scholar] [CrossRef]
- Wood, B.C.; Stavila, V.; Poonyayant, N.; Heo, T.W.; Ray, K.G.; Klebanoff, L.E.; Udovic, T.J.; Lee, J.R.I.; Angboonpong, N.; Sugar, J.D.; et al. Nanointerface-Driven Reversible Hydrogen Storage in the Nanoconfined Li–N–H System. Adv. Mater. Interfaces 2017, 4, 1600803. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, F.; Liu, Y.; Zhang, Y.; Wang, X.; Pang, Y.; Zheng, S. Li2(BH4)(NH2) Nanoconfined in SBA-15 as Solid-State Electrolyte for Lithium Batteries. Nanomaterials 2021, 11, 946. [Google Scholar] [CrossRef]
- de Kort, L.M.; Harmel, J.; de Jongh, P.E.; Ngene, P. The effect of nanoscaffold porosity and surface chemistry on the Li-ion conductivity of LiBH4-LiNH2/metal oxide nanocomposites. J. Mater. Chem. A 2020, 8, 20687–20697. [Google Scholar] [CrossRef]
- White, J.L.; Baker, A.A.; Marcus, M.A.; Snider, J.L.; Wang, T.C.; Lee, J.R.I.; Kilcoyne, D.A.L.; Allendorf, M.D.; Stavila, V.; Gabaly, F.E. The Inside-Outs of Metal Hydride Dehydrogenation: Imaging the Phase Evolution of the Li-N-H Hydrogen Storage System. Adv. Mater. Interfaces 2020, 7, 1901905. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Jia, X.; Guo, J.; Wang, J.; Chen, P. Metathesis of Mg2FeH6 and LiNH2 Leading to Hydrogen Production at Low Temperatures. Phys. Chem. Chem. Phys. 2018, 20, 9833–9837. [Google Scholar] [CrossRef]
- Ali, N.A.; Sazelee, N.A.; Ismail, M. An overview of reactive hydride composite (RHC) for solid-state hydrogen storage materials. Int. J. Hydrogen Energy 2021, 46, 31674–31698. [Google Scholar] [CrossRef]
- Zettl, R.; de Kort, L.; Gombotz, M.; Wilkening, H.M.R.; de Jongh, P.E.; Ngene, P. Combined Effects of Anion Substitution and Nanoconfinement on the Ionic Conductivity of Li-Based Complex Hydrides. J. Phys. Chem. C 2020, 124, 2806–2816. [Google Scholar] [CrossRef] [Green Version]
- Le, T.T.; Pistidda, C.; Abetz, C.; Georgopanos, P.; Garroni, S.; Capurso, G.; Milanese, C.; Puszkiel, J.; Dornheim, M.; Abetz, V.; et al. Enhanced Stability of Li-RHC Embedded in an Adaptive TPX™ Polymer Scaffold. Materials 2020, 13, 991. [Google Scholar] [CrossRef] [Green Version]
- Jin, O.; Shang, Y.; Huang, X.; Mu, X.; Szabó, D.V.; Le, T.T.; Wagner, S.; Kübel, C.; Pistidda, C.; Pundt, A. Microstructural Study of MgB2 in the LiBH4-MgH2 Composite by Using TEM. Nanomaterials 2022, 12, 1893. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Bosenberg, U.; Gosalawit, R.; Dornheim, M.; Cerenius, Y.; Besenbacher, F.; Jensen, T.R. A Reversible Nanoconfined Chemical Reaction. ACS Nano 2010, 4, 3903–3908. [Google Scholar] [CrossRef]
- Gamba, N.S.; Puszkiel, J.; Arneodo Larochette, P.; Gennari, F.C. Dual Application of Ti-Catalyzed Li-RHC Composite for H2 Purification and CO Methanation. Int. J. Hydrogen Energy 2020, 45, 19493–19504. [Google Scholar] [CrossRef]
- Neves, A.M.; Puszkiel, J.; Capurso, G.; Bellosta von Colbe, J.M.; Milanese, C.; Dornheim, M.; Klassen, T.; Jepsen, J. Modeling the Kinetic Behavior of the Li-RHC System for Energy-Hydrogen Storage: (I) Absorption. Int. J. Hydrogen Energy 2021, 46, 32110–32125. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Liu, H.; Yan, M. Enhanced hydrogen storage properties of 2LiBH4-LiAlH4 nanoconfined in resorcinol formaldehyde carbon aerogel. J. Alloys Compd. 2017, 726, 525–531. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Javadian, P.; Jensen, N.D.; Nielsen, U.G.; Jensen, T.R.; Utke, R. Compaction of LiBH4-LiAlH4 nanoconfined in activated carbon nanofibers: Dehydrogenation kinetics, reversibility, and mechanical stability during cycling. Int. J. Hydrogen Energy 2017, 42, 1036–1047. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen Desorption Properties of Bulk and Nanoconfined LiBH4-NaAlH4. Crystals 2016, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Yao, Z.; Xiao, X.; Wang, X.; He, J.; Chen, M.; Cheng, H.; Zhang, L.; Chen, L. Enhanced hydrogen storage properties of high-loading nanoconfined LiBH4-Mg(BH4)2 composites with porous hollow carbon nanospheres. Int. J. Hydrogen Energy 2021, 46, 852–864. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, X.; He, Y.; Yao, Z.; Ye, X.; Kou, H.; Chen, C.; Huang, T.; Fan, X.; Chen, L. Probing an intermediate state by X-ray absorption near-edge structure in nickel-doped 2LiBH4-MgH2 reactive hydride composite at moderate temperature. Mater. Today Nano 2020, 12, 100090. [Google Scholar] [CrossRef]
- Dansirima, P.; Thiangviriya, S.; Plerdsranoy, P.; Utke, O.; Utke, R. Small hydrogen storage tank filled with 2LiBH4-MgH2 nanoconfined in activated carbon: Reaction mechanisms and performances. Int. J. Hydrogen Energy 2019, 44, 10752–10762. [Google Scholar] [CrossRef]
- Utke, R.; Thiangviriya, S.; Javadian, P.; Jensen, T.R.; Milanese, C.; Klassen, T.; Dornheim, M. 2LiBH4–MgH2 nanoconfined into carbon aerogel scaffold impregnated with ZrCl4 for reversible hydrogen storage. Mater. Chem. Phys. 2016, 169, 136–141. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Pistidda, C.; Dornheim, M.; Baricco, M. Exploring Ternary and Quaternary Mixtures in the LiBH4-NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2 System. ChemPhysChem 2019, 20, 1348–1359. [Google Scholar] [CrossRef] [Green Version]
- Bergemann, N.; Pistidda, C.; Uptmoor, M.; Milanese, C.; Santoru, A.; Emmler, T.; Puszkiel, J.; Dornheim, M.; Klassen, T. A new mutually destabilized reactive hydride system: LiBH4–Mg2NiH4. J. Energy Chem. 2019, 34, 240–254. [Google Scholar] [CrossRef] [Green Version]
- Gosalawit-Utke, R.; Milanese, C.; Javadian, P.; Girella, A.; Laipple, D.; Puszkiel, J.; Cattaneo, A.S.; Ferrara, C.; Wittayakhun, J.; Skibsted, J.; et al. 2LiBH4–MgH2–0.13TiCl4 confined in nanoporous structure of carbon aerogel scaffold for reversible hydrogen storage. J. Alloys Compd. 2014, 599, 78–86. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Roedern, E.; Pinatel, E.R.; Corno, M.; Jensen, T.R.; Baricco, M. A thermodynamic investigation of the LiBH4-NaBH4 system. RSC Adv. 2016, 6, 60101–60108. [Google Scholar] [CrossRef]
- Liu, X.; Peaslee, D.; Sheehan, T.P.; Majzoub, E.H. Decomposition Behavior of Eutectic LiBH4-Mg(BH4)2 and Its Confinement Effects in Ordered Nanoporous Carbon. J. Phys. Chem. C 2014, 118, 27265–27271. [Google Scholar] [CrossRef]
- Liu, Y.; Heere, M.; Contreras Vasquez, L.; Paterakis, C.; Sørby, M.H.; Hauback, B.C.; Book, D. Dehydrogenation and Rehydrogenation of a 0.62LiBH4-0.38NaBH4 Mixture with Nano-Sized Ni. Int. J. Hydrogen Energy 2018, 43, 16782–16792. [Google Scholar] [CrossRef]
- Lee, H.S.; Hwang, S.J.; To, M.; Lee, Y.S.; Cho, Y.W. Discovery of Fluidic LiBH4 on Scaffold Surfaces and Its Application for Fast Co-Confinement of LiBH4-Ca(BH4)2 into Mesopores. J. Phys. Chem. C 2015, 119, 9025–9035. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Cheng, H.; Zhu, Y.; Li, L.; Lin, H. Effects of Two-Dimension MXene Ti3C2 on Hydrogen Storage Performances of MgH2-LiAlH4 Composite. Chem. Phys. 2019, 522, 178–187. [Google Scholar] [CrossRef]
- Vajo, J.J.; Tan, H.; Ahn, C.C.; Addison, D.; Hwang, S.J.; White, J.L.; Wang, T.C.; Stavila, V.; Graetz, J. Electrolyte-Assisted Hydrogen Storage Reactions. J. Phys. Chem. C 2018, 122, 26845–26850. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Liu, J.; Zhao, Y.; Zhang, J.; Zhu, Y.; Zhang, Y.; Liu, Y.; Li, L. Enhanced Dehydrogenation Properties of LiAlH4-Mg2NiH4 Nanocomposites via Doping Ti-Based Catalysts. Mater. Res. Express 2019, 6, 075067. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, Y.S.; Suh, J.Y.; Kim, M.; Yu, J.S.; Cho, Y.W. Enhanced Desorption and Absorption Properties of Eutectic LiBH4-Ca(BH4)2 Infiltrated into Mesoporous Carbon. J. Phys. Chem. C 2011, 115, 20027–20035. [Google Scholar] [CrossRef]
- Javadian, P.; Jensen, T.R. Enhanced Hydrogen Reversibility of Nanoconfined LiBH4-Mg(BH4)2. Int. J. Hydrogen Energy 2014, 39, 9871–9876. [Google Scholar] [CrossRef]
- Meethom, S.; Kaewsuwan, D.; Chanlek, N.; Utke, O.; Utke, R. Enhanced Hydrogen Sorption of LiBH4–LiAlH4 by Quenching Dehydrogenation, Ball Milling, and Doping with MWCNTs. J. Phys. Chem. Solids 2020, 136, 109202. [Google Scholar] [CrossRef]
- Lu, C.; Zou, J.; Zeng, X.; Ding, W.; Shao, H. Enhanced Hydrogen Sorption Properties of Core-Shell like Structured Mg@NaBH4/MgB2 Composite. J. Alloys Compd. 2019, 810, 151763. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Yahya, M.S.; Itam Sulaiman, N.N.; Abdul Halim Yap, M.F.A.; Ismail, M. Enhanced the Hydrogen Storage Properties and Reaction Mechanisms of 4MgH2 + LiAlH4 Composite System by Addition with TiO2. Int. J. Energy Res. 2021, 45, 21365–21374. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Sheng, P.; Liu, S.; Zhao, G.; Wang, B.; Wang, X.; Yan, M. Hydrogen Desorption Kinetics of the Destabilized LiBH4–AlH3 Composites. Int. J. Hydrogen Energy 2017, 42, 22358–22365. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Zhang, W.; Gan, Y.; Xia, Y.; Zhang, J.; Huang, H.; Liang, C.; Pan, H. Hydrogen Pressure-Dependent Dehydrogenation Performance of the Mg(NH2)2-2LiH-0.07KOH System. ACS Appl. Mater. Interfaces 2020, 12, 15255–15261. [Google Scholar] [CrossRef]
- Thiangviriya, S.; Plerdsranoy, P.; Wiset, N.; Javadian, P.; Jensen, T.R.; Utke, R. Hydrogen Sorption and Reaction Mechanisms of Nanoconfined 2LiBH4-NaAlH4. J Alloys Compd. 2015, 633, 484–493. [Google Scholar] [CrossRef]
- Peru, F.; Payandeh, S.; Charalambopoulou, G.; Jensen, T.R.; Steriotis, T. Hydrogen Sorption and Reversibility of the LiBH4-KBH4 Eutectic System Confined in a CMK-3 Type Carbon via Melt Infiltration. C J. Carbon Res. 2020, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Heere, M.; GharibDoust, S.H.P.; Brighi, M.; Frommen, C.; Sørby, M.H.; Černý, R.; Jensen, T.R.; Hauback, B.C. Hydrogen Sorption in Erbium Borohydride Composite Mixtures with LiBH4 and/or LiH. Inorganics 2017, 5, 31. [Google Scholar] [CrossRef]
- Thiangviriya, S.; Sitthiwet, C.; Plerdsranoy, P.; Capurso, G.; Pistidda, C.; Utke, O.; Dornheim, M.; Klassen, T.; Utke, R. Hydrogen Sorption Kinetics, Hydrogen Permeability, and Thermal Properties of Compacted 2LiBH4-MgH2 Doped with Activated Carbon Nanofibers. Int. J. Hydrogen Energy 2019, 44, 15218–15227. [Google Scholar] [CrossRef]
- Wang, J.; Lei, G.; Pistidda, C.; He, T.; Cao, H.; Dornheim, M.; Chen, P. Hydrogen Storage Properties and Reaction Mechanisms of K2Mn(NH2)4–8LiH System. Int. J. Hydrogen Energy 2021, 46, 40196–40202. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen Storage Properties of Nanoconfined LiBH4-NaBH4. Int. J. Hydrogen Energy 2015, 40, 14916–14924. [Google Scholar] [CrossRef] [Green Version]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen Storage Properties of Nanoconfined LiBH4-Ca(BH4)2. Nano Energy 2015, 11, 96–103. [Google Scholar] [CrossRef]
- Sartori, S.; Knudsen, K.D.; Hage, F.S.; Heyn, R.H.; Bardaji, E.G.; Zhao-Karger, Z.; Fichtner, M.; Hauback, B.C. Influence of Nanoconfinement on Morphology and Dehydrogenation of the Li11BD4-Mg(11BD4)2 System. Nanotechnology 2012, 23, 255704. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, W.; Pistidda, C.; Puszkiel, J.; Milanese, C.; Santoru, A.; Karimi, F.; Castro Riglos, M.V.; Gizer, G.; Welter, E.; et al. Kinetic Alteration of the 6Mg(NH2)2-9LiH-LiBH4 System by Co-Adding YCl3 and Li3N. Phys. Chem. Chem. Phys. 2017, 19, 32105–32115. [Google Scholar] [CrossRef] [PubMed]
- Gosalawit-Utke, R.; Milanese, C.; Javadian, P.; Jepsen, J.; Laipple, D.; Karmi, F.; Puszkiel, J.; Jensen, T.R.; Marini, A.; Klassen, T.; et al. Nanoconfined 2LiBH4-MgH2-TiCl3 in Carbon Aerogel Scaffold for Reversible Hydrogen Storage. Int. J. Hydrogen Energy 2013, 38, 3275–3282. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Meng, Q.; Guo, Z.; Gu, Q.; Liu, H.; Liu, Z.; Yu, X. Nanoconfinement Significantly Improves the Thermodynamics and Kinetics of Co-Infiltrated 2LiBH4-LiAlH4 Composites: Stable Reversibility of Hydrogen Absorption/Resorption. Acta Mater. 2013, 61, 6882–6893. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Shaw, L. New Insights into the Solid-State Hydrogen Storage of Nanostructured LiBH4-MgH2 System. Chem. Eng. J. 2020, 385, 123856. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Santoru, A.; Poletti, M.G.; Pistidda, C.; Klassen, T.; Dornheim, M.; Baricco, M. Phase Stability and Hydrogen Desorption in a Quinary Equimolar Mixture of Light-Metals Borohydrides. Int. J. Hydrogen Energy 2018, 43, 16793–16803. [Google Scholar] [CrossRef]
- Javadian, P.; Gharibdoust, S.H.P.; Li, H.W.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Reversibility of LiBH4 Facilitated by the LiBH4-Ca(BH4)2 Eutectic. J. Phys. Chem. C 2017, 121, 18439–18449. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismail, M. Significant Effect of TiF3 on the Performance of 2NaAlH4 + Ca(BH4)2 Hydrogen Storage Properties. Int. J. Hydrogen Energy 2019, 44, 21979–21987. [Google Scholar] [CrossRef]
- Xian, K.; Gao, M.; Li, Z.; Gu, J.; Shen, Y.; Wang, S.; Yao, Z.; Liu, Y.; Pan, H. Superior Kinetic and Cyclic Performance of a 2D Titanium Carbide Incorporated 2LiH + MgB2 Composite toward Highly Reversible Hydrogen Storage. ACS Appl. Energy Mater. 2019, 2, 4853–4864. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Utke, R. Ternary LiBH4-MgH2-NaAlH4 Hydride Confined into Nanoporous Carbon Host for Reversible Hydrogen Storage. J. Phys. Chem. Solids 2016, 90, 80–86. [Google Scholar] [CrossRef]
- Li, G.; Matsuo, M.; Takagi, S.; Chaudhary, A.-L.; Sato, T.; Dornheim, M.; Orimo, S.-i. Thermodynamic Properties and Reversible Hydrogenation of LiBH4–Mg2FeH6 Composite Materials. Inorganics 2017, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Zhang, X.; Li, H.-W.; Huang, Z.; Hu, J.; Gao, M.; Pan, H.; Liu, Y. Titanium Hydride Nanoplates Enable 5 Wt% of Reversible Hydrogen Storage by Sodium Alanate below 80 °C. Research 2021, 2021, 9819176. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, X.; Xia, G.; Yu, X. Efficient Chemical Regeneration of LiBH4·NH3 Spent Fuel for Hydrogen Storage. Int. J. Hydrogen Energy 2015, 40, 146–150. [Google Scholar] [CrossRef]
- Li, S.; Sun, W.; Tang, Z.; Guo, Y.; Yu, X. Nanoconfinement of LiBH4·NH3 towards Enhanced Hydrogen Generation. Int. J. Hydrogen Energy 2012, 37, 3328–3337. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Wang, S.; Li, Z.; Guo, X.; Ye, J.; Jiang, L. Current Progress and Research Trends on Lithium Amidoborane for Hydrogen Storage. J. Mater. Sci. 2020, 55, 2645–2660. [Google Scholar] [CrossRef]
- Liu, X.; Peaslee, D.; Majzoub, E.H. Tailoring the Hydrogen Storage Properties of Li4BN3H10 by Confinement into Highly Ordered Nanoporous Carbon. J. Mater. Chem. A Mater. 2013, 1, 3926–3931. [Google Scholar] [CrossRef]
- Salman, M.S.; Rawal, A.; Aguey-Zinsou, K.-F. Tunable NaBH4 Nanostructures Revealing Structure-Dependent Hydrogen Release. Adv. Energy Sustain. Res. 2021, 2, 2100063. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.-F. Controlling the growth of NaBH4 nanoparticles for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 2054–2067. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Vaunois, S.; Pistidda, C.; Dornheim, M.; Baricco, M. Reactive Hydride Composite of Mg2NiH4 with Borohydrides Eutectic Mixtures. Crystals 2018, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Aguey-Zinsou, K.-F. Direct Synthesis of NaBH4 Nanoparticles from NaOCH3 for Hydrogen Storage. Energies 2019, 12, 4428. [Google Scholar] [CrossRef]
- Huang, T.; Zou, J.; Liu, H.; Ding, W. Effect of Different Transition Metal Fluorides TMFx (TM = Nb, Co, Ti) on Hydrogen Storage Properties of the 3NaBH4-GdF3 System. J. Alloys Compd. 2020, 823, 153716. [Google Scholar] [CrossRef]
- Huang, T.; Zou, J.; Zeng, X.; Wang, J.; Liu, H.; Ding, W. Reversible Hydrogen Sorption Behaviors of the 3NaBH4-(x)YF3-(1-x)GdF3 System: The Effect of Double Rare Earth Metal Cations. Int. J. Hydrogen Energy 2019, 44, 4868–4877. [Google Scholar] [CrossRef]
- Huang, T.; Zou, J.; Zhao, N.; Zeng, X.; Ding, W. Reversible Hydrogen Storage System of 3NaBH4-0.5ScF3-0.5YF3: The Synergistic Effect of ScF3 and YF3. J. Alloys Compd. 2019, 791, 1270–1276. [Google Scholar] [CrossRef]
- Orłowski, P.A.; Grochala, W. Effect of Vanadium Catalysts on Hydrogen Evolution from NaBH4. Solids 2022, 3, 21. [Google Scholar] [CrossRef]
- Ali, N.A.; Yahya, M.S.; Mustafa, N.S.; Sazelee, N.A.; Idris, N.H.; Ismail, M. Modifying the Hydrogen Storage Performances of NaBH4 by Catalyzing with MgFe2O4 Synthesized via Hydrothermal Method. Int. J. Hydrogen Energy 2019, 44, 6720–6727. [Google Scholar] [CrossRef]
- Miyazaki, R.; Onishi, K.; Miyagawa, R.; Hihara, T. Static Observation of the Interphase between NaBH4 and LiI during the Conversion Reaction. J. Solid State Chem. 2021, 303, 122496. [Google Scholar] [CrossRef]
- Boran, A.; Erkan, S.; Eroglu, I. Hydrogen Generation from Solid State NaBH4 by Using FeCl3 Catalyst for Portable Proton Exchange Membrane Fuel Cell Applications. Int. J. Hydrogen Energy 2019, 44, 18915–18926. [Google Scholar] [CrossRef]
- Li, L.; Xu, C.; Chen, C.; Wang, Y.; Jiao, L.; Yuan, H. Sodium Alanate System for Efficient Hydrogen Storage. Int. J. Hydrogen Energy 2013, 38, 8798–8812. [Google Scholar] [CrossRef]
- Rossin, A.; Tuci, G.; Luconi, L.; Giambastiani, G. Metal–Organic Frameworks as Heterogeneous Catalysts in Hydrogen Production from Lightweight Inorganic Hydrides. ACS Catal. 2017, 7, 5035–5045. [Google Scholar] [CrossRef]
- NaraseGowda, S.; Brown, C.M.; Tyagi, M.; Jenkins, T.; Dobbins, T.A. Quasi-Elastic Neutron Scattering Studies of Hydrogen Dynamics for Nanoconfined NaAlH4. J. Phys. Chem. C 2016, 120, 14863–14873. [Google Scholar] [CrossRef]
- Luo, X.; Rawal, A.; Aguey-Zinsou, K.-F. Investigating the Factors Affecting the Ionic Conduction in Nanoconfined NaBH4. Inorganics 2021, 9, 2. [Google Scholar] [CrossRef]
- Huen, P.; Peru, F.; Charalambopoulou, G.; Steriotis, T.A.; Jensen, T.R.; Ravnsbæk, D.B. Nanoconfined NaAlH4 Conversion Electrodes for Li Batteries. ACS Omega 2017, 2, 1956–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohstroh, W.; Roth, A.; Hahn, H.; Fichtner, M. Thermodynamic Effects in Nanoscale NaAlH4. ChemPhysChem 2010, 11, 789–792. [Google Scholar] [CrossRef]
- Bonatto Minella, C.; Lindemann, I.; Nolis, P.; Kießling, A.; Baró, M.D.; Klose, M.; Giebeler, L.; Rellinghaus, B.; Eckert, J.; Schultz, L.; et al. NaAlH4 Confined in Ordered Mesoporous Carbon. Int. J. Hydrogen Energy 2013, 38, 8829–8837. [Google Scholar] [CrossRef]
- Verkuijlen, M.H.W.; Gao, J.; Adelhelm, P.; van Bentum, P.J.M.; de Jongh, P.E.; Kentgens, A.P.M. Solid-State NMR Studies of the Local Structure of NaAlH4/C Nanocomposites at Different Stages of Hydrogen Desorption and Rehydrogenation. J. Phys. Chem. C 2010, 114, 4683–4692. [Google Scholar] [CrossRef]
- Javadian, P.; Nielsen, T.K.; Ravnsbæk, D.B.; Jepsen, L.H.; Polanski, M.; Plocinski, T.; Kunce, I.; Besenbacher, F.; Bystrzycki, J.; Jensen, T.R. Scandium Functionalized Carbon Aerogel: Synthesis of Nanoparticles and Structure of a New ScOCl and Properties of NaAlH4 as a Function of Pore Size. J. Solid State Chem. 2015, 231, 190–197. [Google Scholar] [CrossRef]
- Stavila, V.; Bhakta, R.K.; Alam, T.M.; Majzoub, E.H.; Allendorf, M.D. Reversible Hydrogen Storage by NaAlH4 Confined within a Titanium-Functionalized MOF-74(Mg) Nanoreactor. ACS Nano 2012, 6, 9807–9817. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.Z.; Zhang, Y.; Liu, D.M.; Wang, C.Y.; Si, T.Z.; Li, Y.T.; Zhang, Q.A. Coupling of nanoconfinement with metallic catalysis in supported NaAlH4 for low-temperature hydrogen storage. J. Power Sources 2021, 491, 229611. [Google Scholar] [CrossRef]
- EHuang, Y.; Shao, H.; Zhang, Q.; Zang, L.; Guo, H.; Liu, Y.; Jiao, L.; Yuan, H.; Wang, Y. Layer-by-layer uniformly confined Graphene-NaAlH4 composites and hydrogen storage performance. Int. J. Hydrogen Energy 2020, 45, 28116–28122. [Google Scholar] [CrossRef]
- Paskevicius, M.; Filsø, U.; Karimi, F.; Puszkiel, J.; Pranzas, P.K.; Pistidda, C.; Hoell, A.; Welter, E.; Schreyer, A.; Klassen, T.; et al. Cyclic stability and structure of nanoconfined Ti-doped NaAlH4. Int. J. Hydrogen Energy 2016, 41, 4159–4167. [Google Scholar] [CrossRef]
- Beatrice, C.A.G.; Moreira, B.R.; de Oliveira, A.D.; Passador, F.R.; de Almeida Neto, G.R.; Leiva, D.R.; Pessan, L.A. Development of polymer nanocomposites with sodium alanate for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 5337–5346. [Google Scholar] [CrossRef]
- Carr, C.L.; Jayawardana, W.; Zou, H.; White, J.L.; Gabaly, F.E.; Conradi, M.S.; Stavila, V.; Allendorf, M.D.; Majzoub, E.H. Anomalous H2 Desorption Rate of NaAlH4 Confined in Nitrogen-Doped Nanoporous Carbon Frameworks. Chem. Mater. 2018, 30, 2930–2938. [Google Scholar] [CrossRef]
- Do, H.W.; Kim, H.; Cho, E.S. Enhanced hydrogen storage kinetics and air stability of nanoconfined NaAlH4 in graphene oxide framework. RSC Adv. 2021, 11, 32533. [Google Scholar] [CrossRef]
- Ianni, E.; Sofianos, M.V.; Rowles, M.R.; Sheppard, D.A.; Humphries, T.D.; Buckley, C.E. Synthesis of NaAlH4/Al composites and their applications in hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 17309–17317. [Google Scholar] [CrossRef]
- Sun, Y.; Aguey-Zinsou, K.-F. Light-activated hydrogen storage in Mg, LiH and NaAlH4. ChemPlusChem 2018, 83, 904–908. [Google Scholar] [CrossRef]
- Chen, W.; You, L.; Xia, G.; Yu, X. A balance between catalysis and nanoconfinement towards enhanced hydrogen storage performance of NaAlH4. J. Mater. Sci. Technol. 2021, 79, 205–211. [Google Scholar] [CrossRef]
- Meenakshi; Agnihotri, D.; Sharma, H. Carbon nanotubes for improving dehydrogenation from NaAlH4. Comput. Chem. 2016, 1097, 61–69. [Google Scholar] [CrossRef]
- Gao, Q.; Xia, G.; Yu, X. Confined NaAlH4 Nanoparticles inside CeO2 Hollow Nanotubes towards Enhanced Hydrogen Storage. Nanoscale 2017, 9, 14612–14619. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, G.; Fang, F.; Yu, X.; Zhang, Q.; Ouyang, L.; Zhu, M.; Sun, D. De-/Re-Hydrogenation Features of NaAlH4 Confined Exclusively in Nanopores. Acta Mater. 2011, 59, 1829–1838. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, D.; Fan, G.; Chen, Y.; Fan, Y.; Liu, B. Synergistic Effect of CeF3 Nanoparticles Supported on Ti3C2MXene for Catalyzing Hydrogen Storage of NaAlH4. ACS Appl. Energy Mater. 2021, 4, 2820–2827. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, Y.; Chen, Y.; Liu, X.; Liu, B.; Han, S. Two-Dimensional C@TiO2/Ti3C2 Composite with Superior Catalytic Performance for NaAlH4. Int. J. Hydrogen Energy 2020, 45, 21666–21675. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Javadian, P.; Polanski, M.; Besenbacher, F.; Bystrzycki, J.; Jensen, T.R. Nanoconfined NaAlH4: Determination of Distinct Prolific Effects from Pore Size, Crystallite Size, and Surface Interactions. J. Phys. Chem. C 2012, 116, 21046–21051. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Polanski, M.; Zasada, D.; Javadian, P.; Besenbacher, F.; Bystrzycki, J.; Skibsted, J.; Jensen, T.R. Improved Hydrogen Storage Kinetics of Nanoconfined NaAlH4 Catalyzed with TiCl3 Nanoparticles. ACS Nano 2011, 5, 4056–4064. [Google Scholar] [CrossRef]
- Plerdsranoy, P.; Wiset, N.; Milanese, C.; Laipple, D.; Marini, A.; Klassen, T.; Dornheim, M.; Gosalawit-Utke, R. Improvement of Thermal Stability and Reduction of LiBH4/Polymer Host Interaction of Nanoconfined LiBH4 for Reversible Hydrogen Storage. Int. J. Hydrogen Energy 2015, 40, 392–402. [Google Scholar] [CrossRef]
- Llamas Jansa, I.; Kalantzopoulos, G.N.; Nordholm, K.; Hauback, B.C. Destabilization of NaBH4 by Transition Metal Fluorides. Molecules 2020, 25, 780. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, L.; Yang, H.; Züttel, A. Destabilizing Sodium Borohydride with an Ionic Liquid. Mater. Today Energy 2018, 9, 391–396. [Google Scholar] [CrossRef]
- Gao, J.; Ngene, P.; Lindemann, I.; Gutfleisch, O.; de Jong, K.P.; de Jongh, P.E. Enhanced Reversibility of H2 Sorption in Nanoconfined Complex Metal Hydrides by Alkali Metal Addition. J. Mater. Chem. 2012, 22, 13209–13215. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Wan, Q.; Zhang, J.; Li, Y.; Li, R.; Dong, X.; Qu, X. Improved Dehydrogenation Performance of NaAlH4 Using NiFe2O4 Nanoparticles. J. Alloys Compd. 2017, 709, 850–856. [Google Scholar] [CrossRef]
- Sofianos, M.V.; Chaudhary, A.L.; Paskevicius, M.; Sheppard, D.A.; Humphries, T.D.; Dornheim, M.; Buckley, C.E. Hydrogen Storage Properties of Eutectic Metal Borohydrides Melt-Infiltrated into Porous Al Scaffolds. J. Alloys Compd. 2019, 775, 474–480. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Tailoring magnesium based materials for hydrogen storage through synthesis: Current state of the art. Energy Storage Mater. 2018, 10, 168–198. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Yao, L.; Xu, C.; Liu, Y.; Li, L. State of the art multi-strategy improvement of Mg-based hydrides for hydrogen storage. J. Alloys Compd. 2019, 782, 796–823. [Google Scholar] [CrossRef]
- Baran, A.; Polański, M. Magnesium-Based Materials for Hydrogen Storage—A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef]
- Révész, Á.; Gajdics, M. Improved H-Storage Performance of Novel Mg-Based Nanocomposites Prepared by High-Energy Ball Milling: A Review. Energies 2021, 14, 6400. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, Y. Current Research Progress in Magnesium Borohydride for Hydrogen Storage (A Review). Prog. Nat. Sci. Mater. Int. 2021, 31, 809–820. [Google Scholar] [CrossRef]
- Sazelee, N.; Ali, N.A.; Yahya, M.S.; Mustafa, N.S.; Halim Yap, F.A.; Mohamed, S.B.; Ghazali, M.Z.; Suwarno, S.; Ismail, M. Recent Advances on Mg–Li–Al Systems for Solid-State Hydrogen Storage: A Review. Front. Energy Res. 2022, 10, 875405. [Google Scholar] [CrossRef]
- White, J.L.; Strange, N.A.; Sugar, J.D.; Snider, J.L.; Schneemann, A.; Lipton, A.S.; Toney, M.F.; Allendorf, M.D.; Stavila, V. Melting of Magnesium Borohydride under High Hydrogen Pressure: Thermodynamic Stability and Effects of Nanoconfinement. Chem. Mater. 2020, 32, 5604–5615. [Google Scholar] [CrossRef]
- Capurso, G.; Agresti, F.; Crociani, L.; Rossetto, G.; Schiavo, B.; Maddalena, A.; lo Russo, S.; Principi, G. Nanoconfined Mixed Li and Mg Borohydrides as Materials for Solid State Hydrogen Storage. Int. J. Hydrogen Energy 2012, 37, 10768–10773. [Google Scholar] [CrossRef] [Green Version]
- Clémençon, D.; Davoisne, C.; Chotard, J.N.; Janot, R. Enhancement of the Hydrogen Release of Mg(BH4)2 by Concomitant Effects of Nano-Confinement and Catalysis. Int. J. Hydrogen Energy 2019, 44, 4253–4262. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, G.; Zhang, J.; Sun, D.; Guo, Z.; Yu, X. Graphene-Tailored Thermodynamics and Kinetics to Fabricate Metal Borohydride Nanoparticles with High Purity and Enhanced Reversibility. Adv. Energy Mater. 2018, 8, 1702975. [Google Scholar] [CrossRef]
- Jeong, S.; Heo, T.W.; Oktawiec, J.; Shi, R.; Kang, S.Y.; White, J.L.; Schneemann, A.; Zaia, E.W.; Wan, L.F.; Ray, K.G.; et al. A Mechanistic Analysis of Phase Evolution and Hydrogen Storage Behavior in Nanocrystalline Mg(BH4)2 within Reduced Graphene Oxide. ACS Nano 2020, 14, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.T.; Strange, N.A.; Leick, N.; Stavila, V.; Bowden, M.E.; Autrey, T.S.; Gennett, T. Mg(BH4)2-Based Hybrid Metal-Organic Borohydride System Exhibiting Enhanced Chemical Stability in Melt. ACS Appl. Energy Mater. 2021, 4, 1704–1713. [Google Scholar] [CrossRef]
- Dun, C.; Jeong, S.; Liu, Y.-S.; Leick, N.; Mattox, T.M.; Guo, J.; Lee, J.-W.; Gennett, T.; Stavila, V.; Urban, J.J. Additive Destabilization of Porous Magnesium Borohydride Framework with Core-Shell Structure. Small 2021, 17, 2101989. [Google Scholar] [CrossRef]
- Ahmad, M.A.N.; Sazelee, N.; Ali, N.A.; Ismail, M. An Overview of the Recent Advances of Additive-Improved Mg(BH4)2 for Solid-State Hydrogen Storage Material. Energies 2022, 15, 862. [Google Scholar] [CrossRef]
- Feng, X.; Yuan, J.; Lv, Y.; Liu, B.; Huang, H.; Zhang, B.; Yan, Y.; Han, S.; Wu, Y. Improvement of Desorption Performance of Mg(BH4)2 by Two-Dimensional Ti3C2 MXene Addition. Int. J. Hydrogen Energy 2020, 45, 16654–16662. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ray, K.G.; Jørgensen, M.; Mattox, T.M.; Cowgill, D.F.; Eshelman, H.V.; Sawvel, A.M.; Snider, J.L.; York, W.; Wijeratne, P.; et al. Nanoscale Mg–B via Surfactant Ball Milling of MgB2: Morphology, Composition, and Improved Hydrogen Storage Properties. J. Phys. Chem. C 2020, 124, 21761–21771. [Google Scholar] [CrossRef]
- Aditya, M.V.V.S.; Panda, S.; Tatiparti, S.S.V. Boron from net charge acceptor to donor and its effect on hydrogen uptake by novel Mg-B-electrochemically synthesized reduced graphene oxide. Sci. Rep. 2021, 11, 10995. [Google Scholar] [CrossRef]
- Wahab, M.A.; Young, D.J.; Karim, A.; Fawzia, S.; Beltramini, J.N. Low-temperature hydrogen desorption from Mg(BH4)2 catalysed by ultrafine Ni nanoparticles in a mesoporous carbon matrix. Int. J. Hydrogen Energy 2016, 41, 20573–20582. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jia, Y.; Yang, D.; Zhao, H.; Yao, X. Enhanced Hydrogen Desorption from Mg(BH4)2 by Combining Nanoconfinement and a Ni Catalyst. J. Mater. Chem. A Mater. 2013, 1, 3471–3478. [Google Scholar] [CrossRef]
- Schneemann, A.; Wan, L.F.; Lipton, A.S.; Liu, Y.-S.; Snider, J.L.; Baker, A.A.; Sugar, J.D.; Spataru, C.D.; Guo, J.; Autrey, T.S.; et al. Nanoconfinement of Molecular Magnesium Borohydride Captured in a Bipyridine-Functionalized Metal-Organic Framework. ACS Nano 2020, 14, 10294–10304. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.; Leick, N.; Lipton, A.S.; Tran, B.; Strange, N.A.; Bowden, M.; Martinez, M.B.; Moury, R.; Gennett, T.; Hagemann, H.; et al. Thermal Conversion of Unsolvated Mg(B3H8)2 to BH4−in the Presence of MgH2. ACS Appl. Energy Mater. 2021, 4, 3737–3747. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Li, Y.; Zhang, X.; Gao, M.; Pan, H. Towards the Endothermic Dehydrogenation of Nanoconfined Magnesium Borohydride Ammoniate. J. Mater. Chem. A Mater. 2015, 3, 11057–11065. [Google Scholar] [CrossRef]
- Xiao, X.; Qin, T.; Jiang, Y.; Jiang, F.; Li, M.; Fan, X.; Li, S.; Ge, H.; Wang, Q.; Chen, L. Significantly Enhanced Hydrogen Desorption Properties of Mg(AlH4)2 Nanoparticles Synthesized Using Solvent Free Strategy. Prog. Nat. Sci. Mater. Int. 2017, 27, 112–120. [Google Scholar] [CrossRef]
- Møller, K.T.; Jørgensen, M.; Andreasen, J.G.; Skibsted, J.; Łodziana, Z.; Filinchuk, Y.; Jensen, T.R. Synthesis and Thermal Decomposition of Potassium Tetraamidoboranealuminate, K[Al(NH2BH3)4]. Int. J. Hydrogen Energy 2018, 43, 311–321. [Google Scholar] [CrossRef]
- Albanese, E.; Corno, M.; Baricco, M.; Civalleri, B. Simulation of nanosizing effects in the decomposition of Ca(BH4)2 through atomistic thin film models. Res. Chem. Intermed. 2021, 47, 345–356. [Google Scholar] [CrossRef]
- Comanescu, C.; Capurso, G.; Maddalena, A. Nanoconfinement in activated mesoporous carbon of calcium borohydride for improved reversible hydrogen storage. Nanotechnology 2012, 23, 385401. [Google Scholar] [CrossRef]
- Ampoumogli, A.; Steriotis, T.; Trikalitis, P.; Bardaji, E.G.; Fichtner, M.; Stubos, A.; Charalambopoulou, G. Synthesis and Characterisation of a Mesoporous Carbon/Calcium Borohydride Nanocomposite for Hydrogen Storage. Int. J. Hydrogen Energy 2012, 37, 16631–16635. [Google Scholar] [CrossRef]
- Pal, P.; Ting, J.-M.; Agarwal, S.; Ichikawa, T.; Jain, A. The Catalytic Role of D-block Elements and Their Compounds for Improving Sorption Kinetics of Hydride Materials: A Review. Reactions 2021, 2, 22. [Google Scholar] [CrossRef]
- Tang, Z.; Tan, Y.; Chen, X.; Ouyang, L.; Zhu, M.; Sun, D.; Yu, X. Immobilization of Aluminum Borohydride Hexammoniate in a Nanoporous Polymer Stabilizer for Enhanced Chemical Hydrogen Storage. Angew. Chem. Int. Ed. 2013, 52, 12659–12663. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Xia, G.; Ma, X.; Fang, F.; Deng, Y. Advanced H2-storage system fabricated through chemical layer deposition in a well-designed porous carbon scaffold. J. Mater. Chem. A 2014, 2, 15168–15174. [Google Scholar] [CrossRef]
- Wu, D.F.; Ouyang, L.Z.; Huang, J.M.; Liu, J.W.; Wang, H.; Shao, H.; Zhu, M. Synthesis and Hydrogen Storage Property Tuning of Zr(BH4)4·8NH3 via Physical Vapour Deposition and Composite Formation. Int. J. Hydrogen Energy 2018, 43, 19182–19188. [Google Scholar] [CrossRef]
- Contreras, L.; Mayacela, M.; Bustillos, A.; Rentería, L.; Book, D. Hydrogen Sorption and Rehydrogenation Properties of NaMgH3. Metals 2022, 12, 205. [Google Scholar] [CrossRef]
- Xia, G.; Li, L.; Guo, Z.; Gu, Q.; Guo, Y.; Yu, X.; Liu, H.; Liu, Z. Stabilization of NaZn(BH4)3 via Nanoconfinement in SBA-15 towards Enhanced Hydrogen Release. J. Mater. Chem. A Mater. 2013, 1, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Al, S. Investigations of Physical Properties of XTiH3 and Implications for Solid State Hydrogen Storage. Z. Fur Nat. Sect. A J. Phys. Sci. 2019, 74, 1023–1030. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Lin, H.; Liu, Y.; Zhang, Y.; Li, S.; Ma, Z.; Li, L. Metal Hydride Nanoparticles with Ultrahigh Structural Stability and Hydrogen Storage Activity Derived from Microencapsulated Nanoconfinement. Adv. Mater. 2017, 29, 1700760. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Q.; Panda, S.; Zhu, W.; Sun, F.; Khan, D.; Dong, J.; Ding, W.; Zou, J. In-Situ Catalyzed and Nanoconfined Magnesium Hydride Nanocrystals in a Ni-MOF Scaffold for Hydrogen Storage. Sustain Energy Fuels 2020, 4, 4694–4703. [Google Scholar] [CrossRef]
- Callini, E.; Szilagyi, P.A.; Paskevicius, M.; Stadie, N.P.; Rehault, J.; Buckley, C.E.; Borgschulte, A.; Zuttel, A. Stabilization of volatile Ti(BH4)3 by nanoconfinement in a metal–organic framework. Chem. Sci. 2016, 7, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Frommen, C.; Sørby, M.H.; Heere, M.; Humphries, T.D.; Olsen, J.E.; Hauback, B.C. Rare Earth Borohydrides—Crystal Structures and Thermal Properties. Energies 2017, 10, 2115. [Google Scholar] [CrossRef] [Green Version]
- Valero-Pedraza, M.J.; Gascón, V.; Carreón, M.A.; Leardini, F.; Ares, J.R.; Martín, Á.; Sánchez-Sánchez, M.; Bañares, M.A. Operando Raman-mass spectrometry investigation of hydrogen release by thermolysis of ammonia borane confined in mesoporous materials. Microporous Mesoporous Mater. 2016, 226, 454–465. [Google Scholar] [CrossRef]
- Petit, J.-F.; Demirci, U.B. Discrepancy in the thermal decomposition/dehydrogenation of ammonia borane screened by thermogravimetric analysis. Int. J. Hydrogen Energy 2019, 44, 14201–14206. [Google Scholar] [CrossRef]
- Turani-Belloto, K.; Castilla-Martinez, C.A.; Cot, D.; Petit, E.; Benarib, S.; Demirci, U.B. Nanosized ammonia borane for solid-state hydrogen storage: Outcomes, limitations, challenges and opportunities. Int. J. Hydrogen Energy 2021, 46, 7351–7370. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Demirci, U.B. Mechanistic insights into the thermal decomposition of ammonia borane, a material studied for chemical hydrogen storage. Inorg. Chem. Front. 2021, 8, 1900–1930. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier. Energies 2020, 13, 3071. [Google Scholar] [CrossRef]
- Diaz, L.B.; Hanlon, J.M.; Bielewski, M.; Milewska, A.; Gregory, D.H. Ammonia Borane Based Nanocomposites as Solid-State Hydrogen Stores for Portable Power Applications. Energy Technol. 2018, 6, 583–594. [Google Scholar] [CrossRef]
- Valero-Pedraza, M.-J.; Cot, D.; Petit, E.; Aguey-Zinsou, K.-F.; Alauzun, J.G.; Demirci, U.B. Ammonia Borane Nanospheres for Hydrogen Storage. ACS Appl. Nano Mater. 2019, 2, 1129–1138. [Google Scholar] [CrossRef]
- Biswas, P.; Ghildiyal, P.; Kwon, H.; Wang, H.; Alibay, Z.; Xu, F.; Wang, Y.; Wong, B.M.; Zachariah, M.R. Rerouting Pathways of Solid-State Ammonia Borane Energy Release. J. Phys. Chem. C 2022, 126, 48–57. [Google Scholar] [CrossRef]
- Mishra, S.; Kang, P.-C.; Guo, R.-F.; Wang, C.-Y.; Nebhani, L. Combined Effect of Functionality and Pore Size on Dehydrogenation of Ammonia Borane via Its Nanoconfinement in Polyacrylamide-Grafted Organically Modified Mesoporous Silica. ACS Appl. Energy Mater. 2021, 4, 6585–6598. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Segovia, J.J.; Martín, Á. Improvement of the kinetics of hydrogen release from ammonia borane confined in silica aerogel. Microporous Mesoporous Mater. 2017, 237, 189–200. [Google Scholar] [CrossRef]
- Fang, M.H.; Wu, S.Y.; Chang, Y.H.; Narwane, M.; Chen, B.H.; Liu, W.L.; Kurniawan, D.; Chiang, W.H.; Lin, C.H.; Chuang, Y.C.; et al. Mechanistic Insight into the Synergetic Interaction of Ammonia Borane and Water on ZIF-67-Derived Co@Porous Carbon for Controlled Generation of Dihydrogen. ACS Appl. Mater. Interfaces 2021, 13, 47465–47477. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ouyang, L.; Felderhoff, M.; Zhu, M. Low temperature dehydrogenation properties of ammonia borane within carbon nanotube arrays: A synergistic effect of nanoconfinement and alane. RSC Adv. 2020, 10, 19027–19033. [Google Scholar] [CrossRef] [PubMed]
- So, S.H.; Jang, J.H.; Sung, S.J.; Yang, S.J.; Nam, K.T.; Park, C.R. Demonstration of the nanosize effect of carbon nanomaterials on the dehydrogenation temperature of ammonia borane. Nanoscale Adv. 2019, 1, 4697–4703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champet, S.; van den Berg, J.; Szczesny, R.; Godula-Jopek, A.; Gregory, D.H. Nano-inclusion in one step: Spontaneous icetemplating of porous hierarchical nanocomposites for selective hydrogen release. Sustain. Energy Fuels 2019, 3, 396–400. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, S.; Liu, M.; Cui, J.; Wang, Z.; Yu, P. Enhanced dehydrogenation performance of ammonia borane con-catalyzed by novel TiO2(B) nanoparticles and bio-derived carbon with well-organized pores. Int. J. Hydrogen Energy 2020, 45, 28070–28077. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, D.; Chen, B.; Liu, Z.; Xia, Q.; Zhu, Y.; Xia, Y. Improved hydrogen release from ammonia borane confined in microporous carbon with narrow pore size distribution. J. Mater. Chem. A 2017, 5, 15395–15400. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Liao, C.-W.; Chang, Y.-W.; Chang, B.K.; Wang, H.; Li, J.; Wang, C.-Y. Influence of Metal-Organic Framework Porosity on Hydrogen Generation from Nanoconfined Ammonia Borane. J. Phys. Chem. C 2017, 121, 27369–27378. [Google Scholar] [CrossRef]
- Peil, S.; Wisser, D.; Stähle, M.; Roßmann, P.K.; Avadhut, Y.S.; Hartmann, M. Hydrogen Release from Ammonia Borane Nanoconfined in Metal-Organic Frameworks with MIL-53 Topology. J. Phys. Chem. C 2021, 125, 9990–10000. [Google Scholar] [CrossRef]
- Salameh, C.; Moussa, G.; Bruma, A.; Fantozzi, G.; Malo, S.; Miele, P.; Demirci, U.B.; Bernard, S. Robust 3D Boron Nitride Nanoscaffolds for Remarkable Hydrogen Storage Capacity from Ammonia Borane. Energy Technol. 2018, 6, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Lai, Q.; Rawal, A.; Quadir, M.Z.; Cazorla, C.; Demirci, U.B.; Aguey-Zinsou, K.-F. Nanosizing Ammonia Borane with Nickel: A Path toward the Direct Hydrogen Release and Uptake of B-N-H Systems. Adv. Sustain. Syst. 2018, 2, 1700122. [Google Scholar] [CrossRef]
- Ploszajski, A.R.; Billing, M.; Cockcroft, J.K.; Skipper, N.T. Crystalline structure of an ammonia borane–polyethylene oxide cocrystal: A material investigated for its hydrogen storage potential. CrystEngComm 2018, 20, 4436–4440. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.F.; Zhou, X.; Yang, J.-h.; Gao, X.; Yin, L.; Zhao, Y.; Zhang, B. Encapsulation of Ammonia Borane in Pd/Halloysite Nanotubes for Efficient Thermal Dehydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 2122–2129. [Google Scholar] [CrossRef]
- Tang, Z.; Li, S.; Yang, W.; Yu, X. Hypercrosslinked Porous Poly(Styrene-Co-Divinylbenzene) Resin: A Promising Nanostructure-Incubator for Hydrogen Storage. J. Mater. Chem. 2012, 22, 12752–12758. [Google Scholar] [CrossRef]
- Shen, M.; Yu, C.; Guan, H.; Dong, X.; Harris, C.; Xiao, Z.; Yin, Z.; Muzzio, M.; Lin, H.; Robinson, J.R.; et al. Nanoparticle-Catalyzed Green Chemistry Synthesis of Polybenzoxazole. J. Am. Chem. Soc. 2021, 143, 2115–2122. [Google Scholar] [CrossRef]
- Guan, H.; Shen, M.; Harris, C.; Lin, H.; Wei, K.; Morales, M.; Bronowich, N.; Sun, S. Cu2O nanoparticle-catalyzed tandem reactions for the synthesis of robust polybenzoxazole. Nanoscale 2022, 14, 6162–6170. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Karkamkar, A.; Bowden, M.; Besenbacher, F.; Jensen, T.R.; Autrey, T. Methods to Stabilize and Destabilize Ammonium Borohydride. Dalton Trans. 2013, 42, 680–687. [Google Scholar] [CrossRef]
- Lombardo, L.; Ko, Y.; Zhao, K.; Yang, H.; Züttel, A. Direct CO2 Capture and Reduction to High-End Chemicals with Tetraalkylammonium Borohydrides. Angew. Chem. Int. Ed. 2021, 60, 9580–9589. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027. [Google Scholar] [CrossRef]
- Zeleňák, V.; Saldan, I. Factors Affecting Hydrogen Adsorption in Metal–Organic Frameworks: A Short Review. Nanomaterials 2021, 11, 1638. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhang, X.; Jiang, L. 1D Nanoconfined Ordered-Assembly Reaction. Adv. Mater. Interfaces 2019, 6, 1900104. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, H.M. Effects of Carbon on Hydrogen Storage Performances of Hydrides. J. Mater. Chem 2010, 20, 5390–5400. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, F.; Ma, J. Nanoconfinement engineering for enhanced adsorption of carbon materials, metal–organic frameworks, mesoporous silica, MXenes and porous organic polymers: A review. Environ. Chem. Lett. 2022, 20, 563–595. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.H. MXenes: Emerging 2D Materials for Hydrogen Storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Juárez, J.M.; Venosta, L.F.; Anunziata, O.A.; Gómez Costa, M.B. H2 Storage Using Zr-CMK-3 Developed by a New Synthesis Method. Int. J. Energy Res. 2022, 46, 2893–2903. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Moury, R.; Ould-Amara, S.; Demirci, U.B. Destabilization of Boron-Based Compounds for Hydrogen Storage in the Solid-State: Recent Advances. Energies 2021, 14, 7003. [Google Scholar] [CrossRef]
- Leick, N.; Strange, N.A.; Schneemann, A.; Stavila, V.; Gross, K.; Washton, N.; Settle, A.; Martinez, M.B.; Gennett, T.; Christensen, S.T. Al2O3 Atomic Layer Deposition on Nanostructured γ-Mg(BH4)2 for H2 Storage. ACS Appl. Energy Mater. 2021, 4, 1150–1162. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, G.; Yang, H.; Bi, W.; Liang, X.; Zhang, Y.; Zhang, G.; Wu, G. Controlled synthesis of V2O5/MWCNT core/shell hybrid aerogels through a mixed growth and self-assembly methodology for supercapacitors with high capacitance and ultralong cycle life. J. Mater. Chem. A 2015, 3, 15692–15699. [Google Scholar] [CrossRef]
- Bi, W.; Gao, G.; Wu, Y.; Yang, H.; Wang, J.; Zhang, Y.; Liang, X.; Liu, Y.; Wu, G. Novel three-dimensional island-chain structured V2O5/graphene/MWCNT hybrid aerogels for supercapacitors with ultralong cycle life. RSC Adv. 2017, 7, 7179–7187. [Google Scholar] [CrossRef]




















| Host Type | Catalyst/Active Intermediate; H2 Storing Composite | wt% H2 | Ref. |
|---|---|---|---|
| High surface area graphite HSAG, carbon aerogels CA | LiCx (7Li NMR), Li2B12H12 (11B NMR) | 18.5 | [77] |
| Hollow carbon nanospheres—modified by removal of carboxyl/ketone groups (HCNWs) | B, Li2B8H8 and Li2B12H12 (11B NMR) | ~0.3 (reversible (0–2 wt% rev. in similar scaffolds) | [78] |
| N-Doped Graphene-Rich Aerogels Decorated with Nickel and Cobalt Nanoparticles | Ni, Co NPs generate Ni2B and CoB intermediates | 8 wt% H2 at 325 °C(Co-NPs); 8 wt% H2 (Ni-NPs decorated CA, after rehydrogenation at 400 °C) | [79] |
| HNCs spheres (hollow carbon nanospheres) | Li2B12H12 (by FTIR 2470 cm−1 and 756 cm−1) | 0.65–0.47 wt% (LiBH4@HNCs) | [80] |
| Mesoporous carbon hollow spheres (MCHSs) with diameter 300 nm, and nanochannels 2–8 nm | Li2B12H12; No B2H6 in desorption (TPD data). | 9.5 wt% DCLB-3 (70 wt% LiBH4, 30 wt% MCHSs); 10.9 wt% DCLB-2 (20 wt% MCHSs). 8.5 wt% reversible capacity (300 °C) | [81] |
| Activated charcoal (AC) | LiBH4/AC nanocomposite showed no B2H6 release (MS data) | Tonset,des = 190 °C; 13.6 wt% (400 °C); 6 wt% reversible (350 °C, 6 MPa) | [82] |
| Porous Hollow Carbon Nanospheres (PHCNSs) | Li2B12H12 (FTIR spectra, vibration at ~2490 cm−1)—weak, retarded by nanoconfined systems xLiBH4@yPHCNS (x,y)∈{(5,5);(6,4);(7,3);(8,2)} | 8.1 wt% (350 °C,25 min; tonset,des = 200 °C), 4.8 wt% reversible (5th cycle) | [83] |
| Carbon wrapped ultrafine Fe3O4 skeleton (p-Fe3O4@C) | Li3BO3, FeB, Fe2B and B proposed as intermediates; xLiBH4@yp-Fe3O4@C | 7.8 wt% (350 °C, 30 min; tonset = 175 °C, tpeak = 337 °C); 6.2 wt% reversible (20th cycle); 79.4 g/L volumetric hydrogen density | [84] |
| Electrospun nanofibers of polyacrylonitrile (PAN)-titanium (IV) isopropoxide composite (ACNF-Ti) | TiO2; LiBH4-ACNF-Ti compacted (868 MPa) | 5–5.2 wt% (~75% of theoretical for 1:1 LiBH4:ACNF-T; tdes = 352–359 °C) | [85] |
| LiBH4 (5–10 nm)/Graphene/Ni nanocrystals (2–4 nm) | Li2B12H12, B2H6—are avoided; Li (XPS) slows kinetics above 315 °C, hence 300 °C chosen as maximum; nano-LiBH4/10Ni@20G | 9.2 wt% (reversible, 300 °C, 100th cycle); 11.6 wt% (600 °C, tonset = 130 °C). | [86] |
| Carbon matrix prepared by resorcinol-formaldehyde method (4 types mesopores: 6, 10, 15, 25 nm; 0.82 cm3 = Vt,pores) | LiH + B—negatively impact reversible behavior; LiH-catalytic destabilization of LiBH4 | 10 wt% (S10/40); poor reversibility; ~12.5 wt% (50% LiBH4 loading) | [87] |
| LiBH4 50–60 nm prepared/no support | close contact LiH and B crucial for reversibility | 12.1 wt% of reversible (400 °C, tonset,des~190 °C; tonset,rehyd~165 °C, 100 bar H2) | [88] |
| Core–shell structure of CNT@ porous carbon @TiO2 hybrid | TiO2; LiTiO2 and TiB2 destabilize LiBH4 | 17.7 wt% (500 °C); 7.3 wt% H2 (60 min, 320 °C); 5.1 wt% (20th cycle) | [89] |
| Nanosheet-like LiBH4·H2O (20–30 nm thick) by freeze-drying technique | H+ from H2O ligand; LiBO2 identified | 10 wt% (at 70 °C; tonset,des = 50 °C) | [90] |
| LiBH4 heated with Ti(OEt)4 (precatalyst) | TiO in-situ introduced into LiBH4, yielding LiBH4-0.06TiO. Li3BO3 and TiH2 produced can act as catalysts for LiBH4 | 9 wt% (400 °C, 30 min); 9 wt% (reversible, 500 °C, 50 bar H2, tonset,rehyd = 150 °C). | [91] |
| Carbon aerogels (CA): 9.7 nm, 0.843 cm3/g, 147.1 m2/g | CoNiB-NPs loaded into carbon aerogels (CA), forming LiBH4@CA@CoNiB | 15.9 wt% (600 °C); 14.5 wt% (400 °C, 300 min.; tonset = 192 °C, tpeak = 320 °C); 9.33 wt% (350 °C, 30 min) | [92] |
| Nanoporous silica (3.3, 3.6, 4.2, 4.4, 5.9, 6.1 nm) and carbon scaffolds (3.1, 3.8, 5.6, 20 nm) (comparison) | LiBH4@SiO2 (SBA-15); LiBH4@C | –; (thermodynamc investigation study-phase transition shift in solid-solid phase transition) | [93] |
| Ce2S3 scaffolds (solvothermal method, 100–200 nm diameter) | Li2S and CeB6 have co-catalytic effects (rehydrogenation) (Reaction (11)). LiBH4 + 20 wt% Ce3S3 | 4.0 wt% (3000 s, 400 °C; tonset,dehyd = 250 °C); rehydrogenation (400 °C, 5 MPa H2) | [94] |
| Activated carbon nanofibers (ACNFtt, tt = activation time during heating, 15–75; SBET = 2752 m2/g, Vtot (2.17 mL/g). | B2H6 is suppressed; C surface shows catalytic effect. Li2B12H12 formed (lowers H2 wt% during cycling). | 11.7 wt% (1st), 7.1 wt% (2nd cycle); 81% of theoretical H2 capacity (tonset = 125 °C, tpeak = 170 °C) | [95] |
| Ti2C3 MXene | MXene catalyst; LiBH4@2Ti3C2 hybrid investigated showed best results. Ti(0)//Ti(II)/ Ti(III)/Ti(IV)-TiO2 complex redox speciation of Ti with possible catalytic role (XPS data) | 9.6 wt% (380 °C, 1 h; tonset = 172.6 °C); partial reversibility: 6.5 wt%—2nd cycle; 5.5 wt%—3rd cycle (300 °C, 95 bar H2; 48% capacity degradation at 3rd cycle) | [96] |
| Ni(0) | nanoporous Ni-based alloy (np-Ni); 1 LiBH4/5 np-Ni | 11.9 wt% (400 °C; tonset = 70 °C); 8.2 wt% (2nd cycle, tonset = 60 °C). | [97] |
| Porous graphene support | Fe3O4 nanoclusters; Fe3O4@rGO destabilizer and catalyst precursor; Li3BO3 catalyst formed in situ. LiBH4–20 wt% Fe3O4@rGO investigated. | 3.36 wt% (400 °C, 1000s; tonset = 74 °C); 5.74 wt% uptake (400 °C, 5 MPa H2); 3.73 wt% reversible (5th cycle) | [98] |
| Graphene in a mesoporous resorcinol–formaldehyde matrix (600 m2/g, 6.1 nm, 1.53 cm3/g) | B2H6 avoided (IR); graphene@MC catalytic effect | 13 wt% (400 °C; tonset = 253 °C); 6 wt% (2nd cycle); reversible system (rehydrogenation at 400 °C, 5 h, 60 bar H2) | [99] |
| Activated carbon | LiBH4-AC-CeF3; CeF3—catalyst | 13.1 wt% (LiBH4-AC; 8.1 wt% reversible–4th cycle); 12.8 wt% (LiBH4-AC-CeF3; 9.3 wt% reversible–4th cycle) (tonset = 160 °C) | [100] |
| Modified carbon nanotubes: SWCNTs, MWCNTs | CNTs; LiBH4@SWCNT, LiBH4@SWCNTs (BM), LiBH4@MWCNTs, and LiBH4@MWCNTs (BM) | 11 wt% (LiBH4@ MWCNTs, 450 °C); 11 wt% (LiBH4@MWCNTs (BM), 400 °C) | [101] |
| Highly ordered mesoporous carbon, C-SBA-15 replica, 1321 m2/g; 1.25 cm3/g (with NbF5 NPs) | MC-NbF5 (catalytic effect of 10 wt% precatalyst NbF5); presumed active catalysts: Nb2O5, NbHx, NbB2 (Reaction (12)). LiBH4@MC-NbF5 system investigated | 6.52 wt% (200 °C) for LiBH4@MC-NbF5. tonset = 150 °C (LiBH4@MC-NbF5), 205 °C (LiBH4@MC), 282 °C (LiBH4-NbF5-BM); 10.65 wt% rehydrogenation (mild conditions: 200 °C, 60 bar H2). | [102] |
| SiS2 | x LiBH4–SiS2 (x = 2–8) investigated. No B2H6 during release. First principles calculations identified various polymorphs, and point to decomposition Reaction (13). | For x = 6: 8.2 wt% (tonset = 92 °C), 2.4 wt% reversible. For x = 2: 4.3 wt% (385 °C, tonset = 88 °C), 1.5 wt% rehydrog (385 °C, 160 bar H2) | [103] |
| Porous Mg scaffold (sintering a NaMgH3 pellet sintered at 450 °C under dynamic vacuum, removing molten Na) (14) | Mg-porous (26 m2/g; 1.25 cm3/g); Reactions (15) and (16); MgB2 as destabilizing agent for 2LiBH4-Mg Investigated materials: Mgporous- x wt% LiBH4 (x = 12.78–35.05) | 4.81 wt% (2LiBH4:Mg); 7.12 wt% (PMg33); 9.69/9.74 wt% (2 LiBH4:MgH2 powder/pellet) | [104] |
| Surface-Modified AlN | AlN@LiBH4; AlN synthesized by alcoholysis of LiBH4 (by freeze-drying, AlN contains O–H and C–H groups (Equation (17)), best catalytic effect). x AlBH4: y AlN; (x,y)∈{(1,1);(3,2);(2,1);(4,1)}. Li2B12H12 forms only during 1st cycle, then it decomposes due to AlN. Li3BO3 in situ with potential catalytic effect (IR data: 746, 1265, 1315 cm−1). | 5.2 wt% (1:1); 6.8 wt% (3:2); 7.7 wt% (2:1); 8.3 wt% (4:1) for LiBH4-AlN composites. Rehydrogenation at 400 °C, 10 MPa, 24 h, with capacity loss (3.7 wt%). 6.1 wt%—optimized 2:1 composite, stable capacity (400 °C, 10 MPa H2). | [105] |
| C:MSU-H 2D ordered mesoporous carbon replica | C:MSU-H pores; three loadings studied: 8, 20 and 40 wt% LiBH4@C-MSU-H | 1.01 wt% (325 °C, tonset = 150 °C; 92% of theoretical maximum for 8 wt% LiBH4-MSU-H); 2.7 wt% (375 °C, 96.4% of theoretical maximum for 20 wt% LiBH4-C-MSU-H); 0.79 wt% (2nd cycle, 200 °C onset, 8 wt% LiBH4-MSU-H) | [106] |
| Mo:MSU-H | Molibdate precursor decomposes thermally at 550 °C (4 h) to give active MoO3 catalyst. Mass ration LiBH4:Mo-MSU-H = 5:1 (~70% host pore filling) | 11.2 wt% H2 (5.2 wt% rehydrogenation 450 °C, 80 bar H2) | [107] |
| Single-Walled Carbon Nanotubes (SWCNTs) | LiB(OH)4, Li2CO3 and LiBO2 | 4.0 wt% (153–368 °C, SWLiB-A); 4.3 wt% (108–433 °C, SWLiB-N); partical reversibility (100–150 °C, 5–10 bar H2) | [108] |
| Al Derived from AlH3 (Al*) shows superior hydrogenation effect compared to commercial Al. | LiBH4/Al* composite (Equations (18) and (19)): 2LiBH4 + Al → 2LiH + AlB2 + 3H2 (18) LiH + Al → LiAl + 1/2H2 (19). B2H6 and an intriguing “Li-Al-B-H” phase detected. | 6.2 wt% (LiBH4/Al*); 5.5 wt% (LiBH4/Al). Reversibility (5.5 wt%) at 400 °C under 8 MPa H2 (Equation (20)): LiH + LiAl + AlB2 + 7/2H2 ↔ 2LiBH4 + 2Al. (20) | [109] |
| Host Type | Catalyst/Active Intermediate; H2 Storing Composite | wt% H2 | Ref. |
|---|---|---|---|
| Graphite | LiCx (instead of LiH); LiAlH4/HSAG nanocomposite (NC-HSAG) | 10% wt% (300 °C, LiAlH4/HSAG) | [77] |
| High surface area graphite (HSAG) | Li3AlH6 active intermediate; no other catalyst was used | 0.6 wt% partially reversible storage at 300 °C (30 min) (tonset = 135 °C, 7 MPa H2) | [116] |
| NiCo2O4@rGO | NiCo2O4, rGO | 6.28 wt% (tonset = 62.7 °C); 4.0 wt% (isothermal 150 °C, 20 min.) | [117] |
| N-doped CMK-3 carbon (NCMK-3) | N-sites in LiAlH4@NCMK-3; LiAlH4@CMK-3 synthesized for comparison. | ~1.1 wt% (240 °C, tonset = 126 °C); >80% reversibility of LiAlH4 (50 °C, 100 MPa H2) | [118] |
| (2D) layered Ti3C2 | catalytic effect of 2D Ti3C2 in LiAlH4 + 5 wt% Ti3C2, which forms under ball milling active Ti(0) and Ti(+3) catalytic sites (XPS data) | 6.5 wt% (58.6 °C onset); 5.5 wt% (200 °C, 35 min); 3.9 wt% (120 °C, 40 min) | [119] |
| - | SrFe12O19 addition., with active catalyst formed LiFeO2/Sr-phases | 5.54 wt% (130 °C, 20 min) for LiAlH4 + 10 wt% SrFe12O19 | [120] |
| - | ZrC powder, x mol% ZrC-doped LiAlH4 (x = 1,2,5,10). | 6.61 wt% (for 1 mol%-doped LiAlH4); 5.62 wt% (145 °C, 180 min); 5.48 wt% (130 °C, 180 min); 4.08 wt% (115 °C, 180 min). | [121] |
| - | 2LiBH4-M (M = Al, LiAlH4, Li3AlH6) | 8 wt% (2LiBH4-LiAlH4, 150 min) | [122] |
| - | Fe, LiFeO2 and amorphous Ba or Ba-containing species (XRD, after desorption at 250 °C); LiAlH4-10 wt% BaFe12O19 | 4.2 wt% (90 °C, 2.5 h); ~6.0 wt% (250 °C, tonset = 95 °C) | [123] |
| - | K2NbF7 as precatalyst for in situ formed NbF4, LiF, and K-containing species; LiAlH4 + 10 wt% K2NbF7 | ~ 6.2 wt% (250ׄ°C, tonset ~75 °C); 3.2 wt% (120 min, 90 °C, kinetic study) | [124] |
| Nickel-Containing Porous Carbon Sheets (Ni-PCS) | Ni, Cporous; LiAlH4-5 wt% Ni-PCS (11.6 µm) | 8.14 wt% (LiAlH4 + PCS); 7.97 wt% (LiAlH4 + Ni-PCS) | [125] |
| TiO2/Hierarchically Porous Carbon | TiO2/Cnanoporous; LAH-TiO+/HPC with postulated role of defect redox sites Ti4+/Ti3+/Ti2+ in 0.62/0.22/0.16 atomic ration (XPS). | 6.2 wt% H2 (60 min, 160 °C); 4.3 wt% (40 min, 130 °C; tonset = 64 °C, tpeak = 115 °C); partial rehydrogenation possible (300 °C, 4 MPa) | [126] |
| Hollow carbon nanospheres (HCNs) | LiAlH4@HCNs | 5.8 wt%/expected 2.3 wt% (solvent traces; sharp onset 146 °C; full conversion to LiH, 90 min). 0.37 wt% reversible rehydrogenation to LiAlH4 (150 °C, 8 MPa H2; vs 2.4 wt% max theoretical for used loading). | [127] |
| - | LiAlH4.xMe2O; adduct formation excludes Li3AlH6 or other intermediates. | N/A; proof-of-concept regarding rehydrogenation potential of LiAlH4. | [128] |
| - | LiAlH4 · 4THF regeneration; Ti-catalyzed Al (TiCl3). | 4.5 wt& regeneration at 398K using Al* [Al(Ti)- 2 mol% Ti] | [129] |
| - | Surfactant used as stabilizer for NP size manipulation. | –; desolvation from LiAlH4-X-N (X-solvent; N = 0.1, 1, 5, 10) | [59] |
| FGi (Fluorographite) | LiF (and possibly LiAlF4), Al4C3—active catalyst(s), generated in LiAlH4-xFGi composites | 6.25 wt%, with 5.7 wt% (ultra-fast: seconds, tonset = 61.2 °C, 65 °C main step) for LiAlH4-40FGi. | [130] |
| h-BN | LiAlH4/x wt% h-BN (x = 0,4,14,40). Composites. h-BN has a catalytic effect on Li3AlH6 decomposition (second step desorption). | 7.6 wt% (LiAlH4/4 wt% h-BN), 6.8 wt% (LiAlH4/14 wt% h-BN), 4.7 wt% (LiAlH4/40 wt% h-BN). | [131] |
| RHC Type | Components | RHC Type | Components |
|---|---|---|---|
| Binary | Ca(BH4)2 + MgH2 | Ternary | Ca(BH4)2 + 2LiBH4 + 2MgH2 |
| 2LiBH4-MgH2 | NaAlH4-MgH2-LiBH4 | ||
| 2LiBH4-2.5Mg2NiH4 | Ca(BH4)2-LiBH4-MgH2 | ||
| Na3AlH6-3MgH2 | LiNH2-MgH2-Ca(BH4)2 | ||
| 2NaAlH4-Ca(BH4)2 | LiAlH4-MgH2-LiBH4 | ||
| NaBH4-Li3AlH6 | LiBH4-CaH2-MgH2 | ||
| Ca(BH4)2-Mg(AlH4)2 | MgH2-Na3AlH6-LiBH4 | ||
| Ca(BH4)2-LiNH2 | LiBH4-Mg(NH2)2-LiH | ||
| NaAlH4-Ca(BH4)2 | LiNH2-MgH2-LiBH4 | ||
| LiBH4-Mg(BH4)2-(TiF3) | LiNH2-LiH-Mg(BH4)2 | ||
| Na3AlH6-LiBH4 –(MgFe2O4) | |||
| MgH2-LiAlH4-(SeFe12O19) | |||
| MgH2-NaAlH4-(TiF3) | |||
| Ternary | LiBH4-NaBH4-M(BH4)2 M = Mg, Ca | Quaternary | LiBH4-NaBH4-KBH4-Mg(BH4)2 |
| LiBH4-KBH4-M(BH4)2 M = Mg, Ca | LiBH4-NaBH4-KBH4-Ca(BH4)2 | ||
| LiBH4-Mg(BH4)2-Ca(BH4)2 | LiBH4-NaBH4-Mg(BH4)2-Ca(BH4)2 | ||
| NaBH4-KBH4-M(BH4)2 M = Mg, Ca | LiBH4-KBH4-Mg(BH4)2-Ca(BH4)2 | ||
| MBH4-Mg(BH4)2-Ca(BH4)2 M = Na, K | NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2 |
| RHC Type | Chemical Reaction(s) Proposed | |
|---|---|---|
| Binary | Ca(BH4)2 + MgH2 → CaH2 + MgB2 + 4H2 | (37) |
| Ca(BH4)2 + MgH2 → 2/3CaH2 + 1/3CaB6 + Mg+ 13/3 H2 | (38) | |
| Ca(BH4)2 + MgH2 → CaH2 + 2B + Mg + 3H2 | (39) | |
| 2LiBH4 + MgH2 → 2LiBH4 + Mg + H2 | (40) | |
| 2LiBH4 + Mg + H2 → 2LiH + MgB2 + 4H2 | (41) | |
| 2LiBH4 + 2.5Mg2NiH4 → 2LiH + MgNi2.5B2 + 4MgH2 + 4H2 | (42) | |
| Na3AlH6 + 3MgH2 → 3NaMgH3 + Al + 3/2 H2 | (43) | |
| 2NaAlH4 + Ca(BH4)2 → Ca(AlH4)2 + 2NaBH4 | (44) | |
| Tertiary | Ca(BH4)2 + 2LiBH4 + 2MgH2 → 1/3CaH2 + 2/3CaB6 + 2LiH + 2Mg + 26/3 H2 | (45) |
| Host Type | Catalyst/Active Intermediate; H2 Storing Composite RHC | wt% H2 | Ref. |
|---|---|---|---|
| Adaptive poly(4-methyl-1-pentene), TPX™ Polymer | 2LiH + MgB2+ 7.5(3TiCl3·AlCl3) and 2LiH + MgB2+ 7.5(3TiCl3·AlCl3)@TPX | ~9.1 wt% (non-confined); 7.3 wt% (TPX-confined) | [140] |
| Carbon aerogel scaffold with pore size Dmax ∼21 nm | Al12Mg17 and Mg1−xAlxB2 proposed as intermediates; 2 LiBH4: MgH2 | 4.3 wt%; ~4 wt% reversible | [142] |
| - | Li-RHC (2LiH + MgB2/2LiBH4 + MgH2) composite doped with TiO2 (Li-RHC-Ti). | 10.1 wt% (stable after cycles 11–15) | [143] |
| - | 2 LiH + MgB2/2 LiBH4- MgH2. Additive used: 0.05 mol TiCl3/mol MgB2 | - (kinetic study by PCI isotherms in the 350–400 °C temperature interval). | [144] |
| Resorcinol formaldehyde carbon aerogel (RFC): 1.1452 cm3/g, 687.1 m2/g. | 2LiBH4-LiAlH4/RFC; AlB2 as catalyst in-situ. Li2B12H12 was also identified as potential intermediate. | 5.7 wt% (reversible) (tonset, step 1 = 100 °C; tonset,step 2 = 280 °C); rehydrogenation at 350 °C, 5 MPa H2 (5 h) | [145] |
| Activated carbon nanofibers (ACNF) | LiBH4-LiAlH4 | 6.6 wt% (milled LiBH4-LiAlH4); 2.7 wt% (nano RHC-ACNF) | [146] |
| Mesoporous carbon aerogel CA (Dmax = 30 nm, SBET = 689 m2/g and Vtot = 1.21 cm3/g) | 2LiBH4-NaAlH4; AlB2 and Al detected as final dehydrogenation products (can act as catalysts, also Li3AlH6 and LiAl3 was observed); C surface can also exhibit catalytic properties. | 2.4 wt% (2.6 wt% theoretical; 33% pore filling); 7.8 wt% (estimated based on 100% pore filling of CA); tonset = 100 °C; 83% reversible of theoretical wt% H2 (RHC-CA); 47% reversible (RHC). Rehydrogenation at 126 bar H2, 400 °C, up to 10 h (NaBH4 recovered) | [147] |
| Porous hollow carbon nanospheres (HCNS) | LMBH: LiBH4-Mg(BH4)2 in 55:45 mole ratio, eutectic formation reported. (LMBH@HCNS final composite). Stable [B12H12]2− anion was detected by FTIR (dehydrogenated products) | 4.3 wt% (33LMBH@HCNS); 6.1 wt% (50LMBH@HCNS); 8.1 wt% (67LMBH@HCNS). Rehydrogenation (100 bar H2, 6 h, 280 °C) | [148] |
| Nano–Ni(doped)in Ni/C scaffold | 2LiBH4–MgH2; final composite: 2LiBH4–MgH2–15%Ni/C and reactive intermediate (nanosized) Ni4B3 as a pre-catalyst for MgNi3B2 species. | ~9 wt% | [149] |
| Activated carbon | 2LiBH4-MgH2; “LiBH4-MgH2-AC” composite; Li2B12H12—intermediate (incomplete rehydrogenation) | 3.56–4.55 wt% (dehydrogenation); 2.03–3.28 wt% H2 (rehydrogenation) for the first 5 cycles | [150] |
| Carbon aerogel scaffold (CAS) doped with ZrCl4 | 2LiBH4–MgH2; ZrCl4 catalyst/dopant (Zr-P63mmc and ZrB2 detected by XRD in dehydrogenated samples). | 2.5–5.4 wt% (nano-RHC:AC, 1:3–1:1); 8.7 wt% (milled RHC) | [151] |
| - | ternary and quaternary mixtures in the system LiBH4-NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2: | various (structural investigation study) | [152] |
| - | 2 LiBH4–2.5 Mg2NiH4; reversible H2 storage via MgNi2.5B2 (intermediate) | ~4.8 wt% (under H2 pressure of 1 or 5 bar) | [153] |
| Carbon aerogel scaffold (CAS) | 2LiBH4–MgH2–0.13TiCl4; TiCl4 catalyst ([B12H12]2− stable intermediate identified at 2480 cm−1). | 3.6 wt% (1.5 h, tonset= 140 °C, release in range 140–380 °C) | [154] |
| - | LiBH4–NaBH4 | N/A | [155] |
| Ordered Nanoporous Carbon (NPCs) 1012 m2/g and 0.65 cm3/g | LiBH4–Mg(BH4)2 | ~7 wt% (1st cycle), ~ 1 wt% (2nd cycle) | [156] |
| - | 0.62LiBH4-0.38NaBH4; nano-Ni as catalyst, producing 0.91 (0.62LiBH4-0.38NaBH4)-0.09Ni catalyzed RHC. | 8.1 wt% H2 (tpeak = 350 °C, up to 650 °C, Ar). Decreasing performace in subsequent cycles (5.1 wt%, 1.1 wt% and 0.6 wt%). | [157] |
| - | 2LiBH4 + M (M = LiAlH4, Li3AlH6) | 8 wt% (2LiBH4-LiAlH4), 9.1 wt% (2LiBH4-Li3AlH6, 150 min) | [122] |
| Nanoporous silica (fumed silica; mesoporous MCM-41 and SBA-15) | LiBH4–Ca(BH4)2 (4:1, 2.1:1 -eutectic composition) | N/A | [158] |
| 2D-MXene (Ti3C2) | 4MgH2-LiAlH4-Ti3C2 (Ti3C2 MXene acts as pre-catalyst of Ti(0) species in 4MgH2-LiAlH4 RHC). TiH1.942 intermediate identified in situ hints at reaction Ti(0) with RHC. | ~ 6.5 wt% (onset at 336K, peak at 594 K) | [159] |
| - | LiBH4/KBH4 as eutectic catalyst for MgH2/Sn destabilized hydride, and for rehydrogenation of spent MgB2 to Mg(BH4)2. 0.025 MgI2 was found to bring further kinetic enhancement | ~1.7 wt% (tonset = 150 °C; 40 h); ~2 wt% rehydrogenated (920–1000 bar. at 215–175 °C); 1.9 wt% (2nd dehydrogenation). | [160] |
| - | 4LiAlH4–Mg2NiH4; Ti-based catalyst used (3 mol% TiF3); potential intermediacy of Al3Ti (Ti0) phase (in-situ XPS data). [F-] effect possibly due to replacement of H in Al-species, forming [AlF4]− and [AlF6]3− which facilitate dehydrogenation. | 5.62 wt% (TiF3-cat.); 5.02–5.09 (undoped, Ti-or TiO2-cat.), with tonset = 50 °C for 1st step (21); tonset = 128 °C for 2nd step (22); 1.38 wt% (reversible rehydrog., 3 MPa H2, 300 °C, 150 s). | [161] |
| Mesoporous carbon CMK-3 (C-replica of SBA-15 silica) | x LiBH4–(1 − x) Ca(BH4)2 eutectic with x = 0.50, 0.60, 0.65, 0.68, 0.70, 0.75, and 0.80 (mp~200 °C). CMK–3 porosity: 1229 m2/g, 3.5 nm pore size, 1.63 cm3/g–0.59 cm3/g micro & 1.04 cm3/g meso) | ~11 wt% (eutectic RHC); ~ 5 wt% (RHC@CMK-3, up to 400 °C, tpeak~300 °C, 2 h, under 3 bar H2). Rehydrogenation (400 °C, 24 h, 110 bar H2) | [162] |
| Activated carbon aerogel CA (689–2660 m2/g, 1.21–3.13 cm3/g) | 0.55LiBH4–0.45Mg(BH4)2 | 13.3 wt% H2 (RHC@CA); 8.4 wt% (RHC). Reversible: 8.3 wt% (RHC@CA,4th cycle); 3.1 wt% (RHC, 4th cycle). | [163] |
| MWCNTs | LiBH4–LiAlH4; active phases/catalysts formed in-situ: AlB2 and LiAl. | 2.0–3.0 wt% (5.5 h, 400 °C for RHC@MWCNTs), 2.3–2.8 wt% (RHC) decreasing after 3 cycles. | [164] |
| - | Mg@NaBH4/MgB2 core-shell structures | 5.98 wt% | [165] |
| - | 4MgH2 + LiAlH4, TiO2 catalyst. Active catalyst phases are considered Al3Ti and TiH2. | 4.7 wt% (450 °C); 2.5 wt% (13 min, 320 °C); rehydrogenation confirmed (2.9 wt%, 10 min) | [166] |
| - | LiBH4-x AlH3 (x = 0.5, 1.0, 2.0) | 11.0 wt% (release, 450 °C, 6 h) for LiBH4 − 0.5 AlH3 (best result); 3.2 wt% reversible capacity (2nd dehydrogenation, 400 °C, 5 MPa H2, 5 h). | [167] |
| - | Mg(NH2)2–2LiH–0.07KOH | 4.5–4.9 wt% under 0–5 bar H2, | [168] |
| Carbon aerogel scaffold (CAS) | 2LiBH4–NaAlH4; LiNa2AlH6 was identified as short-lived intermediate (60) | 2.39 wt% (400 °C, for 2:1 weight ratio CAS-RHC); ~2.0 wt% reversible (4th cycle, 400 °C, 80 bar H2, 12 h) | [169] |
| CMK-3 Type Carbon (5 nm pore size) | LiBH4-KBH4 Eutectic (0.725 LiBH4–0.275 KBH4) | reversible 2.5–3 wt% (5th cycle) | [170] |
| 3LiBH4 + Er(BH4)3 + 3LiH | 4.2 (1st cycle); 3.7 (2nd cycle); 3.5 (3rd cycle) out of 6 wt% (theoretically accessible for given RHC) | [171] | |
| Activated carbon nanofibers (ACNFs) | 2LiBH4-MgH2; ACNFs as dopant (30 wt%) | 1.8 to 4.5 wt% H2 (reversible) | [172] |
| - | K2Mn(NH2)4–8LiH; K3MnH5 and K–Mn-species (probably hydrides) | 6 wt% (open system); rehydrogenation almost complete (230 °C, 50 bar H2, very fast at 1 wt%/min); 3 wt% reversible (375 °C release, 300 °C uptake) | [173] |
| CO2-activated carbon aerogel (37–38 nm; 690–2358 m2/g) | 0.62LiBH4–0.38NaBH4 (eutectic) | 1.6 wt% (4th cycle, 22% of full theoretical capacity) | [174] |
| Activated carbon aerogel (pristine: 689 m2/g, 1.21 cm3/g and CO2-activated: 2660 m2/g, 3.13 cm3/g) | 0.7LiBH4–0.3Ca(BH4)2 | 12.08 wt% (RHC), 7.71 wt% (RHC-CACO2), 3.36 (RHC-CA) | [175] |
| Carbon nanoscaffold (IRH33, 1.17 cm3 g−1, 2587 m2 g−1, 0.5 to 4.5 nm pores) | Li11BD4–Mg(11BD4)2 | 4.5 wt% (460 °C, 6.7 wt% maximum theoretical based on 46% filling, 14.6 wt% maximum of RHC) | [176] |
| - | NaBH4-Li3AlH6 | 4.2 wt% (330 °C); 6.1 wt% (420 °C, 30 atm, 60 min) | [132] |
| - | 6Mg(NH2)2–9LiH–LiBH4 (additives: YCl3 and Li3N) | 4.2 wt% uptake (180 °C, 85 bar H2, 8 min or 90 °C, 185 bar H2) | [177] |
| - | 2LiH + MgB2/2LiBH4 + MgH2; 0.05 TiCl3 was used as additive | N/A | [144] |
| Resorcinol–formaldehyde carbon aerogel scaffold (RF–CAS) | 2LiBH4–MgH2–TiCl3 | 3.6–3.75 wt% (1st release, 95–98.6% of maximum theoretical; 425 °C, 3.4 bar, 4.5 h); 3.25 wt% (2nd release, 8 h); 3.6–3.75 (3rd, 4th release, 15 h); uptake (425 °C, 130–145 bar) | [178] |
| Mesoporous carbon (MC) scaffolds | 2LiBH4-LiAlH4 | 10 wt% (300 °C, 300 min) | [179] |
| - | LiBH4-MgH2; Fe3B was used as additive. | 4.11 wt% (1st cycle, des.); 2.35 wt% (1st abs); 2.89 wt% (7th cycle, des.); 3.32 wt% (7th cycle, abs.). | [180] |
| - | LiBH4-NaBH4-KBH4-Mg(BH4)2-Ca(BH4)2 | N/A (up tp 500 °C, incomplete desorption) | [181] |
| Carbon aerogel scaffold CAS (SBET = 2421 ± 189 m2/g, Vtot = 2.46 ± 0.46 mL/g, 13 nm pore size) | 0.68LiBH4–0.32Ca(BH4)2 (“LiCa” eutectic) | 5.4 wt% (bulk RHC), 3.7 wt% (RHC@CAS), 1.1 wt% (LiBH4@CAS), at 5th cycle. | [182] |
| - | 2NaAlH4 + Ca(BH4)2; 5 wt% TiF3 as additive and precursor of active catalytic species Al3Ti and CaF2 (synergy) | ~8 wt% (des, RHC-TiF3); ~7.5 wt% (des., RHC); 4.1 wt% (abs, RHC-TiF3); 3.5 wt% (abs, RHC), after 60 min, 420 °C, 30 atm H2. | [183] |
| 2D MXene—nanoadditive (x wt%, x = 1, 3, 5, 7 wt%) | 2LiH + MgB2/2LiBH4 + MgH2; 2D Ti3C2 MXene as additive | 9.0 wt% H2 (400 °C, 20 min). Regeneration by hydrogenated at 350 °C/10 MPa, <5 min. | [184] |
| Manoporous carbon host (CAS; 458 m2/g; 0.56cm3/g; 4.86 nm pore size) | LiBH4-MgH2-NaAlH4 transforming into LiAlH4–MgH2–NaBH4, Intermediate phases detected as Li3Mg7, Mg17Al12 (PXD). | 3.0 wt% (tpeak = 329 °C, up to 450 °C, ~72% of maximum theoretical capacity of RHC@CAS) | [185] |
| – | (1 − x) LiBH4– x Mg2FeH6, x = 0.25, 0.5, 0.75. | 6.0 wt% (580–630K; rehydrogenation with no H2 wt% loss after 4 cycles) | [186] |
| NP-TiH2@G (TiH2 nanoplates 50 nm lateral; 15 nm thick; additive) | NaAlH4-7 wt%NP-TiH2@G | 5 wt% (reversible, full dehydrogenation at 80 °C; rehydrogenation 30 °C, 100 atm H2) | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comanescu, C. Paving the Way to the Fuel of the Future—Nanostructured Complex Hydrides. Int. J. Mol. Sci. 2023, 24, 143. https://doi.org/10.3390/ijms24010143
Comanescu C. Paving the Way to the Fuel of the Future—Nanostructured Complex Hydrides. International Journal of Molecular Sciences. 2023; 24(1):143. https://doi.org/10.3390/ijms24010143
Chicago/Turabian StyleComanescu, Cezar. 2023. "Paving the Way to the Fuel of the Future—Nanostructured Complex Hydrides" International Journal of Molecular Sciences 24, no. 1: 143. https://doi.org/10.3390/ijms24010143