Allogenic Adipose-Derived Stem Cells in Diabetic Foot Ulcer Treatment: Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact
Abstract
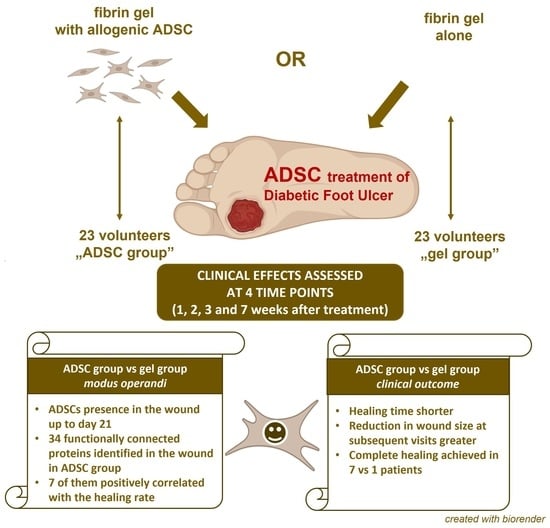
:1. Introduction
2. Results
2.1. Clinical Outcomes
2.2. Proteomic Analysis
2.3. Donor DNA in Wound Samples
3. Discussion
4. Materials and Methods
4.1. Clinical Observation Design
4.2. ADSC Collection and Preparation
4.3. Manufacturing of the Medicinal Product
4.4. Clinical Procedures
4.5. Evaluation of the Clinical Outcomes
- measurement of the wound surface;
- local and systemic tolerance of therapy, including the episodes of wound infection or any other adverse events;
- number of patients in each group who achieved complete wound closure;
- evaluation of the pain scale: evaluation of the changes in pain experienced by the patient due to the wound treated with the tested method, in relation to the wound treated in a standard way through the use of the visual analog scale (VAS).
4.6. Wound Debridement
4.7. Proteomic Analysis
4.8. Search for Donor DNA in Wound Scrapings
4.9. Statistical Analyses
- To compare the healing rate in both groups, the Wilcoxon-Mann–Whitney test was used;
- Exponential regression was used to determine the half-time of wound surface decline;
- A Spearman correlation was used to evaluate the relationship between the rate of healing and upregulated protein expression. The correlations were calculated between the relative wound size and a measure of increase in protein expression. This measure was defined as the difference in the logarithm of the protein intensities from the mass spectrometry measurements. The measurements were performed in weeks 0 and 1.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bandyk, D.F. The Diabetic Foot: Pathophysiology, Evaluation, and Treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.Q.; Shahriar, S.S.; Yan, Z.; Xie, J. Recent Advances in Functional Wound Dressings. Adv. Wound Care 2022. [Google Scholar] [CrossRef] [PubMed]
- FrykbergRobert, G. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Marfia, G.; Navone, S.E.; Di Vito, C.; Ughi, N.; Tabano, S.; Miozzo, M.; Tremolada, C.; Bolla, G.; Crotti, C.; Ingegnoli, F.; et al. Mesenchymal Stem Cells: Potential for Therapy and Treatment of Chronic Non-Healing Skin Wounds. Organogenesis 2015, 11, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J. Precursor Cells of Mechanocytes. Int. Rev. Cytol. 1976, 47, 327–359. [Google Scholar] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone Marrow Osteogenic Stem Cells: In Vitro Cultivation and Transplantation in Diffusion Chambers. Cell Prolif. 1987, 20, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A. Mesenchymal Stromal Cells Derived from Various Tissues: Biological, Clinical and Cryopreservation Aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (Adscs) and Mesenchymal Stem Cells (Mscs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [Green Version]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.; Xian, C.J. Adipose-Derived Stem Cells for Wound Healing. J. Cell. Physiol. 2018, 234, 7903–7914. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Pontecorvi, P.; Anastasiadou, E.; Napoli, C.; Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Liu, Y.; Holmes, C. Tissue Regeneration Capacity of Extracellular Vesicles Isolated from Bone Marrow-Derived and Adipose-Derived Mesenchymal Stromal/Stem Cells. Front. Cell Dev. Biol. 2021, 9, 648098. [Google Scholar] [CrossRef] [PubMed]
- Waldner, M.; Zhang, W.; James, I.B.; Allbright, K.; Havis, E.; Bliley, J.M.; Almadori, A.; Schweizer, R.; Plock, J.A.; Washington, K.M.; et al. Characteristics and Immunomodulating Functions of Adipose-Derived and Bone Marrow-Derived Mesenchymal Stem Cells across Defined Human Leukocyte Antigen Barriers. Front. Immunol. 2018, 9, 1642. [Google Scholar] [CrossRef] [Green Version]
- Gadelkarim, M.; Abushouk, A.I.; Ghanem, E.; Hamaad, A.M.; Saad, A.M.; Abdel-Daim, M.M. Adipose-Derived Stem Cells: Effectiveness and Advances in Delivery in Diabetic Wound Healing. Biomed. Pharmacother. 2018, 107, 625–633. [Google Scholar] [CrossRef]
- Moon, K.-C.; Suh, H.-S.; Kim, K.-B.; Han, S.-K.; Young, K.-W.; Lee, J.-W.; Kim, M.-H. Potential of Allogeneic Adipose-Derived Stem Cell-Hydrogel Complex for Treating Diabetic Foot Ulcers. Diabetes 2019, 68, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, H.; Deng, X.; Chen, M.; Han, X.; Yan, W.; Wang, N. CXCR4 antagonist delivery on decellularized skin scaffold facilitates impaired wound healing in diabetic mice by increasing expression of SDF-1 and enhancing migration of CXCR4-positive cells. Wound Repair Regen. 2017, 25, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, J.; Idlett-Ali, S.; Zhang, L.; Caples, K.; Peddibhotla, S.; Reeves, M.; Zgheib, C.; Malany, S.; Liechty, K.W. Discovery of Small Molecule Activators of Chemokine Receptor CXCR4 That Improve Diabetic Wound Healing. Int. J. Mol. Sci. 2022, 23, 2196. [Google Scholar] [CrossRef] [PubMed]
- Griss, J.; Viteri, G.; Sidiropoulos, K.; Nguyen, V.; Fabregat, A.; Hermjakob, H. Reactomegsa-Efficient Multi-Omics Comparative Pathway Analysis. Mol. Cell. Proteom. 2020, 19, 2115–2125. [Google Scholar] [CrossRef]
- Preda, M.B.; Neculachi, C.A.; Fenyo, I.M.; Vacaru, A.-M.; Publik, M.A.; Simionescu, M.; Burlacu, A. Short Lifespan of Syngeneic Transplanted Msc Is a Consequence of in Vivo Apoptosis and Immune Cell Recruitment in Mice. Cell Death Dis. 2021, 12, 566. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Wang, H.-J.; Li, Z.-H.; Wang, Y.-L.; Wu, X.-P.; Tan, Y.-Z. Mesenchymal Stem Cell-Loaded Cardiac Patch Promotes Epicardial Activation and Repair of the Infarcted Myocardium. J. Cell. Mol. Med. 2017, 21, 1751–1766. [Google Scholar] [CrossRef]
- Ding, Z.; Gau, D.; Deasy, B.; Wells, A.; Roy, P. Both Actin and Polyproline Interactions of Profilin-1 Are Required for Migration, Invasion and Capillary Morphogenesis of Vascular Endothelial Cells. Exp. Cell Res. 2009, 315, 2963–2973. [Google Scholar] [CrossRef]
- González-Pecchi, V.; Valdés, S.; Pons, V.; Honorato, P.; Martinez, L.O.; Lamperti, L.; Aguayo, C.; Radojkovic, C. Apolipoprotein a-I Enhances Proliferation of Human Endothelial Progenitor Cells and Promotes Angiogenesis through the Cell Surface Atp Synthase. Microvasc. Res. 2015, 98, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Goto, S.; Takaoka, Y.; Tseng, H.; Fujimura, T.; Kawamoto, S.; Ono, K.; Chen, C. A Novel Moonlight Function of Glyceraldehyde-3-Phosphate Dehydrogenase (Gapdh) for Immunomodulation. Biofactors 2017, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Uddin, J.; Piao, L.; Hwang, I.; Lee, J.H.; Ha, H. Novel Role of Endogenous Catalase in Macrophage Polarization in Adipose Tissue. Mediat. Inflamm. 2016, 2016, 8675905. [Google Scholar] [CrossRef] [Green Version]
- Karantza, V. Keratins in Health and Cancer: More Than Mere Epithelial Cell Markers. Oncogene 2011, 30, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Hamill, K.J.; Hiroyasu, S.; Colburn, Z.T.; Ventrella, R.V.; Hopkinson, S.B.; Skalli, O.; Jones, J.C. Alpha Actinin-1 Regulates Cell-Matrix Adhesion Organization in Keratinocytes: Consequences for Skin Cell Motility. J. Investig. Dermatol. 2015, 135, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.R.; Gómez, B.I.; McIntyre, M.K.; Dubick, M.A.; Christy, R.J.; Nicholson, S.E.; Burmeister, D.M. The Cutaneous Microbiome and Wounds: New Molecular Targets to Promote Wound Healing. Int. J. Mol. Sci. 2018, 19, 2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdukiewicz, M.; Sidorczuk, K.; Rafacz, D.; Pietluch, F.; Chilimoniuk, J.; Rödiger, S.; Gagat, P. Proteomic Screening for Prediction and Design of Antimicrobial Peptides with Ampgram. Int. J. Mol. Sci. 2020, 21, 4310. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Sidorowicz, A.; Mikosinski, J.; Krzyżanowski, M.; Orleanski, J.; Twardowska-Saucha, K.; Nykaza, A.; Dyaczynski, M.; Belz-Lagoda, B.; Dziwiszek, G.; et al. Evaluation of Ll-37 in Healing of Hard-to-Heal Venous Leg Ulcers: A Multicentric Prospective Randomized Placebo-Controlled Clinical Trial. Wound Repair Regen. 2021, 6, 938–950. [Google Scholar] [CrossRef]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial Peptides and Wound Healing: Biological and Therapeutic Considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Mildmay-White, A.; Khan, W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef]
- Wessel, D.; Flügge, U.I. A Method for the Quantitative Recovery of Protein in Dilute Solution in the Presence of Detergents and Lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Kuzniewska, B.; Cysewski, D.; Wasilewski, M.; Sakowska, P.; Milek, J.; Kulinski, T.M.; Winiarski, M.; Kozielewicz, P.; Knapska, E.; Dadlez, M.; et al. Mitochondrial Protein Biogenesis in the Synapse Is Supported by Local Translation. EMBO Rep. 2020, 21, e48882. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Munir, S.; Mulaw, M.A.; Singh, K.; Crisan, D.; Sindrilaru, A.; Treiber, N.; Wlaschek, M.; Huber-Lang, M.; Gebhard, F.; et al. A Novel S100a8/A9 Induced Fingerprint of Mesenchymal Stem Cells Associated with Enhanced Wound Healing. Sci. Rep. 2018, 8, 6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sroussi, H.Y.; Köhler, G.A.; Agabian, N.; Villines, D.; Palefsky, J.M. Substitution of Methionine 63 or 83 in S100a9 and Cysteine 42 in S100a8 Abrogate the Antifungal Activities of S100a8/A9: Potential Role for Oxidative Regulation. FEMS Immunol. Med. Microbiol. 2009, 55, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehl-Fie, T.E.; Chitayat, S.; Hood, M.I.; Damo, S.; Restrepo, N.; Garcia, C.; Munro, K.A.; Chazin, W.J.; Skaar, E.P. Nutrient Metal Sequestration by Calprotectin Inhibits Bacterial Superoxide Defense, Enhancing Neutrophil Killing of Staphylococcus Aureus. Cell Host Microbe 2011, 10, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100a8/S100a9): A Key Protein between Inflammation and Cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-H.; Yu, N.-N.; Jin, Y.-H.; Mao, Y.-Y.; Feng, L.; Liu, Y.; Wang, A.-G.; Sun, H.-N.; Kwon, T.; Han, Y.-H. Peroxiredoxin Ii with Dermal Mesenchymal Stem Cells Accelerates Wound Healing. Aging 2021, 13, 13926. [Google Scholar] [CrossRef]
- Takayama, Y.; Aoki, R. Roles of Lactoferrin on Skin Wound Healing. Biochem. Cell Biol. 2011, 90, 497–503. [Google Scholar] [CrossRef]
- Lu, J.; Guevara, M.A.; Francis, J.D.; Spicer, S.K.; Moore, R.E.; Chambers, S.A.; Craft, K.M.; Manning, S.D.; Townsend, S.D.; Gaddy, J.A. Analysis of Susceptibility to the Antimicrobial and Anti-Biofilm Activity of Human Milk Lactoferrin in Clinical Strains of Streptococcus Agalactiae with Diverse Capsular and Sequence Types. Front. Cell. Infect. Microbiol. 2021, 11, 740872. [Google Scholar] [CrossRef]
- Gupta, A.K.; Parasar, D.; Sagar, A.; Choudhary, V.; Chopra, B.S.; Garg, R.; Ashish; Khatri, N. Analgesic and Anti-Inflammatory Properties of Gelsolin in Acetic Acid Induced Writhing, Tail Immersion and Carrageenan Induced Paw Edema in Mice. PLoS ONE 2015, 10, e0135558. [Google Scholar] [CrossRef]
- Witke, W.; Sharpe, A.H.; Hartwig, J.H.; Azuma, T.; Stossel, T.P.; Kwiatkowski, D.J. Hemostatic, Inflammatory, and Fibroblast Responses Are Blunted in Mice Lacking Gelsolin. Cell 1995, 81, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-W.; Zhang, C.-N.; Cao, Y.-J.; Qu, Y.-X.; Li, T.-Y.; Yang, T.-G.; Geng, D.; Sun, Y.-K. Bidirectional Regulation of I-Type Lysozyme on Cutaneous Wound Healing. Biomed. Pharmacother. 2020, 131, 110700. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.M.; Harder, J. Antimicrobial Skin Peptides and Proteins. Cell. Mol. Life Sci. CMLS 2006, 63, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Kaijzel, E.L.; Koolwijk, P.; VAN Erck, M.G.M.; VAN Hinsbergh, V.W.M.; DE Maat, M.P.M. Molecular Weight Fibrinogen Variants Determine Angiogenesis Rate in a Fibrin Matrix in Vitro and in vivo. J. Thromb. Haemost. 2006, 4, 1975–1981. [Google Scholar] [CrossRef]
- Duong, L.; Gross, S.P.; Siryaporn, A. A Novel Antibacterial Strategy: Histone and Antimicrobial Peptide Synergy. Microb. Cell 2020, 7, 309. [Google Scholar] [CrossRef]
- Brookes, J.D.L.; Jaya, J.S.; Tran, H.; Vaska, A.; Werner-Gibbings, K.; D’Mello, A.C.; Wong, J.; Lemoh, C.N.; Saunder, A.C.; Yii, M.K. Broad-Ranging Nutritional Deficiencies Predict Amputation in Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2020, 19, 27–33. [Google Scholar] [CrossRef]
- Mockenhaupt, M.; Viboud, C.; Dunant, A.; Naldi, L.; Halevy, S.; Bavinck, J.N.B.; Sidoroff, A.; Schneck, J.; Roujeau, J.C.; Flahault, A. Stevens–Johnson Syndrome and Toxic Epidermal Necrolysis: Assessment of Medication Risks with Emphasis on Recently Marketed Drugs. The Euroscar-Study. J. Investig. Dermatol. 2008, 128, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Suri, B.; Verma, N.; Schmidtchen, A. Toll-Like Receptor 3 Agonist, Polyinosinic-Polycytidylic Acid, Upregulates Carbonic Anhydrase Ii in Human Keratinocytes. Acta Dermato-Venereol. 2018, 98, 762–765. [Google Scholar] [CrossRef] [Green Version]
- Pechter, P.M.; Gil, J.; Valdes, J.; Tomic-Canic, M.; Pastar, I.; Stojadinovic, O.; Kirsner, R.S.; Davis, S.C. Keratin Dressings Speed Epithelialization of Deep Partial-Thickness Wounds. Wound Repair Regen. 2012, 20, 236–242. [Google Scholar] [CrossRef]
- Roth, W.; Hatzfeld, M.; Magin, T.M. Targeting the Palm: A Leap Forward toward Treatment of Keratin Disorders. J. Investig. Dermatol. 2012, 132, 1541–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moll, R.; Divo, M.; Langbein, L. The Human Keratins: Biology and Pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.M.K.; Aryal, E.; Safari, E.; Mojsoska, B.; Jenssen, H.; Prabhala, B.K. Current State of Slc and Abc Transporters in the Skin and Their Relation to Sweat Metabolites and Skin Diseases. Proteomes 2021, 9, 23. [Google Scholar] [CrossRef]
- D’Alessandro, S.; Magnavacca, A.; Perego, F.; Fumagalli, M.; Sangiovanni, E.; Prato, M.; Dell’Agli, M.; Basilico, N. Effect of Hypoxia on Gene Expression in Cell Populations Involved in Wound Healing. BioMed Res. Int. 2019, 2019, 2626374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, J.V.; Howley, P.; Davis, R.; Mashchak, A.; Goheen, S.C. Protease Inhibition by Oleic Acid Transfer from Chronic Wound Dressings to Albumin. Int. J. Pharm. 2007, 340, 42–51. [Google Scholar] [CrossRef]
- Bangalore, N.; Travis, J.; Onunka, V.C.; Pohl, J.; Shafer, W.M. Identification of the Primary Antimicrobial Domains in Human Neutrophil Cathepsin G. J. Biol. Chem. 1990, 265, 13584–13588. [Google Scholar] [CrossRef]
- Cazander, G.; Jukema, G.N.; Nibbering, P.H. Complement Activation and Inhibition in Wound Healing. Clin. Dev. Immunol. 2012, 2012, 534291. [Google Scholar] [CrossRef]
- Rafail, S.; Kourtzelis, I.; Foukas, P.G.; Markiewski, M.M.; DeAngelis, R.A.; Guariento, M.; Ricklin, D.; Grice, E.; Lambris, J.D. Complement Deficiency Promotes Cutaneous Wound Healing in Mice. J. Immunol. 2014, 194, 1285–1291. [Google Scholar] [CrossRef] [Green Version]
- Ganeshan, K.; Chawla, A. Metabolic Regulation of Immune Responses. Annu. Rev. Immunol. 2014, 32, 609. [Google Scholar] [CrossRef] [Green Version]
- Im, M.J.; Hoopes, J.E. Energy Metabolism in Healing Skin Wounds. J. Surg. Res. 1970, 10, 459–464. [Google Scholar] [CrossRef]
- Donaldson, D.J.; Mahan, J.T. Fibrinogen and Fibronectin as Substrates for Epidermal Cell Migration During Wound Closure. J. Cell Sci. 1983, 62, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound Healing--Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.G.; Smith, A.; Kashpur, O.; Yanez, V.; Knight, E.; Mooney, D.J.; Veves, A.; Tomic-Canic, M.; Garlick, J.A. Altered Ecm Deposition by Diabetic Foot Ulcer-Derived Fibroblasts Implicates Fibronectin in Chronic Wound Repair. Wound Repair Regen. 2016, 24, 630–643. [Google Scholar] [CrossRef] [Green Version]
- Dimayuga, P.; Zhu, J.; Oguchi, S.; Chyu, K.Y.; Xu, X.O.H.; Yano, J.; Shah, P.K.; Nilsson, J.; Cercek, B. Reconstituted Hdl Containing Human Apolipoprotein a-1 Reduces Vcam-1 Expression and Neointima Formation Following Periadventitial Cuff-Induced Carotid Injury in Apoe Null Mice. Biochem. Biophys. Res. Commun. 1999, 264, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Vowinkel, T.; Mori, M.; Krieglstein, C.F.; Russell, J.; Saijo, F.; Bharwani, S.; Turnage, R.H.; Davidson, W.; Tso, P.; Granger, D.N.; et al. Apolipoprotein a-Iv Inhibits Experimental Colitis. J. Clin. Investig. 2004, 114, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.R.; Wang, Y.; Adili, R.; Ju, L.; Spring, C.M.; Jin, J.W.; Yang, H.; Neves, M.A.; Chen, P.; Yang, Y.; et al. Apolipoprotein a-Iv Binds Aiibβ3 Integrin and Inhibits Thrombosis. Nat. Commun. 2018, 9, 3608. [Google Scholar] [CrossRef] [Green Version]
- Karahan, A.; AAbbasoğlu, A.; Işık, S.A.; Çevik, B.; Saltan, Ç.; Elbaş, N.Ö.; Yalılı, A. Factors Affecting Wound Healing in Individuals with Pressure Ulcers: A Retrospective Study. Ostomy/Wound Manag. 2018, 64, 32–39. [Google Scholar] [CrossRef]
- Płoski, R.; Pollak, A.; Müller, S.; Franaszczyk, M.; Michalak, E.; Kosinska, J.; Stawinski, P.; Spiewak, M.; Seggewiss, H.; Bilinska, Z.T. Does P. Q247x in Trim63 Cause Human Hypertrophic Cardiomyopathy? Circ. Res. 2014, 114, e2–e5. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The Mutational Constraint Spectrum Quantified from Variation in 141,456 Humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]



| ADSC (N = 23) | Fibrin Gel (N = 23) | Total (N = 46) | p Value | ||
|---|---|---|---|---|---|
| Gender (N, %) | M | 20 (87%) | 17 (74%) | 37 (80%) | 0.459 |
| F | 3 (13%) | 6 (26%) | 9 (20%) | ||
| Age (years) | Mean ± SD | 56.7 (11.1) | 61.7 (7.5) | 59.2 (9.7) | 0.130 |
| Median | 56.0 | 63.0 | 61.0 | ||
| Min, Max | 42.0, 75.0 | 38.0, 74.0 | 38.0, 75.0 | ||
| Body weight (kg) | Mean ± SD | 97.0 (20.4) | 103.6 (18.2) | 100.3 (19.4) | 0.395 |
| Median | 98.0 | 105.0 | 101.0 | ||
| Min, Max | 49.0, 130.0 | 68.0, 140.0 | 49.0, 140.0 | ||
| Height (cm) | Mean ± SD | 179.7 (9.2) | 177.1 (7.6) | 178.4 (8.5) | 0.499 |
| Median | 178.0 | 179.0 | 178.0 | ||
| Min, Max | 160.0, 200.0 | 160.0, 188.0 | 160.0, 200.0 | ||
| Body mass index (kg/m2) | Mean ± SD | 30.0 (5.9) | 33.0 (5.6) | 31.5 (5.9) | 0.068 |
| Median | 30.8 | 33.0 | 31.6 | ||
| Min, Max | 19.1, 39.8 | 21.5, 49.6 | 19.1, 49.6 | ||
| Wound size in week 0 (cm2) | Mean ± SD | 2.72 (2.85) | 2.68 (1.58) | 2.70 (2.28) | 0.420 |
| Median | 1.9 | 2.20 | 1.95 | ||
| Min, Max | 1.0, 14.0 | 1.00, 5.6 | 1.00, 14.00 | ||
| Ulcer location (N, %) | Left limb | 11 (48%) | 11 (48%) | 22 (48%) | 1.000 |
| Right limb | 12 (52%) | 12 (52%) | 24 (52%) | ||
| Forefoot | 18 (78%) | 16 (70%) | 34 (74%) | 0.099 | |
| Hindfoot | 0 (0%) | 4 (17%) | 4 (9%) | ||
| Midfoot | 5 (22%) | 3 (13%) | 8 (17%) | ||
| Plantar | 17 (74%) | 20 (87%) | 37 (80%) | 0.486 | |
| Dorsal | 3 (13%) | 2 (9%) | 5 (11%) | ||
| Both | 3 (13%) | 1 (4%) | 4 (9%) | ||
| Duration of diabetic foot ulcers (months) | Mean ± SD | 19.5 (31.1) | 30.2 (35.9) | 24.9 (33.6) | 0.155 |
| Median | 9.0 | 14.0 | 12.0 | ||
| Min, Max | 1.0, 144.0 | 1.0, 156.0 | 1.0, 156.0 | ||
| Duration of diabetes mellitus (years) | Mean ± SD | 16.1 (8.6) | 19.7 (10.7) | 17.9 (9.8) | 0.424 |
| Median | 16.0 | 18.0 | 16.0 | ||
| Min, Max | 2.0, 39.0 | 3.0, 44.0 | 2.0, 44.0 | ||
| DM type (n, %) | Type 1 | 4 (17%) | 5 (22%) | 9 (20%) | 0.361 |
| Type 2 | 15 (65%) | 17 (74%) | 32 (70%) | ||
| Other | 4 (17%) | 1 (4%) | 5 (11%) | ||
| Neuropathy (N, %) | Yes | 23 (100%) | 23 (100%) | 46 (100%) | --- |
| No | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Retinopathy (N, %) | Yes | 14 (61%) | 13 (57%) | 27 (59%) | 0.811 |
| No | 9 (39%) | 10 (43%) | 19 (41%) | ||
| Coronary artery disease (N, %) | Yes | 3 (13%) | 6 (26%) | 9 (20%) | 0.459 |
| No | 20 (87%) | 17 (74%) | 37 (80%) | ||
| Hypertension (N, %) | Yes | 16 (70%) | 22 (96%) | 38 (83%) | 0.130 |
| No | 7 (30%) | 1 (4%) | 8 (17%) | ||
| Smoking history (N, %) | Yes | 15 (65%) | 14 (61%) | 29 (63%) | 0.811 |
| No | 8 (35%) | 9 (39%) | 17 (37%) | ||
| ABI | Mean ± SD | 1.05 (0.14) | 1.11 (0.18) | 1.08 (0.16) | 0.130 |
| Median | 1.00 | 1.08 | 1.00 | ||
| Min, Max | 0.83, 1.40 | 0.70, 1.45 | 0.70, 1.45 | ||
| tPO2 | Mean ± SD | 54.6 (19.3) | 52.3 (17.9) | 53.4 (18.4) | 0.695 |
| Median | 53.0 | 50.0 | 50.5 | ||
| Min, Max | 25.0, 98.0 | 25.0, 87.0 | 25.0, 98.0 | ||
| Glycated hemoglobin A1c at screening visit (%) | Mean ± SD | 7.25 (1.31) | 7.57 (1.35) | 7.41 (1.33) | 0.536 |
| Median | 7.40 | 7.30 | 7.40 | ||
| Min, Max | 5.00, 10.70 | 5.40, 10.80 | 5.00, 10.80 | ||
| Creatinine level (mg/dL) | Mean ± SD | 1.11 (0.28) | 1.17 (0.38) | 1.14 (0.33) | 0.828 |
| Median | 1.11 | 1.05 | 1.07 | ||
| Min, Max | 0.64, 1.60 | 0.74, 1.87 | 0.64, 1.87 | ||
| GFR (mL/min/1.73 m2) | Mean ± SD | 74.8 (21.2) | 71.3 (24.4) | 73.0 (22.7) | 0.811 |
| Median | 78.0 | 74.0 | 76.0 | ||
| Min, Max | 45.0, 113.0 | 27.0, 105.0 | 27.0, 113.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrozikiewicz-Rakowska, B.; Szabłowska-Gadomska, I.; Cysewski, D.; Rudziński, S.; Płoski, R.; Gasperowicz, P.; Konarzewska, M.; Zieliński, J.; Mieczkowski, M.; Sieńko, D.; et al. Allogenic Adipose-Derived Stem Cells in Diabetic Foot Ulcer Treatment: Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact. Int. J. Mol. Sci. 2023, 24, 1472. https://doi.org/10.3390/ijms24021472
Mrozikiewicz-Rakowska B, Szabłowska-Gadomska I, Cysewski D, Rudziński S, Płoski R, Gasperowicz P, Konarzewska M, Zieliński J, Mieczkowski M, Sieńko D, et al. Allogenic Adipose-Derived Stem Cells in Diabetic Foot Ulcer Treatment: Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact. International Journal of Molecular Sciences. 2023; 24(2):1472. https://doi.org/10.3390/ijms24021472
Chicago/Turabian StyleMrozikiewicz-Rakowska, Beata, Ilona Szabłowska-Gadomska, Dominik Cysewski, Stefan Rudziński, Rafał Płoski, Piotr Gasperowicz, Magdalena Konarzewska, Jakub Zieliński, Mateusz Mieczkowski, Damian Sieńko, and et al. 2023. "Allogenic Adipose-Derived Stem Cells in Diabetic Foot Ulcer Treatment: Clinical Effectiveness, Safety, Survival in the Wound Site, and Proteomic Impact" International Journal of Molecular Sciences 24, no. 2: 1472. https://doi.org/10.3390/ijms24021472